Abstract
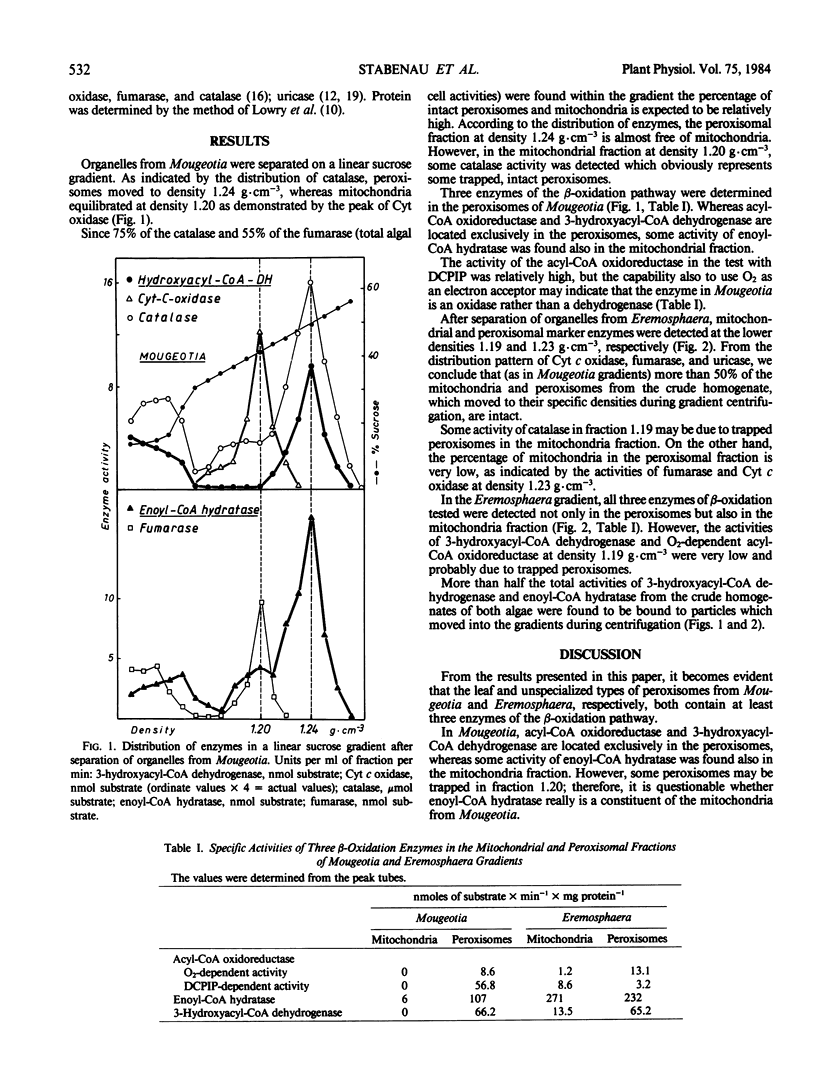
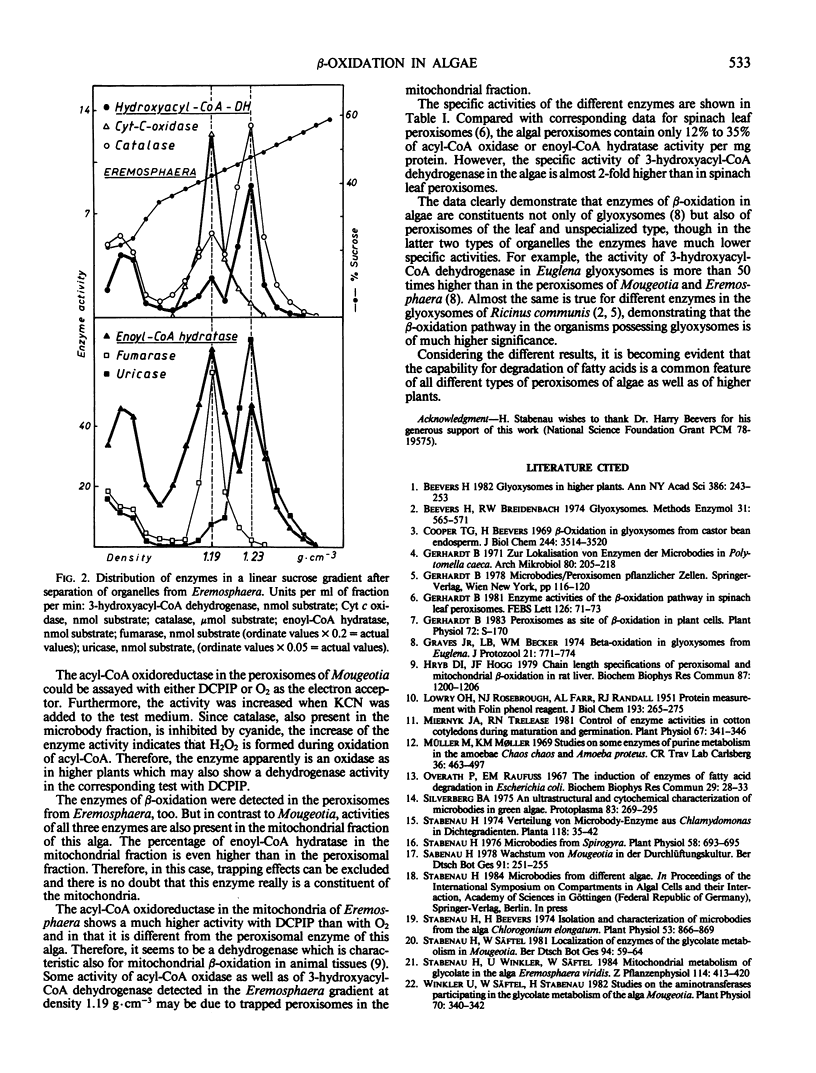
The algae Mougeotia and Eremosphaera were used for isolation of microbodies with the characteristics of leaf peroxisomes and unspecialized peroxisomes, respectively. In both types of organelles, the following enzymes of the β-oxidation pathway were determined: acyl-CoA oxido-reductase, enoyl-CoA hydratase, and 3-hydroxyacyl-CoA dehydrogenase. There are indications that the peroxisomal oxidoreductase of both algae is a H2O2-forming oxidase rather than a dehydrogenase.
The enzymes enoyl-CoA hydratase and acyl-CoA oxidoreductase are located also in the mitochondria from Eremosphaera but not from Mougeotia. The mitochondrial acyl-CoA oxidizing enzyme was found to be a dehydrogenase. The specific activities of acyl-CoA oxidase and enoyl-CoA hydratase are lower than in spinach leaf peroxisomes. However, the activity of 3-hydroxyacyl-CoA dehydrogenase in the peroxisomes of both algae is almost 2-fold higher. The capability for degradation of fatty acids is a common feature of all different types of peroxisomes from algae.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper T. G., Beevers H. Beta oxidation in glyoxysomes from castor bean endosperm. J Biol Chem. 1969 Jul 10;244(13):3514–3520. [PubMed] [Google Scholar]
- Gerhardt B. Zur Lokalisation von Enzymen der Microbodies in Polytomella caeca. Arch Mikrobiol. 1971;80(3):205–218. [PubMed] [Google Scholar]
- Graves L. B., Jr, Becker W. M. Beta-oxidation in glyoxysomes from Euglena. J Protozool. 1974 Nov;21(5):771–774. doi: 10.1111/j.1550-7408.1974.tb03750.x. [DOI] [PubMed] [Google Scholar]
- Hryb D. J., Hogg J. F. Chain length specificities of peroxisomal and mitochondrial beta-oxidation in rat liver. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1200–1206. doi: 10.1016/s0006-291x(79)80034-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miernyk J. A., Trelease R. N. Control of Enzyme Activities in Cotton Cotyledons during Maturation and Germination : IV. beta-OXIDATION. Plant Physiol. 1981 Feb;67(2):341–346. doi: 10.1104/pp.67.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M., Moller K. M. Studies on some enzymes of purine metabolism in the amoebae Chaos chaos and Amoeba proteus. C R Trav Lab Carlsberg. 1969;36(24):463–497. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- Silverberg B. A. An ultrastructural and cytochemical characterization of microbodies in the green algae. Protoplasma. 1975;83(3):269–295. doi: 10.1007/BF01282559. [DOI] [PubMed] [Google Scholar]
- Stabenau H., Beevers H. Isolation and Characterization of Microbodies from the Alga Chlorogonium elongatum. Plant Physiol. 1974 Jun;53(6):866–869. doi: 10.1104/pp.53.6.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabenau H. Microbodies from spirogyra: organelles of a filamentous alga similar to leaf peroxisomes. Plant Physiol. 1976 Nov;58(5):693–695. doi: 10.1104/pp.58.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler U., Säftel W., Stabenau H. Studies on the aminotransferases participating in the glycolate metabolism of the alga mougeotia. Plant Physiol. 1982 Aug;70(2):340–343. doi: 10.1104/pp.70.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]


