Abstract
Disruption of the HSP104 gene in a mutant which cannot accumulate trehalose during heat shock treatment caused trehalose accumulation (H. Iwahashi, K. Obuchi, S. Fujii, and Y. Komatsu, Lett. Appl. Microbiol 25:43–47, 1997). This implies that Hsp104 affects trehalose metabolism. Thus, we measured the activities of enzymes involved in trehalose metabolism. The activities of trehalose-synthesizing and -hydrolyzing enzymes are low in the HSP104 disruption mutant during heat shock. This data is correlated with intracellular trehalose and glucose levels observed in the HSP104 disruption mutant. These results suggest that during heat shock, Hsp104 contributes to the simultaneous increase in both accumulation and degradation of trehalose.
In cells of yeasts and some other organisms, exposure to temperatures higher than the optimum for growth results in enhancement of the synthesis of heat shock proteins and the metabolism of trehalose (17). These cells then acquire the ability to survive under more extreme conditions, a phenomenon referred to as transitory thermotolerance (17). Trehalose and neutral trehalase (Nth1 and Nth2) are suggested to be protectants against thermal and other stress conditions. Trehalose biosynthesis is catalyzed by the sequential action of trehalose-6-phosphate synthase (Tps1) and trehalose-6-phosphate phosphatase (Tps2) activities (called the trehalose synthase complex) (12), with UDP-glucose and glucose-6-phosphate used as substrates. These activities are increased by heat shock treatment, and yeast accumulates trehalose (12, 17). It has been observed that trehalose prevents protein denaturation (4) and stabilizes the membrane (5, 13). Neutral trehalase catalyzes the hydrolysis of trehalose to glucose, and its expression is induced by heat shock treatment (12). Mutants deficient in neutral trehalase show decreased thermotolerance in spite of high trehalose levels in cells (9–11). Thus, neutral trehalase activity possibly supplies energy to rescue certain cellular systems during and after exposure to supraoptimal temperatures (12). It has also been reported that trehalose inhibits disaggregation of proteins by Hsp104 (20), thus suggesting that neutral trehalase is an important factor in thermotolerance. In Saccharomyces cerevisiae, Hsp104, Hsp90, Hsp70, and Hsp26 are the major heat shock proteins (17). However, only Hsp104 has been genetically confirmed to be an important factor in transitory thermotolerance (14, 17). Hsp104 is considered to be in the same family as the ClpA and ClpB proteins of Escherichia coli, which are believed to assist in protein degradation (15). Hsp104 forms an oligomeric structure in the presence of ATP in a way similar to that of the ClpA protein (16). Thus, Hsp104 may regulate proteases or be involved in preventing or resolving the aggregation of vital cellular proteins during exposure to supraoptimal temperatures (8, 15, 16). Much work has centered on the role of heat shock proteins and trehalose metabolism in the heat shock response, but no direct relationship or interplay between the two systems has been clearly demonstrated. With respect to Hsp104 and trehalose, at least three studies (3, 7, 22) have shown no effect of Hsp104 on trehalose accumulation in yeast. Thus, the function of Hsp104 is considered to be independent of trehalose metabolism (8). However, Hottiger et al. observed about 30% slower trehalose degradation during recovery from heat shock in an HSP104 disruption mutant (hsp104 mutant) (3). Recently, synergy between Hsp104 and trehalose was documented based on studies with mutants carrying disruptions of HSP104 and TPS1, encoding trehalose-6-phosphate synthase (Tps1) (20). In these studies, the activities of trehalose-metabolizing enzymes (which regulate trehalose concentration) were not determined.
Recently, we studied mutants which are not able to accumulate trehalose and/or Hsp104, in order to examine the contributions of these factors to barotolerance (7). The double mutant could neither synthesize Hsp104 nor accumulate trehalose in the stationary phase; however, it accumulated trehalose during heat shock treatment (7). This suggested the important control of Hsp104 over trehalose metabolism. In this study, we show the effect of Hsp104 on trehalose metabolism by using mutants carrying a disruption of HSP104. Data on the cellular contents of trehalose and glucose as well as on the activities of neutral trehalase, trehalose-6-phosphate synthase, and trehalose-6-phosphate phosphatase are presented.
Strains and growth conditions.
S. cerevisiae strains used in this work are listed in Table 1. Cells were grown on yeast extract-peptone-dextrose medium at 30°C, as previously described (6). Heat shock was performed on logarithmic-phase cells by shifting them from 30°C to 43°C for 0 to 120 min.
TABLE 1.
Yeast strains
| Strain | Genotype (phenotype) | Source or reference |
|---|---|---|
| W303aLEU2+ | MATα can1 ade2 his3 LEU2+ trp1 ura3 | S. Lindquist |
| Δhsp104 LEU2 | MATα can1 ade2 his3 leu2 trp1 ura3 hsp104::LEU2+ | S. Lindquist |
| 224A-12D | MATα his4 leu2 ura3 (trehalose deficient) | C. De Virgilio |
| CWG12 | MATα ade2 his3 (and/or his4) leu2 ura3 hsp104::LEU2+ (trehalose deficient) | 7 |
| CWG13 | MATα ade2 his3 (and/or his4) leu2 ura3 | 7 |
| CWG14 | MATα ade2 his3 (and/or his4) leu2 ura3 (trehalose deficient) | 7 |
| CWG15 | MATα ade2 his3 (and/or his4) leu2 ura3 hsp104::LEU2+ | 7 |
Enzyme activities.
Enzyme activities were measured in crude extracts. Data presented are averages and standard deviations (SD) of at least three independent experiments. The crude extracts were prepared by breaking the cells (30 s each for 10 times) with glass beads in an equal volume of 50 mM imidazole hydrochloride buffer, pH 7.0, containing protease inhibitors and Complete TM (Boehringer, Mannheim, Germany). Broken cells were centrifuged at 12,000 × g for 20 min, and the supernatants were used as the crude extracts. Neutral trehalase activity was measured at 37°C, according to App and Holzer (1), by using a glucose test kit purchased from Wako Co., Osaka, Japan. One unit of neutral trehalase is the amount of enzyme that hydrolyzes 1 μmol of trehalose in 1 min. Trehalose-6-phosphate synthase activity was measured spectrophotometrically, as reported by Vandercammen et al. (21). A reaction mixture containing 2 mM UDP-glucose, 10 mM glucose-6-phosphate, 1 mM EDTA, 50 mM KCl, and 10 mM Mg acetate was incubated at 42°C for 20 min. The reaction was stopped by heating, and the solution was then centrifuged. UDP was measured in the supernatant by the decrease in absorbance at 340 nm in a mixture containing 0.15 mM NADH, 0.25 mM phosphoenolpyruvate, 100 mM KCl, 5 mM MgCl2, 10 μg of pyruvate kinase per ml, 10 μg of lactate dehydrogenase per ml, and 25 mM HEPES (pH 7.1) (18). One unit of trehalose-6-phosphate synthase is the amount of enzyme that produces 1 μmol of UDP in 1 min. Trehalose-6-phosphate phosphatase activity was measured according to Vandercammen et al. (21). One unit of trehalose-6-phosphate phosphatase is the amount of enzyme that produces 1 μmol of trehalose in 1 min. Trehalose in the reaction mixture was measured as glucose after hydrolysis by acid trehalase (5). The reaction mixture contained 25 mM sodium phosphate buffer (pH 6.0), 0.5 mM trehalose-6-phosphate, 10 mM MgCl2, and 50 mM KCl, and the crude extract was incubated at 30°C.
Trehalose, glucose, and protein measurements.
Cellular trehalose was measured by liquid chromatography after extraction in a boiling-water bath, as previously described (5). Cellular glucose was measured in the crude extracts by using the glucose test kit from Wako Co. Protein was measured with a DC protein assay kit (Bio-Rad Japan). Data presented are averages and SD values of at least three independent experiments.
A double mutant deficient in HSP104 and trehalose accumulates trehalose during heat shock treatment.
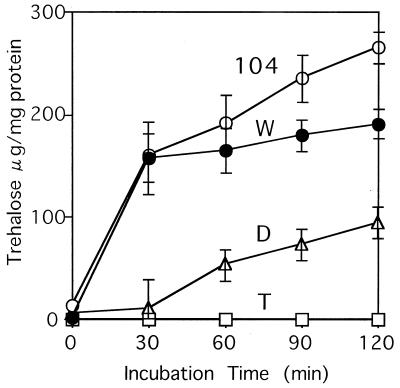
In the course of studying the contribution of trehalose and Hsp104 to barotolerance, we isolated a double mutant unable to accumulate trehalose (in the logarithmic and stationary phases) and unable to synthesize Hsp104 (Table 1) (7). However, this mutant accumulates trehalose during heat shock. This showed a correlation between trehalose metabolism and Hsp104 (7). To understand the accumulation of trehalose in the double mutant, we estimated the cellular contents of trehalose in CWG13 (wild type), CWG14 (trehalose deficient), CWG15 (HSP104 disruption), and CWG12 (trehalose and HSP104 deficient) under heat shock conditions (Fig. 1). The wild type and the hsp104 mutant accumulated trehalose within 30 min of heat shock and gradually increased the amount. The hsp104 mutant accumulated more trehalose than the wild-type strain after 30 min. As expected, the trehalose-deficient strain was unable to accumulate trehalose during the entire heat shock period. In the double mutant, trehalose accumulation started after 30 min of heat shock, but the level was much lower than in the single hsp104 mutant and the wild type.
FIG. 1.
Accumulation of trehalose in trehalose-deficient and/or hsp104 mutant cells during heat shock treatment. Logarithmic-phase cells of the wild-type strain (CWG13 [W]), the trehalose-deficient mutant (CWG14 [T]), the hsp104 mutant (CWG15 [104]), and the double mutant (CWG12 [D]), were incubated at 43°C, and trehalose accumulation was estimated at the indicated times. The error bar for each sample represents the SD of at least three independent experiments.
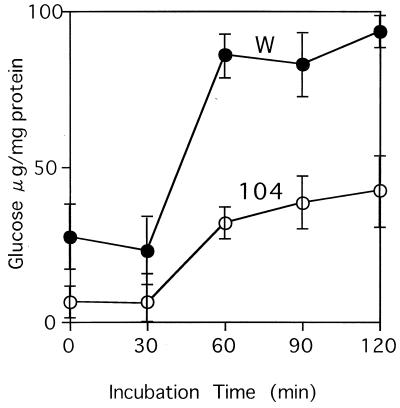
Glucose levels in the hsp104 mutant.
Trehalose is synthesized from UDP-glucose and glucose-6-phosphate and is broken down to glucose (12). Thus, we also estimated the cellular content of glucose in the hsp104 mutant during the heat shock conditions. As shown in Fig. 2, the glucose level in the wild type increased within 60 min of heat shock and remained fairly constant thereafter. Similar patterns of glucose accumulation were observed in the hsp104 mutant. However, the levels were lower in both logarithmic-phase cells and heat-shocked cells than in the wild-type strain. These results suggest that Hsp104 affects glucose levels especially during heat shock.
FIG. 2.
Accumulation of glucose in hsp104 mutant cells during heat shock treatment. Logarithmic-phase cells of the wild type (CWG13 [W]) and the hsp104 mutant (CWG15 [104]) were incubated at 43°C, and intracellular glucose levels were estimated at the indicated times. The error bar for each sample represents the SD of at least three independent experiments.
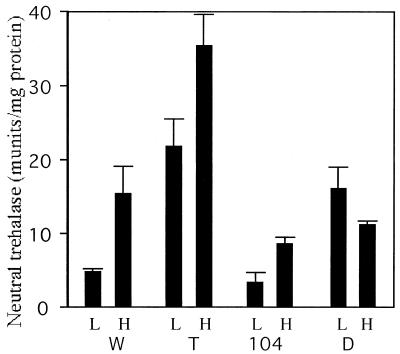
Disruption of HSP104 affects neutral trehalase activity.
The levels of trehalose and glucose observed in the various mutants during heat shock imply that disruption of HSP104 reduced the ability to degrade trehalose to glucose. To examine this possibility, we measured neutral trehalase activities in the mutants in logarithmic phase and after heat shock treatment for 60 min (Fig. 3). The trehalose-deficient mutant showed higher neutral trehalase activities in the logarithmic phase and during heat shock than did the wild-type strain (Fig. 3). In contrast, the hsp104 mutant clearly showed lower neutral trehalase activity after heat shock treatment than did the wild-type strain. This suggests that Hsp104 positively contributes to neutral trehalase activity. The trehalase activities of the double mutants were higher than that of the wild-type strain in logarithmic-phase cells but lower in the heat-shocked cells. This result explains why the double mutant accumulates trehalose during heat shock.
FIG. 3.
Neutral trehalase activities in trehalose-deficient and/or hsp104 mutant cells. Logarithmically growing (L) and heat-shocked (H) cells of the wild type (CWG13 [W]), the trehalose-deficient mutant (CWG14 [T]), the hsp104 mutant (CWG15 [104]), and the double mutant (CWG12 [D]) were harvested, and crude extracts were prepared. The enzyme activities of neutral trehalase were measured in the crude extracts as described in the text. The error bar for each sample represents the SD of at least three independent experiments.
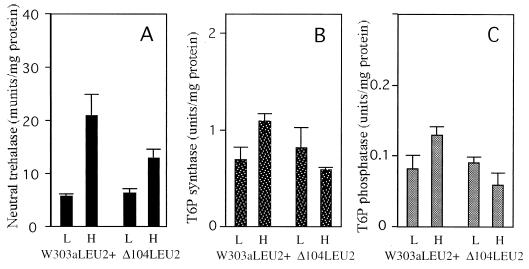
Hsp104 contributes to trehalose metabolism during heat shock.
The neutral trehalase activities, trehalose content, and glucose content of the mutants during heat shock suggest that Hsp104 contributes to trehalose metabolism. However, the trehalose-deficient strain is not genetically well characterized. Thus, we examined trehalose-metabolizing enzyme activities in a different HSP104 disruption background. Figure 4A shows neutral trehalase activity in the logarithmic phase and after heat shock of the hsp104 mutant. As expected, the hsp104 mutant shows lower neutral trehalase activity after heat shock treatment. We confirmed the contribution of Hsp104 to neutral trehalase in the hsp104 mutant and its isogenic strain system. In addition, the hsp104 mutant showed lower trehalose-6-phosphate synthase and phosphatase activities after heat shock treatment than did the wild type (Fig. 4B and C). This suggests that Hsp104 also contributes to trehalose biosynthesis during heat shock conditions. In our experiments, heat shock treatment did not increase trehalose-6-phosphate synthase activity up to three- to fivefold, as reported previously by Hottiger et al. (2) and Ribeiro et al. (19). This is due probably to the different heat shock conditions (43°C) and assay methods used for the present study.
FIG. 4.
Activities of enzymes of trehalose metabolism in hsp104 mutant cells. Logarithmically growing (L) and heat-shocked (H) cells of the wild-type strain (W303aLEU2+) and the hsp104 mutant (Δ104LEU2) were harvested, and crude extracts were prepared. The enzyme activities of neutral trehalase (A), trehalose-6-phosphate (T6P) synthase (B), and trehalose-6-phosphate phosphatase (C) were measured in crude extracts as described in the text. The error bar for each sample represents the SD of at least three independent experiments.
We have defined the effects of Hsp104 on neutral trehalase, trehalose-6-phosphate synthase, and trehalose-6-phosphate phosphatase activities. Disruption of HSP104 caused a decrease in the activities of these enzymes (Fig. 3 and 4). Earlier reports suggested that Hsp104 has no effect on the accumulation of trehalose because the hsp104 mutant accumulates a normal level of trehalose under heat shock conditions (3, 7, 22). Our data do not contradict those observations but explain the reasons for the normal accumulation of trehalose, i.e., the decreased synthesizing and degrading abilities of the Hsp104 mutant. It seems that the contribution of Hsp104 to trehalose metabolism is not the only target of Hsp104, as the hsp104 mutant showed low levels of glucose in the logarithmic phase (Fig. 2). Our data show an interaction between trehalose metabolism and Hsp104; however, we are not certain how this happens. Recently, data have accumulated which show that not only trehalose accumulation but also trehalose degradation is important in order for yeast cells to acquire thermotolerance (9–12). It has been suggested that disruption of NTH1, encoding neutral trehalase, reduces the intracellular glucose level of yeast during heat shock (12) and that the glucose supply is important for thermotolerance (4). Hsp104 seems to contribute to the supply of glucose in the cells through its effect on neutral trehalase, because glucose (Fig. 2) and neutral trehalase activity (Fig. 4) are low in the hsp104 mutant. We suggest that the molecular chaperone, Hsp104, interacts directly or indirectly (e.g., cyclic AMP-dependent protein kinase) with neutral trehalase, trehalose-6-phosphate synthase, and trehalose-6-phosphate phosphatase under different temperature conditions to bring about the increased stability. This speculation is based on results showing that Hsp104 contributes to trehalose-metabolizing enzymes under heat shock conditions (Fig. 4). Several proteins in yeast cells are induced to function at high temperatures; such proteins may need Hsp104 for expressing or maintaining their activities at high temperatures.
Acknowledgments
We thank S. Lindquist, C. De Virgilio, and A. Wiemken for strains and Tomiko Kazama for expert technical assistance.
REFERENCES
- 1.App H, Holzer H. Purification and characterization of neutral trehalase from yeast ABYS1 mutant. J Biol Chem. 1989;264:17583–17588. [PubMed] [Google Scholar]
- 2.Hottiger T, Schmutz P, Wiemken A. Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J Bacteriol. 1987;169:5518–5522. doi: 10.1128/jb.169.12.5518-5522.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hottiger T, De Virgilio C, Bell W, Boller T, Wiemken A. The 70-kilodalton heat-shock proteins of the SSA subfamily negatively modulate heat-shock-induced accumulation of trehalose and promote recovery from heat stress in the yeast Saccharomyces cerevisiae. Eur J Biochem. 1992;210:125–132. doi: 10.1111/j.1432-1033.1992.tb17399.x. [DOI] [PubMed] [Google Scholar]
- 4.Hottiger T, De Virgilio C, Hall M, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur J Biochem. 1994;219:4766–4744. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- 5.Iwahashi H, Obuchi K, Fujii S, Komatsu Y. The correlative evidence suggesting that trehalose stabilizes membrane structure in the yeast Saccharomyces cerevisiae. Cell Mol Biol. 1995;41:763–769. [PubMed] [Google Scholar]
- 6.Iwahashi H, Yang W, Tanguay R M. Detection and expression of the 70kDa heat shock protein ssb1p at different temperatures in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1995;213:484–488. doi: 10.1006/bbrc.1995.2157. [DOI] [PubMed] [Google Scholar]
- 7.Iwahashi H, Obuchi K, Fujii S, Komatsu Y. Barotolerance is dependent on both trehalose and heat shock protein 104 but is essentially different from thermotolerance in Saccharomyces cerevisiae. Lett Appl Microbiol. 1997;25:43–47. doi: 10.1046/j.1472-765x.1997.t01-1-00069.x. [DOI] [PubMed] [Google Scholar]
- 8.Lindquist S, Kim G. Heat-shock protein 104 expression is sufficient for thermotolerance in yeast. Proc Natl Acad Sci USA. 1996;93:5301–5306. doi: 10.1073/pnas.93.11.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nwaka S, Kopp M, Burgert M, Deuchler I, Holzer H. Is thermotolerance of yeast dependent on trehalose accumulation? FEBS Lett. 1994;344:225–228. doi: 10.1016/0014-5793(94)00385-8. [DOI] [PubMed] [Google Scholar]
- 10.Nwaka S, Mechlen B, Destruelle M, Holzer H. Phenotypic features of trehalase mutants in Saccharomyces cerevisiae. FEBS Lett. 1995;360:286–290. doi: 10.1016/0014-5793(95)00105-i. [DOI] [PubMed] [Google Scholar]
- 11.Nwaka S, Kopp M, Holzer H. Expression and function of the trehalase genes NTH1 and YBR0106 in Saccharomyces cerevisiae. J Biol Chem. 1995;270:10193–10198. doi: 10.1074/jbc.270.17.10193. [DOI] [PubMed] [Google Scholar]
- 12.Nwaka S, Holzer H. Molecular biology of trehalose and trehalases in the yeast Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol. 1998;58:197–237. doi: 10.1016/s0079-6603(08)60037-9. [DOI] [PubMed] [Google Scholar]
- 13.Obuchi K, Iwahashi H, Kaul S C, Komatu Y. Heat shock response of yeast confers high pressure tolerance through structural modification of membrane. In: Balny C, Hayashi R, Hermans K, Masson P, editors. Pressure and biotechnology. Montrouge, France: Colloque INSERM/Jhon Libbey Eurotext; 1992. pp. 77–81. [Google Scholar]
- 14.Parsel D A, Sanchez Y, Stitzel D, Lindquist S. Hsp104 is a highly conserved protein with two essential nucleotide-binding sites. Nature. 1991;353:270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- 15.Parsel D A, Kowal A S, Singer M A, Lindquist S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature. 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 16.Parsel D A, Kowal A S, Lindquist S. Saccharomyces cerevisiae Hsp104 protein. J Biol Chem. 1994;269:4480–4487. [PubMed] [Google Scholar]
- 17.Piper P W. Molecular events associated with acquisition of heat tolerance by the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev. 1993;11:339–356. doi: 10.1111/j.1574-6976.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 18.Reinders A, Buckert N, Hohmann S, Thevelein J M, Boller T, Wiemken A, De Virgilio C. Structural analysis of the subunits of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae and their function during heat shock. Mol Microbiol. 1997;24:687–695. doi: 10.1046/j.1365-2958.1997.3861749.x. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro M J S, Silva J T, Panek A D. Trehalose metabolism in Saccharomyces cerevisiae during heat-shock. Biochim Biophys Acta. 1994;1200:139–147. doi: 10.1016/0304-4165(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 20.Singer M A, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–638. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 21.Vandercammen A, Francois J, Hers H-G. Characterization of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase of Saccharomyces cerevisiae. Eur J Biochem. 1989;182:613–620. doi: 10.1111/j.1432-1033.1989.tb14870.x. [DOI] [PubMed] [Google Scholar]
- 22.Winkler K, Kienle I, Burgert M, Wagner J-C, Holzer H. Metabolic regulation of the trehalose content of vegetative yeast. FEBS Lett. 1991;291:269–272. doi: 10.1016/0014-5793(91)81299-n. [DOI] [PubMed] [Google Scholar]
- 23.Zahringer H, Holzer H, Nwaka S. Stability of neutral trehalase during heat stress in Saccharomyces cerevisiae is dependent on the activity of the catalytic subunit of cAMP dependent protein kinase, Tpk1 and Tpk2. Eur J Biochem. 1988;255:544–551. doi: 10.1046/j.1432-1327.1998.2550544.x. [DOI] [PubMed] [Google Scholar]