Abstract
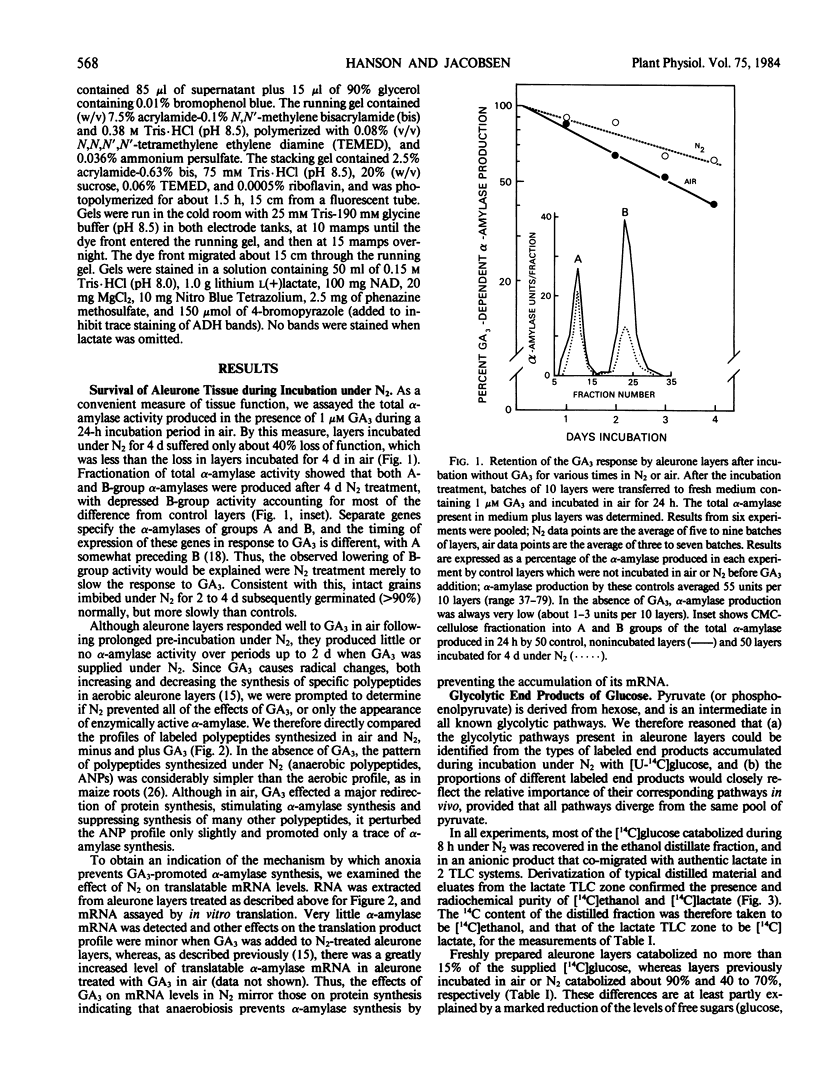
After 4 days in an atmosphere of N2, aleurone layers of barley (Hordeum vulgare L. cv Himalaya) remained viable as judged by their ability to produce near normal amounts of α-amylases when incubated with gibberellic acid (GA3) in air. However, layers did not produce α-amylase when GA3 was supplied under N2, apparently because α-amylase mRNA failed to accumulate.
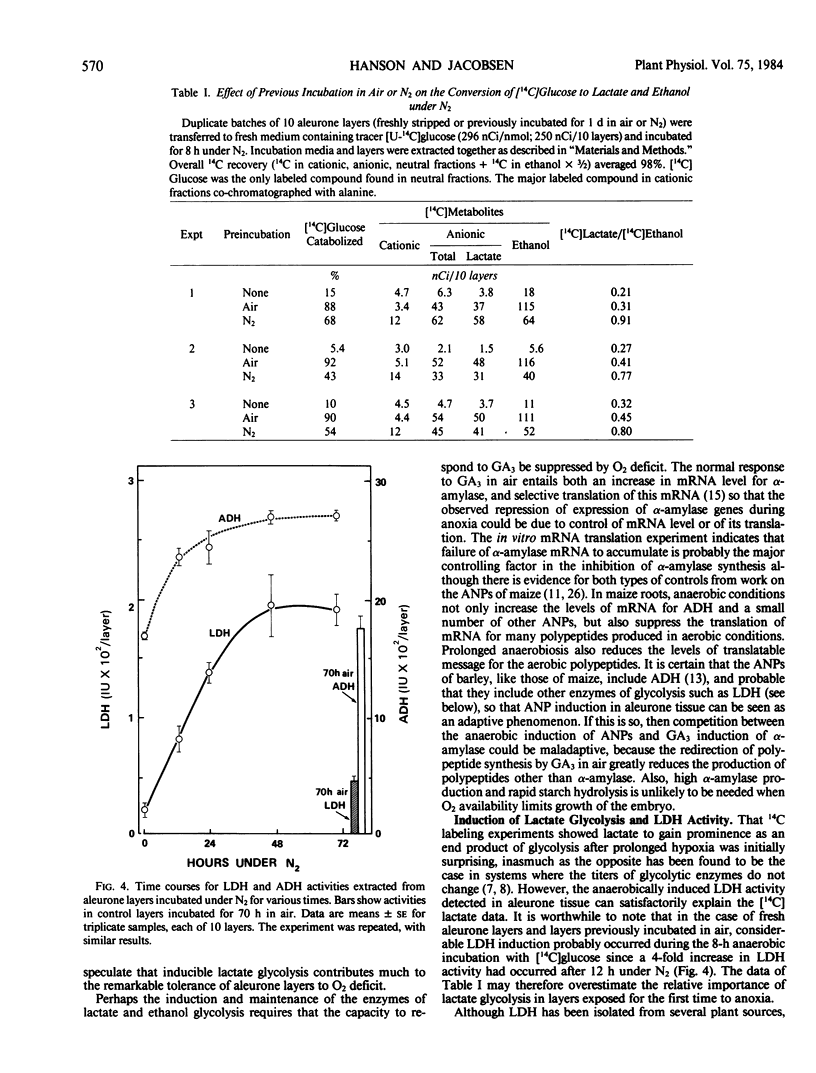
When an 8-hour pulse of [U-14C]glucose was supplied under N2 to freshly prepared aleurone layers, both [14C]lactate and [14C]ethanol accumulated; the [14C]lactate/[14C]ethanol ratio was about 0.3. Prior incubation of layers for 1 day under N2 changed this ratio to about 0.8, indicating an increase in the relative importance of the lactate branch of glycolysis.
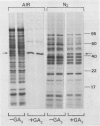
l(+)Lactate dehydrogenase (LDH) activity was low in freshly prepared aleurone layers and increased 10-fold during 2 days under N2, whereas alcohol dehydrogenase activity (ADH) was high initially and rose by 60%. The responses of LDH and ADH activities to O2 tension were dissimilar; when layers were incubated in various O2/N2 mixtures, LDH activity peaked at 2 to 5% O2 whereas ADH activity was highest at 0% O2. The LDH activity was resolved into several enzymically active bands by native polyacrylamide gel electrophoresis.
We conclude that barley aleurone layers are highly adapted to O2 deficiency, that they possess an inducible LDH system as well as an ADH system, and we infer that the LDH and ADH systems are independently regulated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- CANVIN D. T., BEEVERS H. Sucrose synthesis from acetate in the germinating castor bean: kinetics and pathway. J Biol Chem. 1961 Apr;236:988–995. [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. D., Davies S. Purification and properties of L(+)-lactate dehydrogenase from potato tubers. Biochem J. 1972 Oct;129(4):831–839. doi: 10.1042/bj1290831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everse J., Kaplan N. O. Lactate dehydrogenases: structure and function. Adv Enzymol Relat Areas Mol Biol. 1973;37:61–133. doi: 10.1002/9780470122822.ch2. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Jacobsen J. V., Zwar J. A. Regulated expression of three alcohol dehydrogenase genes in barley aleurone layers. Plant Physiol. 1984 Jul;75(3):573–581. doi: 10.1104/pp.75.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P. W. Design of metabolic and enzymic machinery to fit lifestyle and environment. Biochem Soc Symp. 1976;(41):3–31. [PubMed] [Google Scholar]
- Hochachka P. W., Mommsen T. P. Protons and anaerobiosis. Science. 1983 Mar 25;219(4591):1391–1397. doi: 10.1126/science.6298937. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. V., Higgins T. J. Characterization of the alpha-Amylases Synthesized by Aleurone Layers of Himalaya Barley in Response to Gibberellic Acid. Plant Physiol. 1982 Dec;70(6):1647–1653. doi: 10.1104/pp.70.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jervis L. Characterization of potato (Solanum tuberosum)lactate dehydrogenase isoenzymes by affinity chromatography and hybridization. Biochem J. 1981 Sep 1;197(3):755–758. doi: 10.1042/bj1970755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poerio E., Davies D. D. A comparison of potato and vertebrate lactate dehydrogenases. Biochem J. 1980 Nov 1;191(2):341–348. doi: 10.1042/bj1910341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Spencer D., Higgins T. J., Button S. C., Davey R. A. Pulse-labeling Studies on Protein Synthesis in Developing Pea Seeds and Evidence of a Precursor Form of Legumin Small Subunit. Plant Physiol. 1980 Sep;66(3):510–515. doi: 10.1104/pp.66.3.510. [DOI] [PMC free article] [PubMed] [Google Scholar]