Abstract
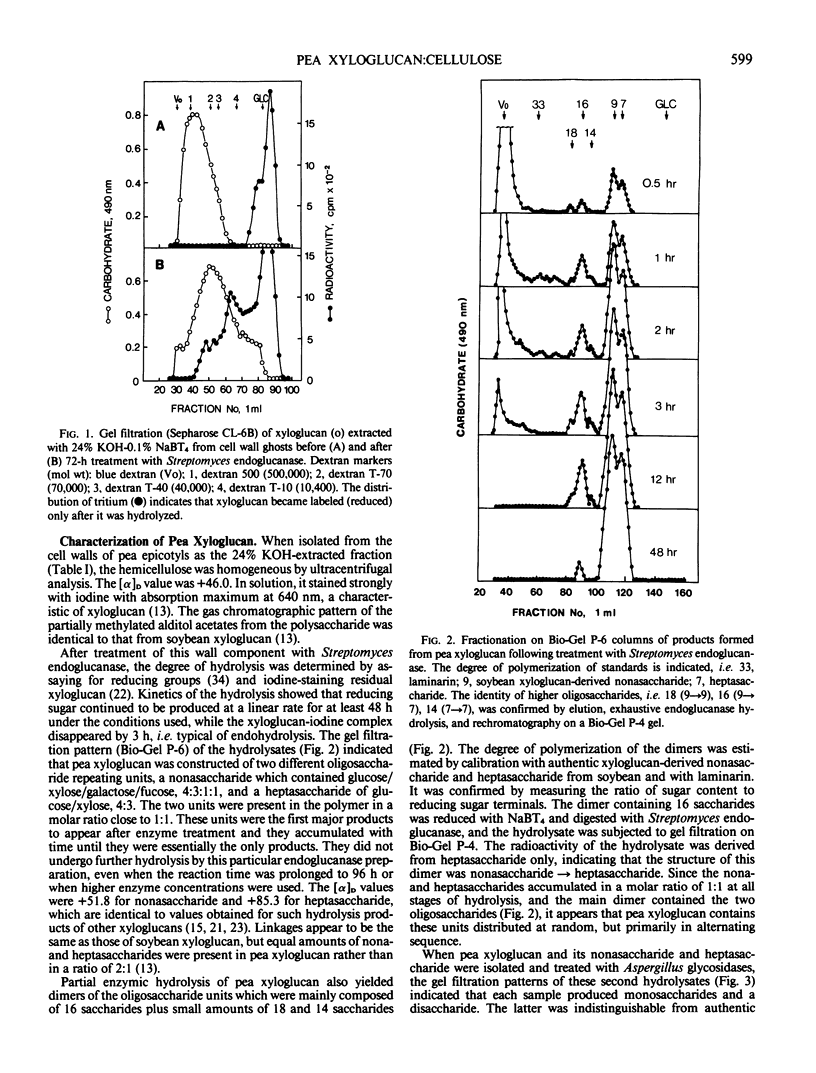
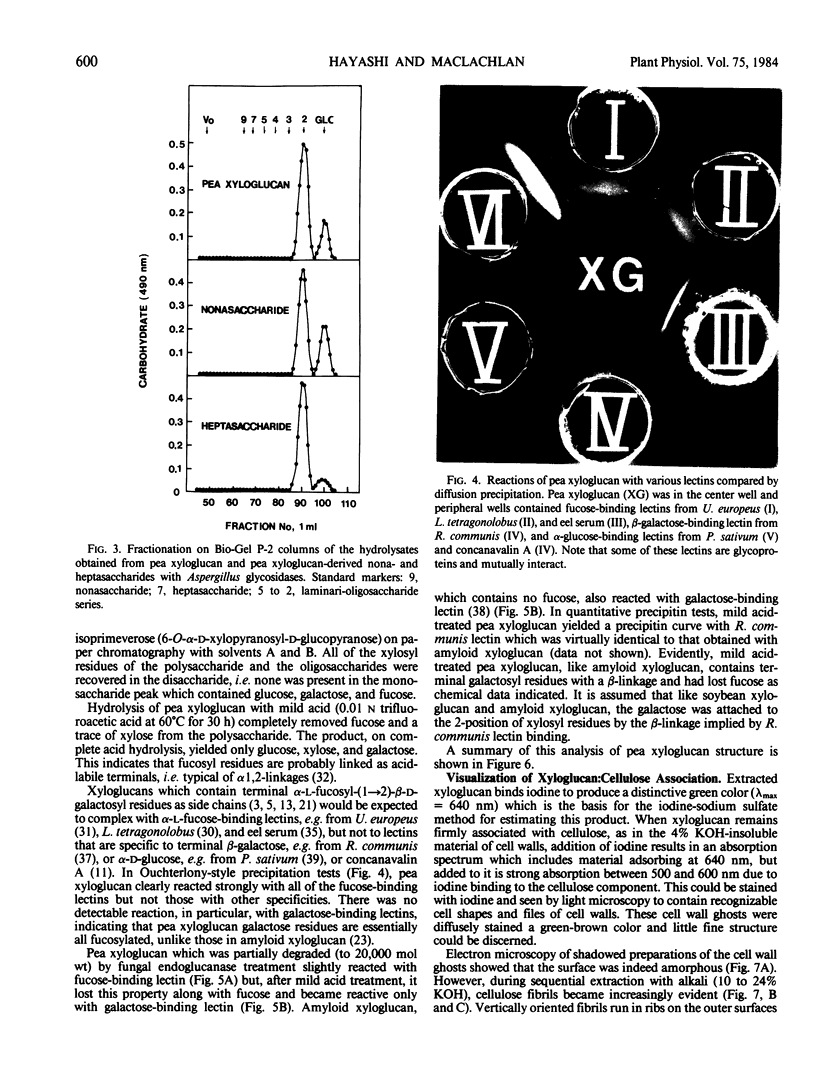
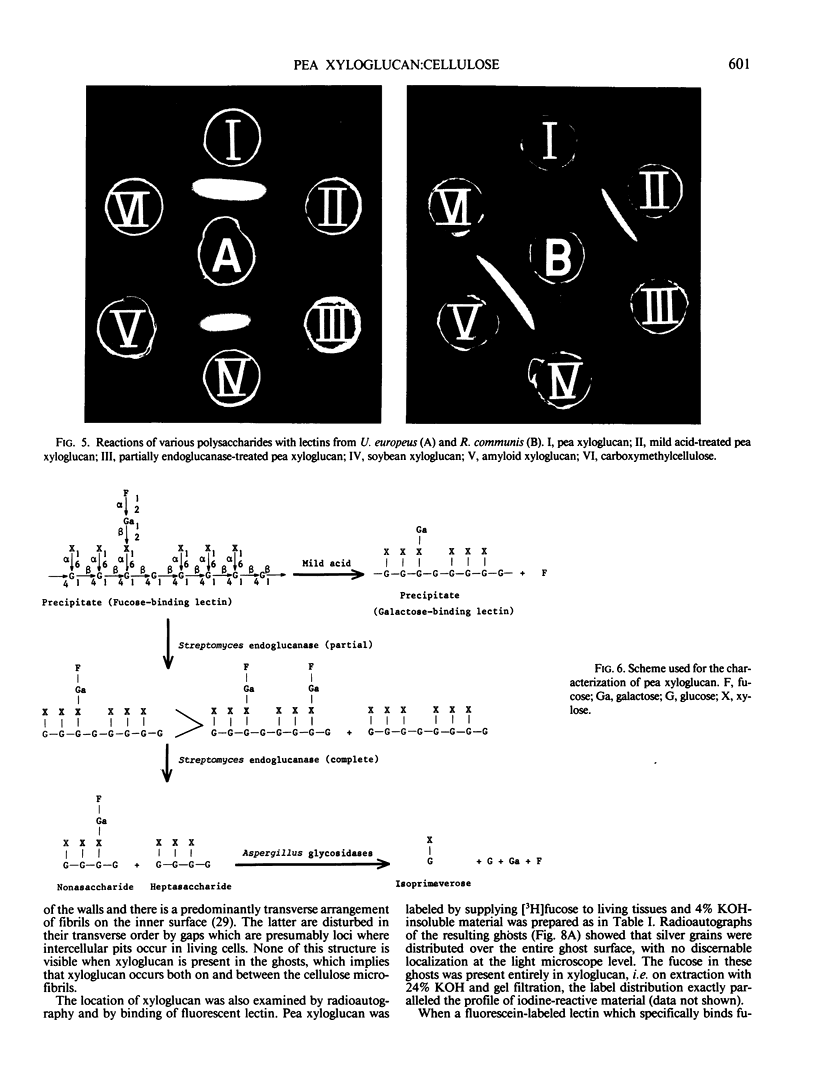
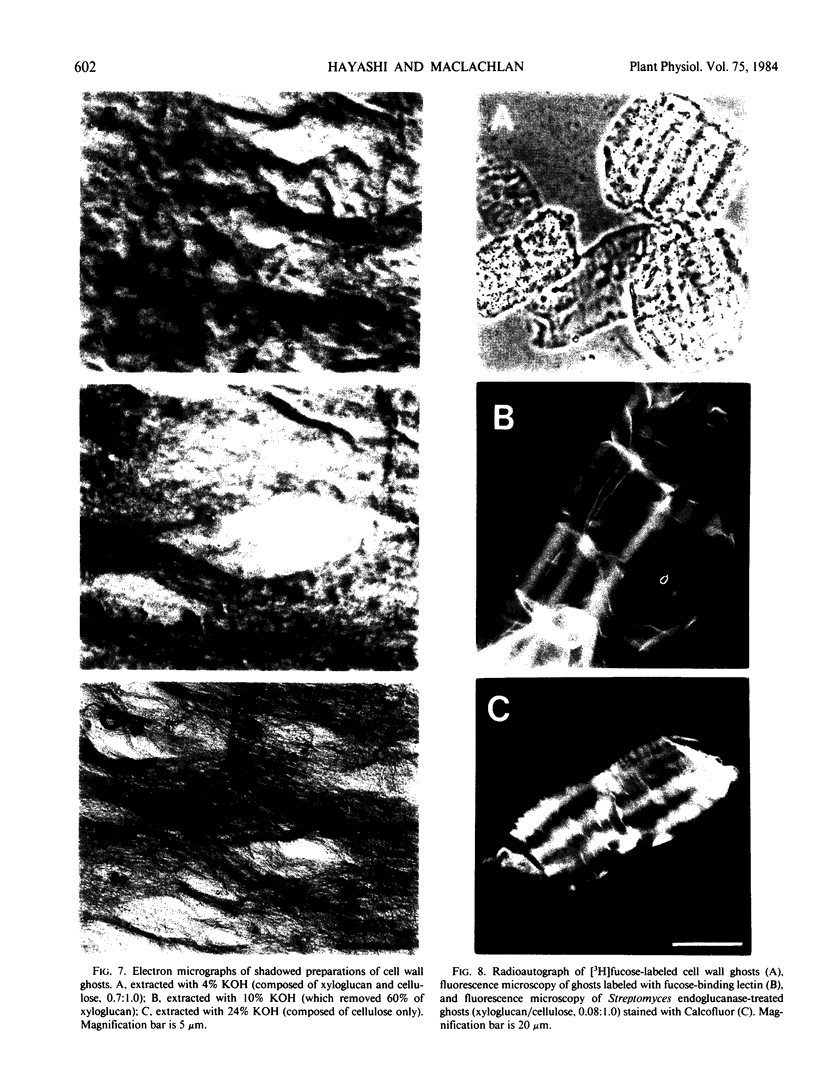
A macromolecular complex composed of xyloglucan and cellulose was obtained from elongating regions of etiolated pea (Pisum sativum L. var. Alaska) stems. Xyloglucan could be solubilized by extraction of this complex with 24% KOH-0.1% NaBH4 or by extended treatment with endo-1,4-β-glucanase. The polysaccharide was homogeneous by ultracentrifugal analysis and gel filtration on Sepharose CL-6B, molecular weight 330,000. The structure of pea xyloglucan was examined by fragmentation analysis of enzymic hydrolysates, methylation analysis, and precipitation tests with fucose- or galactose-binding lectins. The polysaccharide was composed of equal amounts of two subunits, a nonasaccharide (glucose/xylose/galactose/fucose, 4:3:1:1) and a heptasaccharide (glucose/xylose, 4:3), which appeared to be distributed at random, but primarily in alternating sequence. The xyloglucan:cellulose complex was examined by light microscopy using iodine staining, by radioautography after labeling with [3H]fucose, by fluorescence microscopy using a fluorescein-lectin (fucose-binding) as probe, and by electron microscopy after shadowing. The techniques all demonstrated that the macromolecule was present in files of cell shapes, referred to here as cell-wall `ghosts,' in which xyloglucan was localized both on and between the cellulose microfibrils. Since the average chain length of pea xyloglucan was many times the diameter of cellulose microfibrils, it could introduce cross-links by binding to adjacent fibrils and thereby contribute rigidity to the wall.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspinall G. O., Krishnamurthy T. N., Rosell K. G. A fucogalactoxyloglucan from rapeseed hulls. Carbohydr Res. 1977 May;55:11–19. doi: 10.1016/s0008-6215(00)84439-0. [DOI] [PubMed] [Google Scholar]
- Aspinall G. O., Molloy J. A., Craig J. W. Extracellular polysaccharides from suspension-cultured sycamore cells. Can J Biochem. 1969 Nov;47(11):1063–1070. doi: 10.1139/o69-170. [DOI] [PubMed] [Google Scholar]
- Bal A. K., Verma D. P., Byrne H., Maclachlan G. A. Subcellular localization of cellulases in auxin-treated pea. J Cell Biol. 1976 Apr;69(1):97–105. doi: 10.1083/jcb.69.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W. D., Talmadge K. W., Keegstra K., Albersheim P. The Structure of Plant Cell Walls: II. The Hemicellulose of the Walls of Suspension-cultured Sycamore Cells. Plant Physiol. 1973 Jan;51(1):174–187. doi: 10.1104/pp.51.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hayashi T., Kato Y., Matsuda K. Biosynthesis of xyloglucan in suspension-cultured soybean cells. An assay method for xyloglucan xylosyltransferase and attempted synthesis of xyloglucan from UDP-D-xylose. J Biochem. 1981 Jan;89(1):325–328. doi: 10.1093/oxfordjournals.jbchem.a133198. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Matsuda K. Biosynthesis of xyloglucan in suspension-cultured soybean cells. Occurrence and some properties of xyloglucan 4-beta-D-glucosyltransferase and 6-alpha-D-xylosyltransferase. J Biol Chem. 1981 Nov 10;256(21):11117–11122. [PubMed] [Google Scholar]
- Hurn B. A., Chantler S. M. Production of reagent antibodies. Methods Enzymol. 1980;70(A):104–142. doi: 10.1016/s0076-6879(80)70044-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Turnover of cell wall polysaccharides in elongating pea stem segments. Plant Physiol. 1974 May;53(5):669–673. doi: 10.1104/pp.53.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. E., Kabat E. A. Specificity of purified hemagglutinin (lectin) from Lotus tetragonolobus. Biochemistry. 1974 Jul 16;13(15):3184–3192. doi: 10.1021/bi00712a029. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Kisailus E. C., Gruezo F., Kabat E. A. Immunochemical studies on the combining site of the blood group H-specific lectin 1 from Ulex europeus seeds. Arch Biochem Biophys. 1978 Jan 15;185(1):108–115. doi: 10.1016/0003-9861(78)90149-2. [DOI] [PubMed] [Google Scholar]
- REGE W. P., PAINTER T. J., WATKINS W. M., MORGAN W. T. ISOLATION OF SEROLOGICALLY ACTIVE FUCOSE-CONTAINING OLIGOSACCHARIDES FROM HUMAN BLOOD-GROUP H SUBSTANCE. Nature. 1964 Jul 25;203:360–363. doi: 10.1038/203360a0. [DOI] [PubMed] [Google Scholar]
- Sandford P. A., Conrad H. E. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. I. A reexamination using improved procedures for methylation analysis. Biochemistry. 1966 May;5(5):1508–1517. doi: 10.1021/bi00869a009. [DOI] [PubMed] [Google Scholar]
- Terry M. E., Jones R. L. Soluble Cell Wall Polysaccharides Released from Pea Stems by Centrifugation : I. EFFECT OF AUXIN. Plant Physiol. 1981 Sep;68(3):531–537. doi: 10.1104/pp.68.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wauwe J. P., Loontiens F. G., De Bruyne C. K. Carbohydrate binding specificity of the lectin from the pea (Pisum sativum). Biochim Biophys Acta. 1975 Feb 27;379(2):456–461. doi: 10.1016/0005-2795(75)90152-x. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., Loontiens F. G., De Bruyne C. K. The interaction of Ricinus communis hemagglutinin with polysaccharides and low molecular weight carbohydrates. Biochim Biophys Acta. 1973 Jun 20;313(1):99–105. doi: 10.1016/0304-4165(73)90191-8. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- van Wauwe J. P., Loontiens F. G., De Bruyne C. K. On the interaction of Ricinus communis lectin with plant amyloids. Biochim Biophys Acta. 1974 Jun 20;354(1):148–151. doi: 10.1016/0304-4165(74)90063-4. [DOI] [PubMed] [Google Scholar]