Abstract
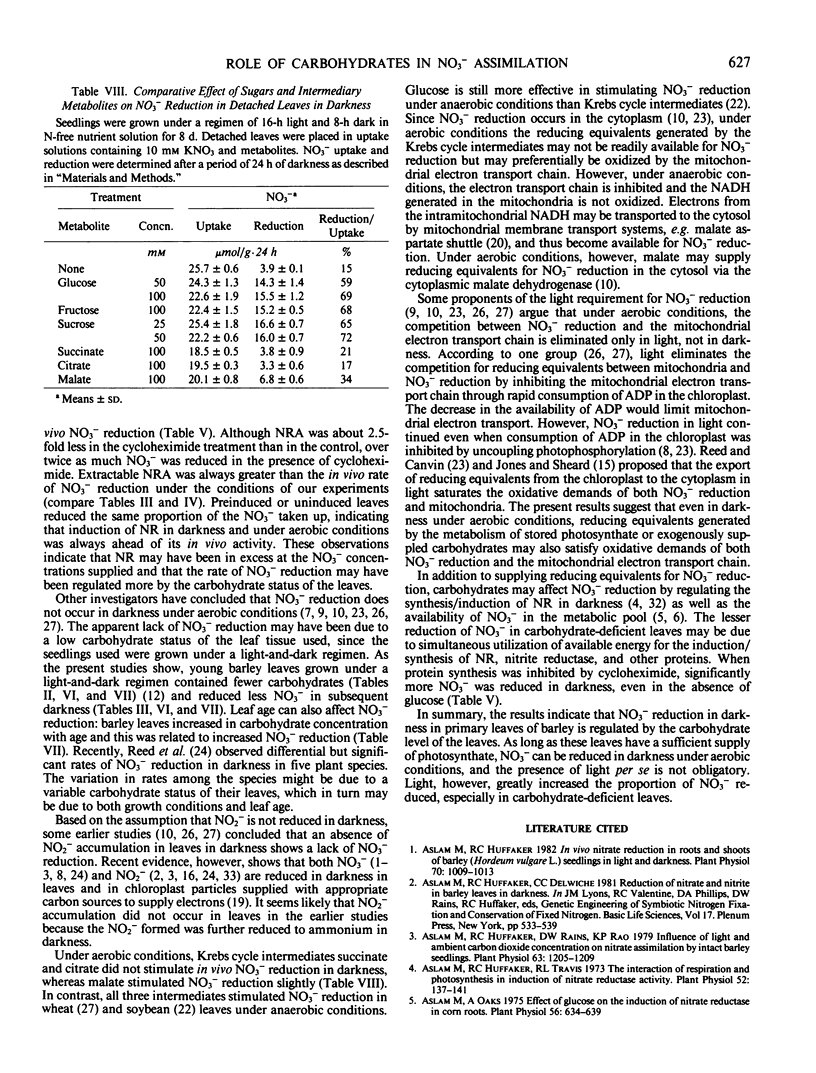
Nitrate reduction was studied as a function of carbohydrate concentration in detached primary leaves of barley (Hordeum vulgare L. cv Numar) seedlings under aerobic conditions in light and darkness. Seedlings were grown either in continuous light for 8 days or under a regimen of 16-hour light and 8-hour dark for 8 to 15 days. Leaves of 8-day-old seedlings grown in continuous light accumulated 4 times more carbohydrates than leaves of plants grown under a light and dark regimen. When detached leaves from these seedlings were supplied with NO3− in darkness, those with the higher levels of carbohydrates reduced a greater proportion of the NO3− that was taken up. In darkness, added glucose increased the percentage of NO3− reduced up to 2.6-fold depending on the endogenous carbohydrate status of the leaves. Both NO3− reduction and carbohydrate content of the leaves increased with age. Fructose and sucrose also increased NO3− reduction in darkness to the same extent as glucose. Krebs cycle intermediates, citrate and succinate, did not increase NO3− reduction, whereas malate slightly stimulated it in darkness.
In light, 73 to 90% of the NO3− taken up was reduced by the detached leaves; therefore, an exogenous supply of glucose had little additional effect on NO3− reduction. The results indicate that in darkness the rate of NO3− reduction in primary leaves of barley depends upon the availability of carbohydrates.
Full text
PDF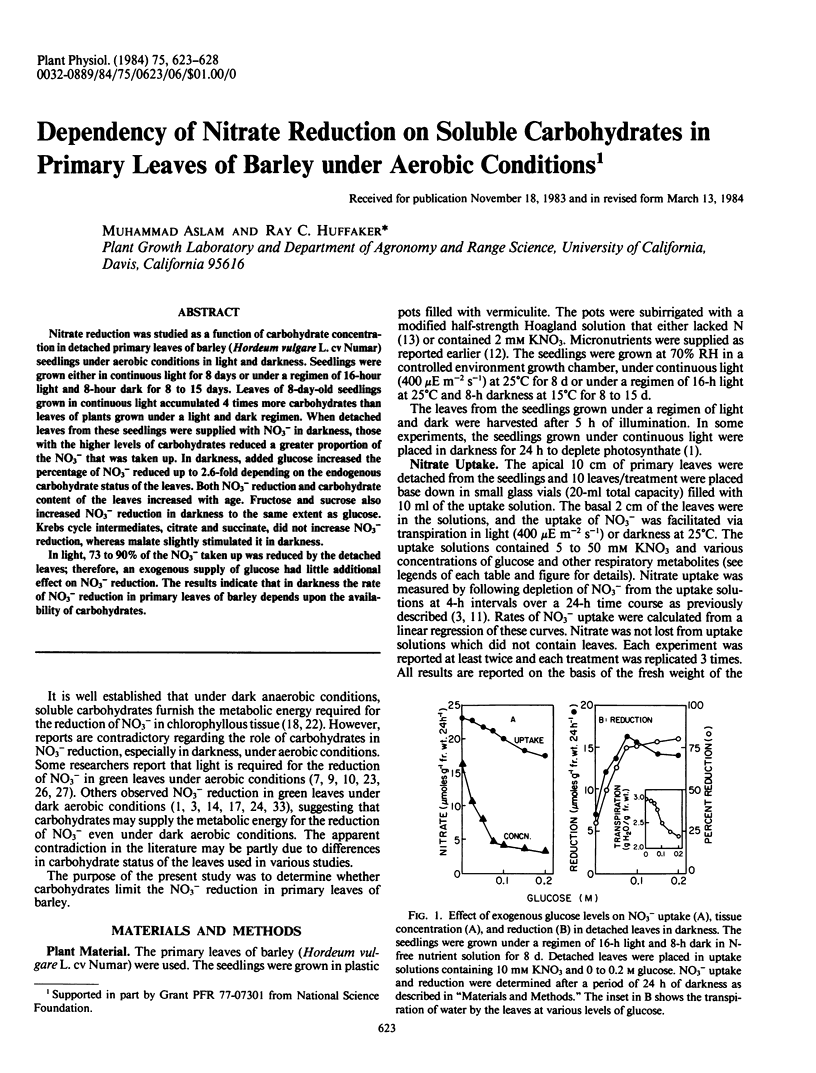


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C. In Vivo Nitrate Reduction in Roots and Shoots of Barley (Hordeum vulgare L.) Seedlings in Light and Darkness. Plant Physiol. 1982 Oct;70(4):1009–1013. doi: 10.1104/pp.70.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Huffaker R. C., Rains D. W., Rao K. P. Influence of light and ambient carbon dioxide concentration on nitrate assimilation by intact barley seedlings. Plant Physiol. 1979 Jun;63(6):1205–1209. doi: 10.1104/pp.63.6.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Huffaker R. C., Travis R. L. The interaction of respiration and photosynthesis in induction of nitrate reductase activity. Plant Physiol. 1973 Aug;52(2):137–141. doi: 10.1104/pp.52.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Oaks A. Effect of glucose on the induction of nitrate reductase in corn roots. Plant Physiol. 1975 Nov;56(5):634–639. doi: 10.1104/pp.56.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Oaks A., Huffaker R. C. Effect of light and glucose on the induction of nitrate reductase and on the distribution of nitrate in etiolated barley leaves. Plant Physiol. 1976 Oct;58(4):588–591. doi: 10.1104/pp.58.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shalom N., Huffaker R. C., Rappaport L. Effect of photosynthetic inhibitors and uncouplers of oxidative phosphorylation on nitrate and nitrite reduction in barley leaves. Plant Physiol. 1983 Jan;71(1):63–66. doi: 10.1104/pp.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantarotwong W., Huffaker R. C., Miller B. L., Granstedt R. C. In vivo nitrate reduction in relation to nitrate uptake, nitrate content, and in vitro nitrate reductase activity in intact barley seedlings. Plant Physiol. 1976 Apr;57(4):519–522. doi: 10.1104/pp.57.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. W., Huffaker R. C. Photosynthesis, leaf resistances, and ribulose-1,5-bisphosphate carboxylase degradation in senescing barley leaves. Plant Physiol. 1980 Jun;65(6):1103–1107. doi: 10.1104/pp.65.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepper L., Flesher D., Hageman R. H. Generation of reduced nicotinamide adenine dinucleotide for nitrate reduction in green leaves. Plant Physiol. 1971 Nov;48(5):580–590. doi: 10.1104/pp.48.5.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kow Y. W., Erbes D. L., Gibbs M. Chloroplast Respiration : A MEANS OF SUPPLYING OXIDIZED PYRIDINE NUCLEOTIDE FOR DARK CHLOROPLASTIC METABOLISM. Plant Physiol. 1982 Feb;69(2):442–447. doi: 10.1104/pp.69.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. C., Harper J. E., Hageman R. H. Nitrate Reductase Activity in Soybeans (Glycine max [L.] Merr.): II. Energy Limitations. Plant Physiol. 1976 Dec;58(6):736–739. doi: 10.1104/pp.58.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A. J., Canvin D. T. Light and Dark Controls of Nitrate Reduction in Wheat (Triticum aestivum L.) Protoplasts. Plant Physiol. 1982 Feb;69(2):508–513. doi: 10.1104/pp.69.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A. J., Canvin D. T., Sherrard J. H., Hageman R. H. Assimilation of [N]Nitrate and [N]Nitrite in Leaves of Five Plant Species under Light and Dark Conditions. Plant Physiol. 1983 Feb;71(2):291–294. doi: 10.1104/pp.71.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney S. K., Naik M. S., Nicholas D. J. Regulation of NADH supply for nitrate reduction in green plants via photosynthesis and mitochondrial respiration. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1209–1216. doi: 10.1016/0006-291x(78)91265-2. [DOI] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner D. L., Boyer J. S. Nitrate Reductase Activity in Maize (Zea mays L.) Leaves: I. Regulation by Nitrate Flux. Plant Physiol. 1976 Oct;58(4):499–504. doi: 10.1104/pp.58.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. R., Huffaker R. C. Determination of nitrate and nitrite by high-pressure liquid chromatography: comparison with other methods for nitrate determination. Anal Biochem. 1980 Feb;102(1):110–119. doi: 10.1016/0003-2697(80)90325-5. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Key J. L. Correlation between Polyribosome Level and the Ability to Induce Nitrate Reductase in Dark-grown Corn Seedlings. Plant Physiol. 1971 Nov;48(5):617–620. doi: 10.1104/pp.48.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]


