Abstract
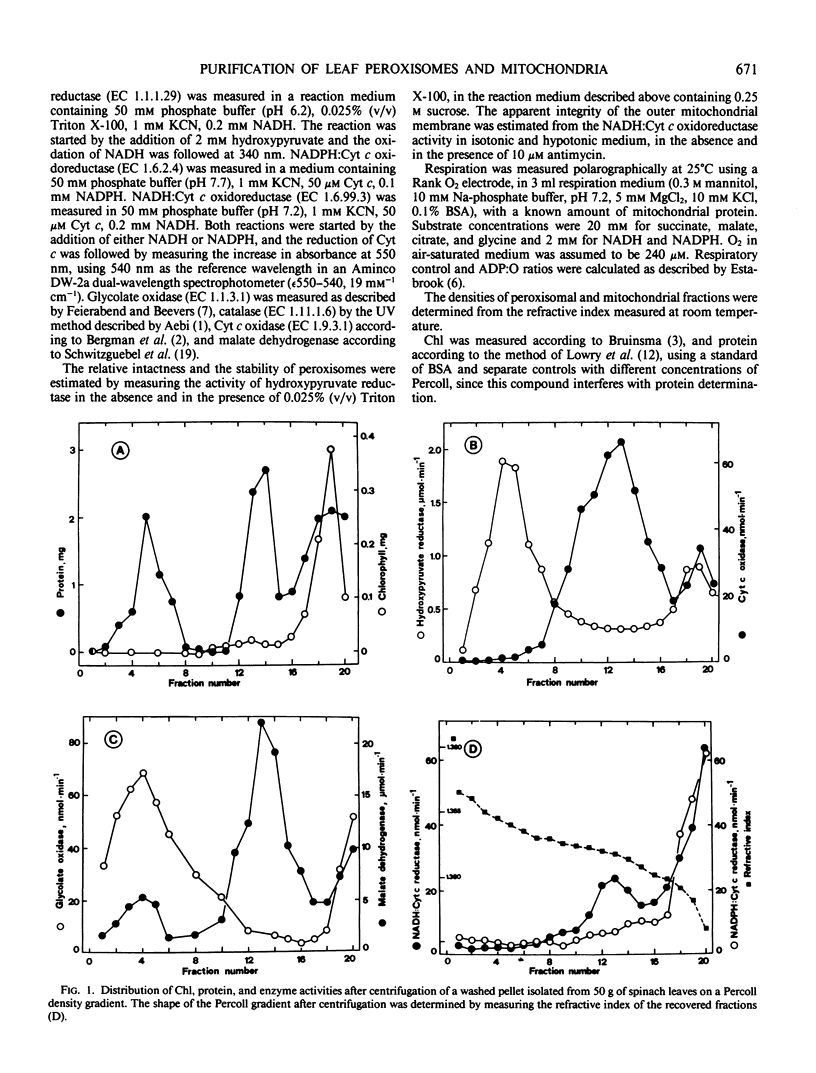
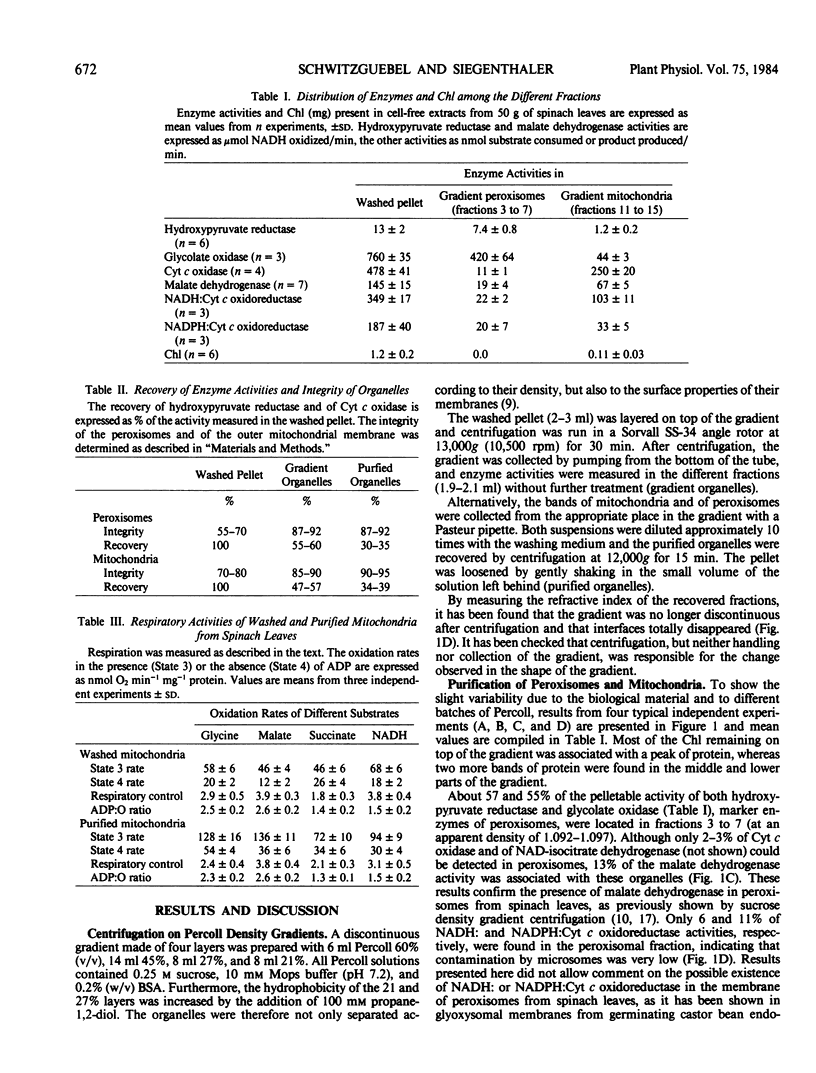
A procedure was developed to purify simultaneously peroxisomes and mitochondria from spinach (Spinacia oleracea L.) leaf under isoosmotic and low viscosity conditions. This method involved differential centrifugation and density gradient centrifugation on four layers of Percoll. Chlorophyll-free preparations of highly intact and active organelles were obtained and cross-contamination was negligible. Both organelles were stable for several hours, even if they remained in Percoll. Purified mitochondria were able to carry out the oxidation of different substrates with excellent respiratory control and ADP:O ratios. The method described in the present work was also suitable to purify mitochondria and peroxisomes from potato (Solanum tuberosum L.) tubers.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUINSMA J. A comment on the spectrophotometric determination of chlorophyll. Biochim Biophys Acta. 1961 Sep 30;52:576–578. doi: 10.1016/0006-3002(61)90418-8. [DOI] [PubMed] [Google Scholar]
- Bergman A., Gardeström P., Ericson I. Method to Obtain a Chlorophyll-free Preparation of Intact Mitochondria from Spinach Leaves. Plant Physiol. 1980 Sep;66(3):442–445. doi: 10.1104/pp.66.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. C., Huang A. H. Metabolism of Glycolate in Isolated Spinach Leaf Peroxisomes : KINETICS OF GLYOXYLATE, OXALATE, CARBON DIOXIDE, AND GLYCINE FORMATION. Plant Physiol. 1981 May;67(5):1003–1006. doi: 10.1104/pp.67.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Moore A. L., Neuburger M. Isolation and oxidative properties of intact mitochondria isolated from spinach leaves. Plant Physiol. 1977 Oct;60(4):625–628. doi: 10.1104/pp.60.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks D. B., Donaldson R. P. Electron transport in glyoxysomal membranes. Arch Biochem Biophys. 1982 Apr 15;215(1):280–288. doi: 10.1016/0003-9861(82)90306-x. [DOI] [PubMed] [Google Scholar]
- Jackson C., Dench J. E., Hall D. O., Moore A. L. Separation of mitochondria from contaminating subcellular structures utilizing silica sol gradient centrifugation. Plant Physiol. 1979 Jul;64(1):150–153. doi: 10.1104/pp.64.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Journet E. P., Neuburger M., Douce R. Role of Glutamate-oxaloacetate Transaminase and Malate Dehydrogenase in the Regeneration of NAD for Glycine Oxidation by Spinach leaf Mitochondria. Plant Physiol. 1981 Mar;67(3):467–469. doi: 10.1104/pp.67.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liang Z., Yu C., Huang A. H. Isolation of spinach leaf peroxisomes in 0.25 molar sucrose solution by percoll density gradient centrifugation. Plant Physiol. 1982 Oct;70(4):1210–1212. doi: 10.1104/pp.70.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler I. J., Beevers H. Oxidation of NADH in Glyoxysomes by a Malate-Aspartate Shuttle. Plant Physiol. 1980 Oct;66(4):555–560. doi: 10.1104/pp.66.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash D., Wiskich J. T. Properties of substantially chlorophyll-free pea leaf mitochondria prepared by sucrose density gradient separation. Plant Physiol. 1983 Mar;71(3):627–634. doi: 10.1104/pp.71.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Douce R., Akazawa T. Isolation and characterization of metabolically competent mitochondria from spinach leaf protoplasts. Plant Physiol. 1982 Apr;69(4):916–920. doi: 10.1104/pp.69.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Graham D., Akazawa T. Isolation of intact chloroplasts and other cell organelles from spinach leaf protoplasts. Plant Physiol. 1976 Sep;58(3):309–314. doi: 10.1104/pp.58.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Isolation and purification of intact peroxisomes from green leaf tissue. Plant Physiol. 1982 Oct;70(4):1213–1217. doi: 10.1104/pp.70.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler P. A., Depéry F. Influence of unsaturated fatty acids in chloroplasts. Shift of the pH optimum of electron flow and relations to deltapH, thylakoid internal pH and proton uptake. Eur J Biochem. 1976 Jan 15;61(2):573–580. doi: 10.1111/j.1432-1033.1976.tb10052.x. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]


