Abstract
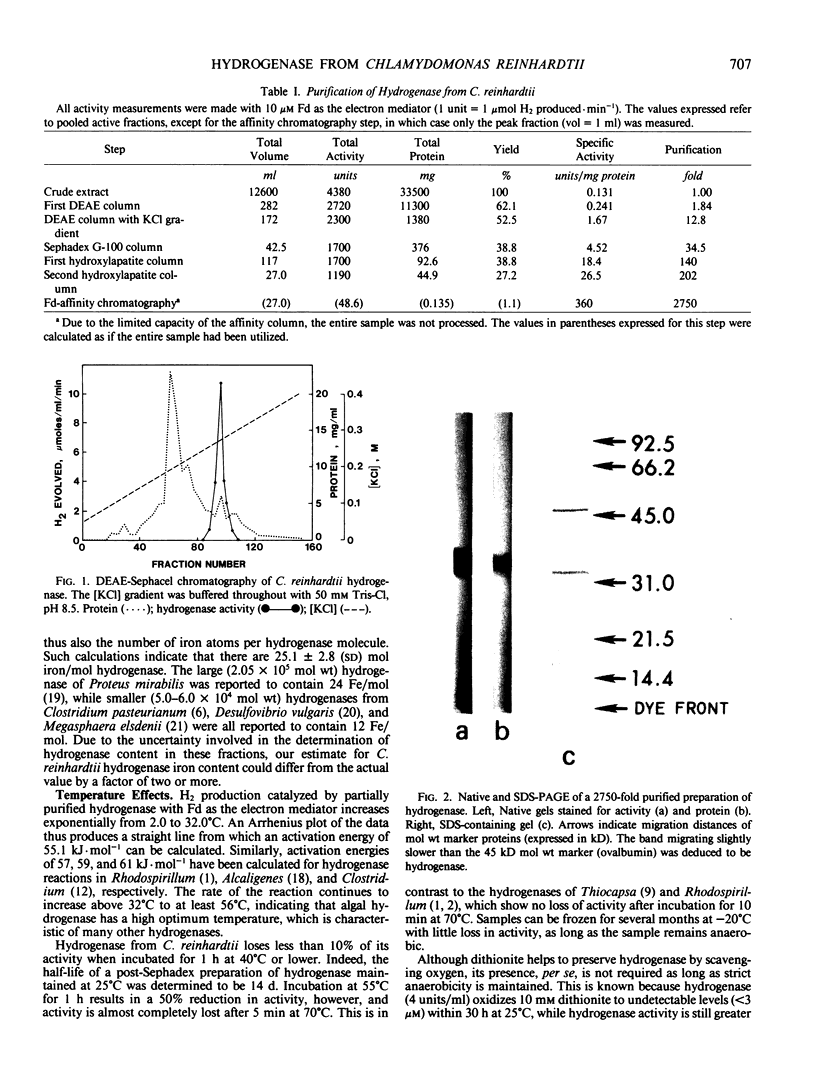
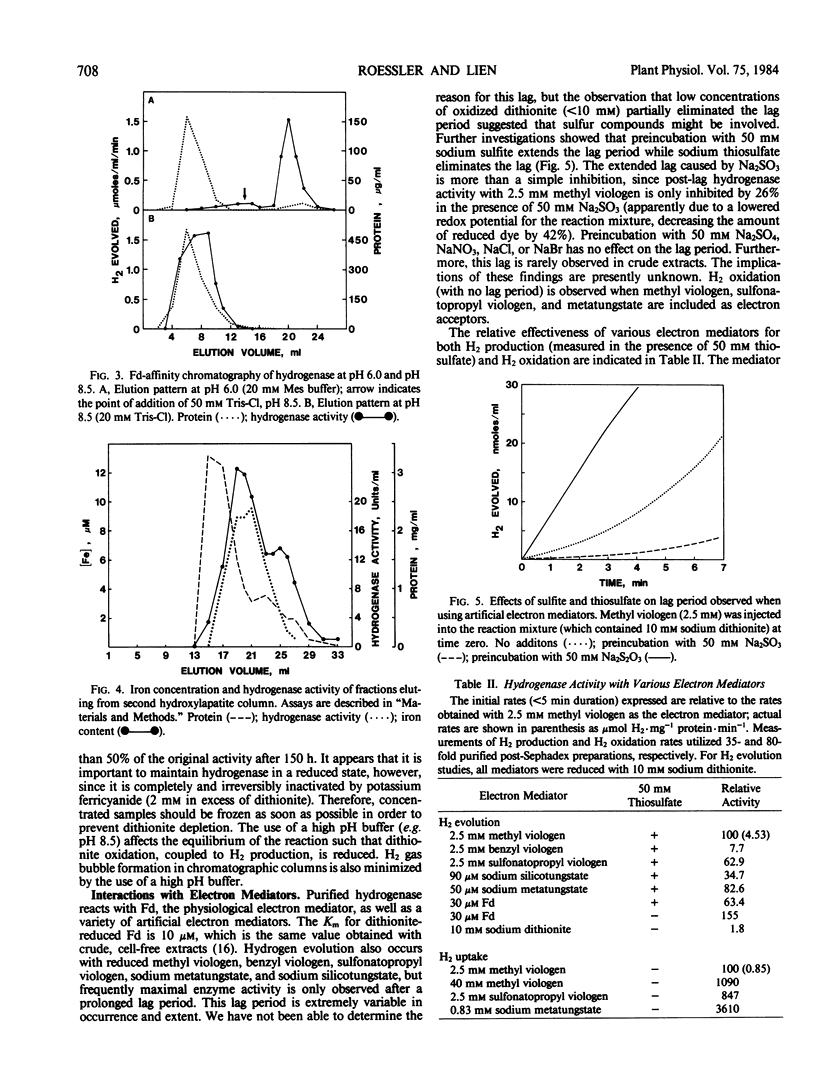
A method is described which results in a 2750-fold purification of hydrogenase from Chlamydomonas reinhardtii, yielding a preparation which is approximately 40% pure. With a saturating amount of ferredoxin as the electron mediator, the specific activity of pure enzyme was calculated to be 1800 micromoles H2 produced per milligram protein per minute. The molecular weight was determined to be 4.5 × 104 by gel filtration and 4.75 × 104 by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The enzyme has an abundance of acidic side groups, contains iron, and has an activation energy of 55.1 kilojoules per mole for H2 production; these properties are similar to those of bacterial hydrogenases. The enzyme is less thermally stable than most bacterial hydrogenases, however, losing 50% of its activity in 1 hour at 55°C. The Km of purified hydrogenase for ferredoxin is 10 micromolar, and the binding of these proteins to each other is enhanced under slightly acidic conditions. Purified hydrogenase also accepts electrons from a variety of artificial electron mediators, including sodium metatungstate, sodium silicotungstate, and several viologen dyes. A lag period is frequently observed before maximal activity is expressed with these artificial electron mediators, although the addition of sodium thiosulfate at least partially overcomes this lag.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. W., Hall D. O. Isolation of the membrane-bound hydrogenase from Rhodospirillum rubrum. Biochem Biophys Res Commun. 1977 Jul 25;77(2):730–737. doi: 10.1016/s0006-291x(77)80039-9. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Properties of the solubilized membrane-bound hydrogenase from the photosynthetic bacterium Rhodospirillum rubrum. Arch Biochem Biophys. 1979 Jul;195(2):288–299. doi: 10.1016/0003-9861(79)90355-2. [DOI] [PubMed] [Google Scholar]
- Adams M. W., Hall D. O. Purification of the membrane-bound hydrogenase of Escherichia coli. Biochem J. 1979 Oct 1;183(1):11–22. doi: 10.1042/bj1830011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams M. W., Mortenson L. E., Chen J. S. Hydrogenase. Biochim Biophys Acta. 1980 Dec;594(2-3):105–176. doi: 10.1016/0304-4173(80)90007-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Purification and properties of hydrogenase from Clostridium pasteurianum W5. Biochim Biophys Acta. 1974 Dec 18;371(2):283–298. doi: 10.1016/0005-2795(74)90025-7. [DOI] [PubMed] [Google Scholar]
- Erbes D. L., King D., Gibbs M. Inactivation of Hydrogenase in Cell-free Extracts and Whole Cells of Chlamydomonas reinhardi by Oxygen. Plant Physiol. 1979 Jun;63(6):1138–1142. doi: 10.1104/pp.63.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogotov I. N., Zorin N. A., Serebriakova L. T., Kondratieva E. N. The properties of hydrogenase from Thiocapsa roseopersicina. Biochim Biophys Acta. 1978 Apr 12;523(2):335–343. doi: 10.1016/0005-2744(78)90036-0. [DOI] [PubMed] [Google Scholar]
- Hatchikian E. C., Bruschi M., Le Gall J. Characterization of the periplasmic hydrogenase from Desulfovibrio gigas. Biochem Biophys Res Commun. 1978 May 30;82(2):451–461. doi: 10.1016/0006-291x(78)90896-3. [DOI] [PubMed] [Google Scholar]
- Kleiner D., Burris R. H. The hydrogenase of Clostridium pasteurianum. Kinetic studies and the role of molybdenum. Biochim Biophys Acta. 1970 Sep 16;212(3):417–427. doi: 10.1016/0005-2744(70)90247-0. [DOI] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Roessler P., Lien S. Anionic modulation of the catalytic activity of hydrogenase from Chlamydomonas reinhardtii. Arch Biochem Biophys. 1982 Jan;213(1):37–44. doi: 10.1016/0003-9861(82)90436-2. [DOI] [PubMed] [Google Scholar]
- Roessler P., Lien S. Effects of electron mediator charge properties on the reaction kinetics of hydrogenase from Chlamydomonas. Arch Biochem Biophys. 1984 Apr;230(1):103–109. doi: 10.1016/0003-9861(84)90090-0. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Schoenmaker G. S., Oltmann L. F., Stouthamer A. H. Purification and properties of the membrane-bound hydrogenase from Proteus mirabilis. Biochim Biophys Acta. 1979 Apr 12;567(2):511–521. doi: 10.1016/0005-2744(79)90137-2. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- van der Westen H. M., Mayhew S. G., Veeger C. Separation of hydrogenase from intact cells of Desulfovibrio vulgaris. Purification and properties. FEBS Lett. 1978 Feb 1;86(1):122–126. doi: 10.1016/0014-5793(78)80112-4. [DOI] [PubMed] [Google Scholar]