The first recognized outbreak of pneumonia due to Legionella pneumophila occurred in Philadelphia, Pa., in July of 1976 among 180 persons attending the 56th annual American Legion Convention. Twenty-nine patients died, and the disease became known as Legionnaires’ disease (23). Guinea pigs were infected with postmortem lung tissue from the patients with fatal Legionnaires’ disease, and embryonated yolk sacs were inoculated with spleen homogenates from the infected guinea pigs. In January of 1977, a gram-negative bacterium was isolated and designated L. pneumophila (36). The source of the infection during the Legionnaires’ convention was later found to be the air-conditioning system in the hotel.
It has been documented that the hallmark of Legionnaires’ disease is the intracellular replication of L. pneumophila in alveolar spaces. At least 39 species of legionellae have been identified. Some of these are associated with disease, while others are environmental isolates; whether the latter can cause disease is not known. L. pneumophila is responsible for more than 80% of cases of Legionnaires’ disease, and among the 13 serogroups of L. pneumophila, serogroup 1 is responsible for more than 95% of Legionnaires’ disease cases. It is estimated that L. pneumophila is responsible for at least 25,000 cases of pneumonia per year in the United States, which is very probably an underestimate due to the difficulty of isolating bacteria from clinical samples. Since L. pneumophila is the most frequent cause of Legionnaires’ disease, most pathogenic and environmental studies have focused on L. pneumophila.
In 1980, Rowbotham described the ability of L. pneumophila to multiply intracellularly within protozoa (40). Since then, L. pneumophila has been described to multiply in many species of protozoa, and this host-parasite interaction is central to the pathogenesis and ecology of L. pneumophila.
Intracellular replication of L. pneumophila within mammalian and protozoan cells has been shown to occur in a ribosome-studded phagosome that does not fuse to lysosomes. Fields hypothesized that the L. pneumophila phagosome fuses to the rough endoplasmic reticulum (RER) (20). Immunocytochemistry has proven this prediction by demonstrating the presence of an RER-specific chaperon, the Bip protein, in ribosome-studded phagosomes within macrophages (45) and protozoa (1). Based on these characteristics, the L. pneumophila phagosome may be accurately described as an endosomal maturation-blocked (EMB) phagosome.
ECOLOGY AND EPIDEMIOLOGY OF LEGIONELLAE
After isolation of L. pneumophila from the air-conditioning system during the first outbreak in Philadelphia, the bacteria have been isolated from numerous sources in the environment. Legionella species have been repeatedly shown to be ubiquitous, particularly in aquatic environments (21). Bacterial transmission to humans occurs through droplets generated from an environmental source such as cooling towers, shower heads, whirlpools, and other human-made devices that generate aerosols (21). Person-to-person transmission has never been documented.
In the environment, Legionella species cannot multiply extracellularly and have been shown to be parasites of protozoa. In 1980, Rowbotham was the first to describe the ability of L. pneumophila to multiply intracellularly within protozoa (40), and this host-parasite interaction has been shown to be central to the pathogenesis and ecology of L. pneumophila (21). Thirteen species of amoebae and two species of ciliated protozoa that allow intracellular bacterial replication have been shown to be potential environmental hosts for legionellae. These findings are rather intriguing, since protozoa normally phagocytose other bacteria and use them as sources of nutrition. This rather sophisticated host-parasite interaction indicates a tremendous adaptation of legionellae to parasitize protozoa. Although other intracellular pathogens such as Chlamydia pneumonia and Mycobacterium avium have been shown to exhibit slight multiplication in amoebae, legionellae remain the only bacterial species that are prolific in their intracellular replication within amoebae. Furthermore, this host-parasite interaction is central to the pathogenesis and ecology of these bacteria (see below).
At least 39 species of legionellae, many of which have been associated with disease, have now been identified (21). In addition, 12 phylogenetic groups of bacteria belonging to five species have been designated legionella-like amoebic pathogens (LLAPs) (10). The LLAPs are genetically related to legionellae, and many of them have been associated with Legionnaires’ disease. In contrast to Legionella species, the LLAPs cannot be cultured in vitro on artificial media. The LLAPs are isolated by coculture with protozoa (21). LLAPs have been isolated from sputum samples derived from patients with Legionnaires’ disease because of the ability of these bacteria to multiply in protozoa, since they cannot be grown on artificial media (10). In consideration of the fact that approximately 50% of the 0.5 million annual cases of pneumonia in the United States are of unknown etiology, the LLAPs may be responsible for at least some of these cases. The recent developments in using PCR to identify bacteria in environmental samples will facilitate better identification of Legionella species and LLAPs.
CAN LEGIONELLAE BE ERADICATED FROM THE ENVIRONMENT?
Many strategies have been used to eradicate legionellae from the sources of infections in the water and plumbing systems that have been associated with disease outbreaks. These strategies include chemical biocides such as chlorine, overheating of the water, and UV irradiation. These strategies have been successful for short periods, after which the bacteria have again been found in these sources. Thus, eradication of L. pneumophila from environmental sources of infection may require continuous treatment of the water with the effective agent used to eradicate the bacteria. It is clear that the sophisticated association of legionellae with protozoa is a major factor in the continuous presence of the bacteria in the environment. Compared to in vitro-grown L. pneumophila, amoeba-grown bacteria have been shown to be highly resistant to chemical disinfectants and to treatment with biocides (11). Amoeba-grown L. pneumophila has been shown to manifest a dramatic increase in its resistance to harsh environmental conditions such as fluctuation in temperature, osmolarity, pH, and exposure to oxidizing agents (6). Protozoa have been shown to release vesicles containing L. pneumophila organisms that are highly resistant to biocides (14). The ability of L. pneumophila to survive within an ameobic cyst, which is a highly resistant developmental stage of amoebae, further contributes to the resistance of L. pneumophila to physical and biochemical agents used in bacterial eradication. It is very possible that eradication of the bacteria from the environment should start by preventing the protozoan infection, which seems to be the integral part of the infectious cycle of L. pneumophila. Recent identification of the lectin protozoan receptor involved in attachment and invasion by L. pneumophila (47) (see below) and further characterization of the mechanisms of bacterial invasion into protozoa may allow the design of strategies to block the protozoan receptor from attaching to legionellae and thus prevent bacterial entry. Extracellular L. pneumophila is more susceptible to environmental conditions and is not protected from biocides and disinfectants. Furthermore, blockage of bacterial entry into amoebae would render the bacteria less infective and virulent to mammalian cells. Alternatively, treatment of water sources contaminated with L. pneumophila with safe agents that block certain essential bacterial metabolic pathways, such as peptidoglycan biosynthesis pathways, may prove to be useful (27).
INITIAL INTERACTIONS BETWEEN L. PNEUMOPHILA AND ITS PRIMITIVE PROTOZOAN HOSTS
In general, initial interactions between intracellular pathogens and host cells are mediated through attachment of a bacterial ligand, such as a pilus, to a receptor on the surface of a host cell. Genetic evidence for the expression of at least two distinct pili on the surface of L. pneumophila has recently been provided (44). One of these pili is a type IV pilus, designated CAP (competence- and adherence-associated pili) (44). Mutants of L. pneumophila defective in expression of the CAP manifest reduced attachment to protozoan cells but are not affected in their intracellular replication (44). Thus, the CAP of L. pneumophila is involved in adherence to protozoan cells. The CAP may provide L. pneumophila with a selective advantage in adhering to surfaces and biofilms in the environment. The host cell receptor to which the CAP binds is not known, but it is possible that the newly described lectin receptor on Hartmannella vermiformis (described below), to which L. pneumophila adheres, is the receptor for the CAP.
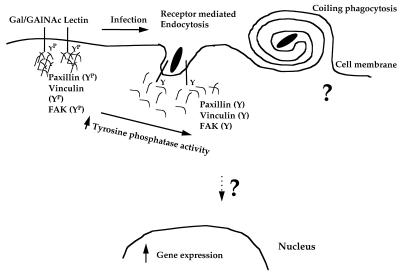
Bacterial attachment to H. vermiformis is mediated by adherence to a protozoan receptor that has been described to be a galactose/N-acetylgalactosamine (Gal/GalNAc) lectin with similarity to the β2 integrin-like Gal/GalNAc lectin of the pathogenic protozoan Entamoeba histolytica (9, 28, 35, 47). Integrins are heterodimeric protein tyrosine kinase receptors that undergo tyrosine phosphorylation upon ligand binding, which subsequently results in recruitment and rearrangements of the cytoskeleton. Interestingly, attachment of L. pneumophila to the Gal/GalNAc of H. vermiformis triggers signal transduction events in H. vermiformis that are manifested in dramatic tyrosine dephosphorylation of the lectin receptor and other proteins (47). Similar observations have been made upon infection of H. vermiformis by another species of legionella, Legionella micdadei (8). Among the L. pneumophila-induced tyrosine-dephosphorylated proteins in H. vermiformis are the cytoskeletal proteins paxillin, vinculin, and focal adhesion kinase (46). Tyrosine phosphatases have been shown to disrupt the cytoskeleton in a mammalian cell. Thus, the induced tyrosine phosphatase activity in H. vermiformis is probably manifested during disruption of the protozoan cytoskeleton to facilitate entry through cytoskeleton-independent receptor-mediated endocytosis (Fig. 1) (33). Interestingly, in addition to these manipulations of the signal transduction of H. vermiformis by L. pneumophila, bacterial invasion is also associated with specific induction of gene expression in protozoa and inhibition of this gene expression blocks entry of the bacteria (5). Following this initial host-parasite interaction, uptake of L. pneumophila by protozoan cells occurs by conventional and coiling phagocytosis (in which the bacterium is surrounded by a multilayer coil-like structure) (1, 15). A proposed model for initial bacterial attachment and uptake by H. vermiformis is depicted in Fig. 1.
FIG. 1.
Model illustrating the signal transduction mechanisms used by L. pneumophila during invasion of its protozoan host H. vermiformis. The 170-kDa Gal/GalNAc lectin is basally tyrosine phosphorylated (YP) and is associated with several phosphorylated proteins. Several cytoskeletal proteins such as paxillin, vinculin, and focal adhesion kinase (FAK) are also tyrosine phosphorylated in resting H. vermiformis and can potentially interact with the Gal/GalNAc lectin. Attachment to and invasion of the host by L. pneumophila is mediated by noncoated-receptor-mediated endocytosis and involves an increase in bacterium-induced tyrosine phosphatase activity in H. vermiformis. This results in tyrosine dephosphorylation of several host cell proteins, including the 170-kDa Gal/GalNAc lectin and cytoskeletal proteins such as paxillin, vinculin, and focal adhesion kinase. This process is associated with disruption of the interaction between the Gal/GalNAc lectin and its associated proteins. Less than 10% of bacterial uptake is mediated by coiling phagocytosis, and we think it is unlikely that the lectin is involved in this process, since it also occurs in mammalian monocytes. The exact signaling mechanisms involved in uptake of L. pneumophila by coiling phagocytosis are not known. Bacterial entry is associated with induction of host cell gene expression, which is necessary for the invasion of H. vermiformis by L. pneumophila.
Uptake of L. pneumophila by another protozoan host, Acanthamoeba polyphaga, is not completely blocked by Gal or GalNAc and is associated with partial tyrosine dephosphorylation of a 170-kDa protein, which may be related to the Gal/GalNAc lectin of H. vermiformis (28). Thus, entry of the bacteria into A. polyphaga is partially mediated by the Gal/GalNAc lectin and additional receptors may be involved in bacterial attachment and entry. The heterogeneity in the uptake mechanisms of L. pneumophila into H. vermiformis and A. polyphaga has been confirmed with invasion-defective mutants of L. pneumophila. Several mutants that were severely defective in attachment to A. polyphaga exhibited minor reductions in attachment to H. vermiformis (28). These data indicate a remarkable adaptation of L. pneumophila to attachment to and invasion of different protozoan hosts.
INTRACELLULAR REPLICATION WITHIN PROTOZOA
After bacterial entry into protozoa, the bacterium is enclosed in a phagosome surrounded by mitochondria and host cell vesicles during the first 60 min (1). The bacterial phagosome is blocked from fusing to the lysosomes (15). In addition, by 4 h postinfection, the phagosome is surrounded by a multilayer membrane derived from the RER (Fig. 2) (1). Based on these characteristics, the L. pneumophila phagosome may be designated an EMB phagosome. Following formation of the EMB phagosome, bacterial replication is initiated (Fig. 2). The 4-h period prior to initiation of intracellular replication may be the time required to recruit the host cell organelles that may be required for replication. Alternatively, the 4-h period may be a lag phase of metabolic and environmental adjustment of the bacteria to a new niche. Interestingly, infection of protozoa by another species of legionella, L. micdadei, results in the formation of an RER-free replicative phagosome (8).
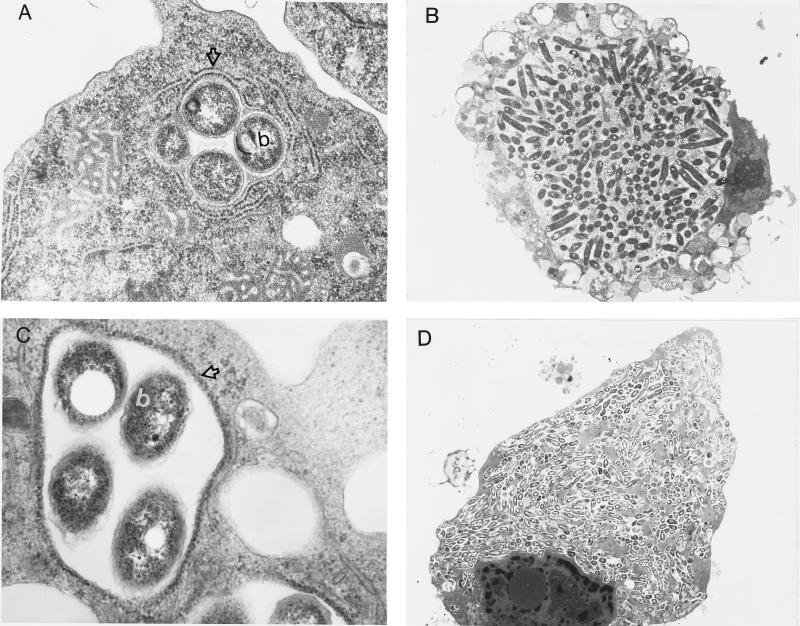
FIG. 2.
Transmission electron micrographs of H. vermiformis (A and B) and WI-26 type I human alveolar epithelial cells (C and D) infected with L. pneumophila AA100 at 4 h (A and C) and 12 h (B and D) postinfection. The open arrows in panels A and C indicate RER-surrounded phagosomes, while the b’s indicate bacteria. Note that the whole cell (B and D) becomes heavily infected with numerous bacteria (a few hundred to a thousand) by 18 h postinfection. Magnifications, ×20,400 (A), ×3,400 (B), ×27,200 (C), ×1,700 (D).
ROLE OF PROTOZOA IN LEGIONNAIRES’ DISEASE
It has been proposed that the infectious particle for Legionnaires’ disease is an amoeba infected with the bacteria (40). Although this has not yet been proven, there are many lines of evidence to suggest that protozoa play major roles in transmission of L. pneumophila. First, many protozoan hosts that allow intracellular bacterial replication, the only means of bacterial amplification in the environment, have been identified (21). Second, in outbreaks of Legionnaires’ disease, amoebae and bacteria have been isolated from the same source of infection and the isolated amoebae support intracellular replication of the bacteria (22). Third, following intracellular replication within protozoa, L. pneumophila organisms exhibit a dramatic increase in resistance to harsh conditions, including high temperature, acidity, and high osmolarity, which may facilitate bacterial survival in the environment (6). Fourth, intracellular L. pneumophila bacteria within protozoa are more resistant to chemical disinfection and biocides than in vitro-grown bacteria (11–13). Fifth, protozoa have been shown to release vesicles of respirable size that contain numerous L. pneumophila organisms, the vesicles are resistant to freeze-thawing and sonication, and the bacteria within the vesicles are highly resistant to biocides (14). Sixth, following their release from the protozoan host, the bacteria exhibit a dramatically enhanced ability to infect mammalian cells in vitro (19). In addition, it has been demonstrated with mice that intracellular bacteria within H. vermiformis are dramatically more infectious and are highly lethal (16). Seventh, the number of bacteria isolated from the source of infection of Legionnaires’ disease is usually very low or undetectable and thus enhanced infectivity of intracellular bacteria within protozoa may compensate for the low infectious dose (38). Eighth, viable but nonculturable L. pneumophila can be resuscitated by coculture with protozoa (42). This observation may suggest that failure to isolate the bacteria from environmental sources of infection may be due to this dormant phase of the bacteria, which makes them unrecoverable on artificial media. Ninth, there has been no documented case of bacterial transmission between individuals. The only known source of transmission is environmental droplets generated from many human-made devices such as shower heads, water fountains, whirlpools, and cooling towers of air-conditioning systems (21).
MOLECULAR BASES OF INVASION OF PROTOZOA
Similar to what occurs in protozoan infection, following entry of the bacteria into macrophages, monocytes, and alveolar epithelial cells, L. pneumophila EMB phagosomes are surrounded by host cell organelles such as mitochondria, vesicles, and RERs (Fig. 2) (30, 45). As with the trafficking of L. pneumophila within protozoa, the EMB phagosome within mammalian macrophages does not fuse to lysosomes (21). The role of the RER in intracellular infection is not known, but the RER is not required as a source of proteins for the bacteria (2). Whether other Legionella species replicate within RER-free phagosomes is still to be determined.
The remarkable similarities in the models of intracellular infection of the two evolutionarily distant host cells (macrophages and protozoa) (Fig. 2) suggest that L. pneumophila may utilize similar molecular mechanisms to manipulate host cell processes of macrophages and protozoa (25). It has been hypothesized that L. pneumophila has evolved as a parasite of protozoa in the environment and that its adaptation to this primitive phagocytic unicellular host was sufficient to allow the bacteria to survive and replicate within the biologically similar phagocytic cells of the more evolved mammalian host (1, 18). In order to test this hypothesis, a collection of 5,200 miniTn10::kan insertion mutants of L. pneumophila have been isolated and examined for their replication within macrophages and protozoa (24–26). It was reasoned that if the molecular bases of the intracellular infection of macrophages and protozoa are similar, defective mutants should exhibit similar phenotypes within both evolutionarily distant host cells. Among 121 distinct insertion mutants with various degrees of defects in survival and replication within macrophages, 89 exhibit very similar phenotypic defects within both macrophages and H. vermiformis. The loci have been designated pmi (for protozoan and macrophage infectivity) (25). These observations showed that many of the molecular aspects of the intracellular infection of macrophages and protozoa are similar. However, 32 mutants with various degrees of defects within macrophages exhibit wild-type phenotypes within protozoa, and the defective loci have been designated mil (for macrophage-specific infectivity loci) (26). Importantly, many of the mil mutants have been tested in peripheral blood monocytes, A/J mouse-derived macrophages, and other protozoa (26). The macrophage-defective and protozoan-wild-type phenotypes of the mil mutants are consistent. Thus, the mil loci are species specific. These data showed that L. pneumophila possesses genetic loci that are not required for infection of protozoa. Therefore, we hypothesize that L. pneumophila evolved in the environment as a protozoan parasite but that it acquired the mil loci that have allowed the bacteria to adapt to the intracellular environment of macrophages (26). It is also possible that ecological coevolution with protozoa has allowed L. pneumophila to develop multiple redundant mechanisms to parasitize protozoa and that some of these mechanisms are essential for survival within macrophages. These speculations may suggest a pathogenic evolution in L. pneumophila through acquisition of the mil loci during its adaptation within protozoa (26). The recent discoveries that L. pneumophila is naturally competent for DNA transformation, which is associated with expression of the type IV CAP (43), and that it is able to conjugate DNA (32, 48) support these speculations. Further characterization of the mil loci may yield interesting information that may help to elucidate these hypotheses.
Many loci of L. pneumophila designated dot (defect in organelle trafficking) and icm (intracellular multiplication) have also been shown to be required for intracellular replication, but it is not known whether they are required for infection of protozoa (32, 48).
One of the first-characterized genes that is partially required for intracellular infection is the mip gene (17, 18, 49). Strains with mutations in the mip gene are partially defective in early survival in macrophages, epithelial cells, and protozoa. Such mutants are also partially attenuated in guinea pigs. The Mip protein is similar to members of a class of proteins designated peptidyl-prolyl cis-trans isomerase (PPIase) and has been shown to possess this enzymatic activity (49). The conserved amino acids in the catalytic domain of PPIase have recently been shown to be conserved among 35 Legionella species. PPIases have been found in other intracellular pathogenic bacteria as well as nonpathogenic bacteria. With site-directed point mutations to alter the PPIase catalytic and conserved domains, it has been demonstrated that the PPIase activity of Mip is not involved in Mip’s function in intracellular infection (49).
ROLE OF IRON IN INTRACELLULAR INFECTION
Iron is an essential nutrient for all living organisms. L. pneumophila requires relatively high concentrations of iron for growth in vitro. How L. pneumophila obtains iron during intracellular growth in the EMB phagosome is not known. Fur is a conserved protein that functions as a repressor of factors involved in iron uptake in several bacteria. A fur gene and Fur-regulated genes have been described for L. pneumophila (29). One of these genes is a homolog of the aerobactin synthetase, raising the possibility that L. pneumophila utilizes siderophores to acquire iron (29). Importantly, a mutant defective in expression of the aerobactin synthetase is defective in intracellular replication within macrophages but it is not known whether this mutant is defective in protozoa. In addition, mutants defective in iron acquisition and assimilation have been isolated through transposon mutagenesis. Many of the mutants are defective in intracellular replication within macrophages and protozoa, but the functions of the defective genes in the mutants are not yet known (39). Further characterization of the iron uptake and assimilation systems in L. pneumophila will yield important information about how these systems are utilized within the phagosome.
PHENOTYPIC MODULATIONS BY INTRACELLULAR BACTERIA WITHIN MAMMALIAN AND PROTOZOAN CELLS
Pathogenic bacteria such as L. pneumophila respond and adapt to the various local environmental conditions they encounter by coordinate regulation of gene expression (2, 3). These phenotypic modulations allow intracellular bacteria to survive and adapt to environmental conditions that may be encountered within a cell.
Intracellular L. pneumophila undergoes a dramatic phenotypic modulation in gene expression in response to the intracellular environment within the EMB phagosomes of macrophages (2, 3). These alterations are manifested through the induction or repression of expression of many genes. Many of the macrophage-induced (MI) genes are also induced in response to one or more in vitro stress stimuli, which indicates that intracellular L. pneumophila is exposed to stress stimuli in vivo (3). In order to examine the molecular aspects of the MI genes, many strategies have been utilized to clone the MI genes, including reverse genetics (2, 4) and, most recently, differential display PCR (7). A recent strategy of selective radiolabeling of proteins of intracellular bacteria has been developed for Salmonella typhimurium. This strategy is based on the use of radioactive diaminopimelic acid (DAP), which is a major component of peptidoglycan and is also a precursor for lysine. Bacterial auxotrophs for DAP are used to infect the host cell in the presence of radioactive DAP. DAP is decarboxylated into lysine by the bacteria, which subsequently radiolabels the bacterial proteins selectively, since DAP cannot be utilized by mammalian cells. In contrast to the successful use of DAP auxotrophs to selectively radiolabel proteins of intracellular S. typhimurium, DAP does not accumulate in the L. pneumophila EMB phagosome in concentrations sufficient to sustain growth of the L. pneumophila DAP auxotroph (27). These observations may indicate differences in the levels of permeability of the EMB phagosome occupied by L. pneumophila and of the phagosome occupied by S. typhimurium, although several other possibilities exist (27).
One of the MI genes that has been cloned by reverse genetics is the global stress gene (gspA) of L. pneumophila, which is induced in response to in vitro stress stimuli and is also induced throughout the intracellular infection period (4, 6). Transcription of gspA is regulated by two promoters, one of which is a ς32-regulated promoter. L. pneumophila exhibits differential levels of expression of gspA by the ς32-regulated promoter throughout intracellular infection, which indicates continuous exposure of the bacterium to stress stimuli throughout intracellular infection (6). Mutation in gspA has no effect on the survival of L. pneumophila within mammalian macrophages and protozoan cells (6). However, the mutant exhibits a dramatic increase in susceptibility to stress stimuli in vitro. Interestingly, an intracellular wild-type strain derived from macrophages or from H. vermiformis exhibits a dramatic increase in its resistance to in vitro stress stimuli (6). The intracellular gspA mutant is similar to the wild-type strain in being equally resistant to in vitro stress stimuli (6). These observations suggest that expression of other stress-induced genes during the intracellular infection may compensate for the loss of gspA. The inorganic pyrophosphatase gene of L. pneumophila has also been shown to be induced within macrophages (2). This enzyme is required for macromolecular biosynthesis, and thus its induction within a host cell is consistent with faster replication in vivo than that in rich medium in vitro (2). In addition to stress and metabolic genes, some of the virulence genes of L. pneumophila may be specifically expressed or their expression may be induced within the EMB phagosome. An example of these genes is the early MI locus (eml), the function of which is not known (7).
It has been demonstrated that L. pneumophila within acanthamoebae undergoes phenotypic changes. Amoeba-grown L. pneumophila is more resistant to antimicrobial agents and possesses altered fatty acid profiles and surface proteins (11–13). Multiplication of L. pneumophila within acanthamoebae enhances infectivity and invasiveness to mammalian cells in vitro (19). How this intra-amoebic growth of L. pneumophila enhances its ability to infect mammalian cells is not known. During intracellular growth within acanthamoebae, L. pneumophila has been shown to express at least five proteins that are not expressed by in vitro-grown bacteria (19). It is possible that prior adaptation of L. pneumophila to the intracellular environment of protozoa, which involves phenotypic modulation by the bacteria in response to the intracellular niche, allows the bacteria to exhibit better adaptation to the intracellular niche within mammalian cells. However, although at the ultrastructural level phagosomes seem to be similar in both mammalian and protozoan cells, whether the biochemical natures of the microenvironments in phagosomes are similar in mammalian and protozoan cells remains to be determined.
KILLING OF THE HOST CELL
The mechanisms by which L. pneumophila kills the protozoan host are not known. In contrast, the mechanisms by which the bacterium kills the mammalian host cell are just starting to emerge. Muller et al. (37) have shown that within 24 to 48 h postinfection of HL-60 macrophage-like cells by L. pneumophila at a multiplicity of infection (MOI) of 10 to 100, the infected cells undergo apoptosis, or programmed cell death. Apoptosis is a strictly regulated suicide program within the dying cell and involves the activation of a family of cysteine proteases (caspases) that subsequently lead to DNA fragmentation of the host cell (41). Whether L. pneumophila induces apoptosis earlier than 24 h in HL-60 macrophages is not known.
It is well documented that intracellular replication of L. pneumophila within macrophages is associated with cytopathogenicity or loss of viability of the cells, as measured with dyes that detect the metabolic activities of the cells. In addition, at high MOIs, the bacteria are cytotoxic to mammalian cells (31). The bacterial factors responsible for this effect on the host cells are not known. Kirby et al. have recently shown that L. pneumophila induces the formation of a pore in bone marrow-derived macrophages from A/J mice when the cells are infected at an MOI of 500, which also results in necrosis of the cells within 20 to 60 min (34). Based on the observations made by Muller et al. (37) and Kirby et al. (34), it was proposed that a biphasic mode of killing of the host cell is mediated by L. pneumophila (34). Those investigators proposed a rapid necrotic cell death during early stages of high MOIs and a later second phase of apoptotic cell death (34). This is a highly unlikely reflection of natural infection, in which the infectious dose has been repeatedly shown to be very low (38); the high MOI is most likely to be attained later in the infection, after the release of intracellular bacteria. Thus, the role of apoptosis in the intracellular infection is still to be determined. The mechanisms of killing of the protozoan host are still to be determined, but it is unlikely that apoptosis plays a role in protozoa, since they are unicellular organisms. Apoptosis is thought to be required by multicellular organisms to eliminate unwanted cells to avoid injury to the rest of the organism. Therefore, protozoan apoptosis, if it occurs, is a host suicide.
CONCLUSIONS
The ability of L. pneumophila to survive and replicate within the EMB phagosome in two evolutionarily distant hosts (mammals and protozoa) is quite intriguing. It will be interesting to characterize the mil loci and their potential genetic transfer into L. pneumophila as a mode of pathogenic evolution of a protozoan parasite that may not have become a human pathogen simply due to the generation of aerosols. Further characterization of the functions of the pmi, mil, dot, and icm loci in intracellular infection and of their roles in alteration of endocytic trafficking of the bacteria will help microbiologists to understand the manipulations of host cell processes by a proficient intracellular pathogen. Understanding the functions of these loci and their roles in blocking maturation of the L. pneumophila EMB phagosome through the endosomal-lysosomal degradation pathway will allow both microbiologists and cell biologists to exploit them as tools to study endocytic trafficking and vesicular fusion.
REFERENCES
- 1.Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect Immun. 1998;66:203–212. doi: 10.1128/iai.66.1.203-212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. doi: 10.1111/j.1365-2958.1994.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Abu Kwaik Y, Fields B S, Engleberg N C. Protein expression by the protozoan Hartmannella vermiformis upon contact with its bacterial parasite Legionella pneumophila. Infect Immun. 1994;62:1860–1866. doi: 10.1128/iai.62.5.1860-1866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu Kwaik Y, Gao L-Y, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 7.Abu Kwaik Y, Pederson L L. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol Microbiol. 1996;21:543–556. doi: 10.1111/j.1365-2958.1996.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 8.Abu Kwaik Y, Venkataraman C, Harb O S, Gao L-Y. Signal transduction in the protozoan host Hartmannella vermiformis upon attachment and invasion by Legionella micdadei. Appl Environ Microbiol. 1998;64:3134–3139. doi: 10.1128/aem.64.9.3134-3139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams S A, Robson S C, Gathiram V, Jackson T F H G, Pillay T S, Kirsch R E, Makgoba M W. Immunological similarity between the 170 kDa amoebic adherence glycoprotein and human β2 integrins. Lancet. 1993;341:17–19. doi: 10.1016/0140-6736(93)92483-a. [DOI] [PubMed] [Google Scholar]
- 10.Adeleke A, Pruckler J, Benson R, Rowbotham T, Halablab M, Fields B S. Legionella-like amoebal pathogens—phylogenetic status and possible role in respiratory disease. Emerg Infect Dis. 1996;2:225–229. doi: 10.3201/eid0203.960311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker J, Brown M R W, Collier P J, Farrell I, Gilbert P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol. 1992;58:2420–2425. doi: 10.1128/aem.58.8.2420-2425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barker J, Lambert P A, Brown M R W. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1993;61:3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barker J, Scaife H, Brown M R W. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berk S G, Ting R S, Turner G W, Ashburn R J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol. 1998;64:279–286. doi: 10.1128/aem.64.1.279-286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellanii: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brieland J K, Fantone J C, Remick D G, LeGendre M, McClain M, Engleberg N C. The role of Legionella pneumophila-infected Hartmanella vermiformis as an infectious particle in a murine model of Legionnaires’ disease. Infect Immun. 1997;65:4892–4896. doi: 10.1128/iai.65.12.5330-5333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cianciotto N P, Eisenstein B I, Mody C H, Toews G B, Engleberg N C. A Legionella pneumophila gene encoding a species-specific surface protein potentiates initiation of intracellular infection. Infect Immun. 1989;57:1255–1262. doi: 10.1128/iai.57.4.1255-1262.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cianciotto N P, Fields B S. Legionella pneumophila mip gene potentiates intracellular infection of protozoa and human macrophages. Proc Natl Acad Sci USA. 1992;89:5188–5191. doi: 10.1073/pnas.89.11.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cirillo J D, Tompkins L S, Falkow S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields B S. Legionella and protozoa: interaction of a pathogen and its natural host. In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella: current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 129–136. [Google Scholar]
- 21.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 22.Fields B S, Nerad T A, Sawyer T K, King C H, Barbaree J M, Martin W T, Morrill W E, Sanden G N. Characterization of an axenic strain of Hartmannella vermiformis obtained from an investigation of nosocomial legionellosis. J Protozool. 1990;37:581–583. doi: 10.1111/j.1550-7408.1990.tb01269.x. [DOI] [PubMed] [Google Scholar]
- 23.Fraser D W, Tsai T R, Orenstein W, Parkin W E, Beecham H J, Sharrar R G, Harris J, Mallison G F, Martin S M, McDade J E, Shepard C C, Brachman P S. Legionnaires’ disease: description of an epidemic of pneumonia. N Engl J Med. 1977;297:1189–1197. doi: 10.1056/NEJM197712012972201. [DOI] [PubMed] [Google Scholar]
- 24.Gao, L.-Y., M. Gutzman, J. K. Brieland, and Y. Abu Kwaik. Different fates of Legionella pneumophila mutants within human-derived macrophages and alveolar epithelial cells. Submitted for publication. [DOI] [PubMed]
- 25.Gao L-Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant hosts, mammalian and protozoan cells. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao L-Y, Harb O S, Abu Kwaik Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harb O S, Abu Kwaik Y. Identification of the aspartate-β-semialdehyde dehydrogenase gene of Legionella pneumophila and characterization of a null mutant. Infect Immun. 1998;66:1898–1903. doi: 10.1128/iai.66.5.1898-1903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harb O S, Venkataraman C, Haack B J, Gao L-Y, Abu Kwaik Y. Heterogeneity in the attachment and uptake mechanisms of the Legionnaires’ disease bacterium, Legionella pneumophila, by protozoan hosts. Appl Environ Microbiol. 1998;64:126–132. doi: 10.1128/aem.64.1.126-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hickey E K, Cianciotto N P. An iron- and Fur-repressed Legionella pneumophila gene that promotes intracellular infection and encodes a protein with similarity to the Escherichia coli aerobactin synthetase. Infect Immun. 1997;65:133–143. doi: 10.1128/iai.65.1.133-143.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz M A. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob T, Escallier J C, Sanguedolce M V, Chicheportiche C, Bongrand P, Capo C, Mege J L. Legionella pneumophila inhibits superoxide generation in human monocytes via the down-modulation of α and β protein kinase C isotypes. J Leukoc Biol. 1994;55:310–312. doi: 10.1002/jlb.55.3.310. [DOI] [PubMed] [Google Scholar]
- 33.King C H, Fields B S, Shotts E B, Jr, White E H. Effects of cytochalasin D and methylamine on intracellular growth of Legionella pneumophila in amoebae and human monocyte-like cells. Infect Immun. 1991;59:758–763. doi: 10.1128/iai.59.3.758-763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 35.Mann B J, Torian B E, Vedvick T S, Petri W A., Jr Sequence of a cysteine-rich galactose-specific lectin of Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:3248–3252. doi: 10.1073/pnas.88.8.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowdle W R. Legionnaires’ disease: isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1977;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 37.Muller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Brein S J, Bhopal R S. Legionnaires’ disease: the infective dose paradox. Lancet. 1993;342:5–6. doi: 10.1016/0140-6736(93)91877-o. [DOI] [PubMed] [Google Scholar]
- 39.Pope C D, O’Connell W A, Cianciotto N P. Legionella pneumophila mutants that are defective for iron acquisition and assimilation and intracellular infection. Infect Immun. 1996;64:629–636. doi: 10.1128/iai.64.2.629-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowbotham T J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1998;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 42.Steinert M, Emody L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone, B. J., and Y. Abu Kwaik. Natural competency for DNA uptake by Legionella pneumophila and its association with expression of type IV pili. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 44.Stone B J, Abu Kwaik Y. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson M S, Isberg R R. Formation of the Legionella pneumophila replicative phagosome. Infect Agents Dis. 1995;2:269–271. [PubMed] [Google Scholar]
- 46.Venkataraman C, Gao L-Y, Bondada S, Abu Kwaik Y. Identification of cytoskeletal proteins in the protozoan Hartmannella vermiformis as substrates for induced tyrosine phosphatase activity mediated by attachment and invasion by the Legionnaires’ disease bacterium, Legionella pneumophila. J Exp Med. 1998;188:1–10. doi: 10.1084/jem.188.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkataraman C, Haack B J, Bondada S, Abu Kwaik Y. Identification of a Gal/GalNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the Legionnaires’ disease bacterium, Legionella pneumophila. J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 49.Wintermeyer E, Ludwig B, Steinert M, Schmidt B, Fischer G, Hacker J. Influence of site specifically altered Mip proteins on intracellular survival of Legionella pneumophila in eukaryotic cells. Infect Immun. 1995;63:4576–4583. doi: 10.1128/iai.63.12.4576-4583.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]