Abstract
Acute respiratory distress syndrome (ARDS) is a life-threatening pulmonary condition characterized by the sudden onset of respiratory failure, pulmonary edema, dysfunction of endothelial and epithelial barriers, and the activation of inflammatory cascades. Despite the increasing number of deaths attributed to ARDS, a comprehensive therapeutic approach for managing patients with ARDS remains elusive. To elucidate the pathological mechanisms underlying ARDS, numerous studies have employed various preclinical models, often utilizing lipopolysaccharide as the ARDS inducer. Accumulating evidence emphasizes the pivotal role of reactive oxygen species (ROS) in the pathophysiology of ARDS. Both preclinical and clinical investigations have asserted the potential of antioxidants in ameliorating ARDS. This review focuses on various sources of ROS, including NADPH oxidase, uncoupled endothelial nitric oxide synthase, cytochrome P450, and xanthine oxidase, and provides a comprehensive overview of their roles in ARDS. Additionally, we discuss the potential of using antioxidants as a strategy for treating ARDS.
Keywords: acute respiratory distress syndrome, reactive oxygen species, antioxidant, acute lung injury, superoxide dismutase, glutathione, vitamins
1. Introduction
Acute respiratory distress syndrome (ARDS) imposes a huge burden in the context of critical care medicine, demanding immediate attention and a comprehensive approach due to its life-threatening nature. Initially described in the 1960s by Petty and Ashbaugh [1], ARDS is characterized by the sudden onset of tachypnea, hypoxemia, and reduced lung compliance. The absence of a standardized or precise definition for ARDS has posed limitations in patient treatment and the statistical analysis of clinical outcomes [2]. In response to this challenge, the Berlin definition was introduced in 2012 as a novel diagnostic framework for ARDS [3]. This definition includes criteria such as (I) acute onset within 1 week; (II) bilateral lung infiltrates observable upon chest radiography; (III) the absence of a fully explanatory cause related to cardiac failure or fluid overload; and (IV) the categorization of ARDS severity based on a positive end-expiratory pressure (PEEP) level of at least 5 cm H2O, with lung injury classified into three grades of severity according to the PaO2/FiO2 ratio: mild (201–300 mm Hg), moderate (101–200 mm Hg), and severe (≤100 mm Hg). Historically, acute lung injury (ALI) referred to a milder form of lung injury, while ARDS signified a more severe lung condition [4]. However, the clinical use of the term ALI has been discontinued, and the classification is now based on the severity of ARDS, providing a clear and consistent categorization for clinical purposes.
ARDS can be attributed to various conditions, with trauma, aspiration, pneumonia, and sepsis serving as primary triggers for its development [5,6,7]. Additionally, the inhalation of harmful substances, aspiration of vomit, pancreatitis, blood transfusion, and near-drowning episodes can also induce ARDS. Recent retrospective observational studies have revealed that 67% of patients with severe COVID-19 develop ARDS, further emphasizing its significance as a primary cause of mortality [8]. Presently, ARDS stands as a leading cause of morbidity and mortality, with no effective treatments available [9]. Therefore, there is an urgent need to elucidate the pathogenesis of ARDS and develop innovative treatment strategies.
1.1. ARDS Incidence and Mortality
The incidence of ARDS varies globally, with data from population-based studies revealing significant diversity [9,10]. A study conducted in the United States from 1999 to 2000 reported an incidence rate of 78.9 per 100,000 person-years [11], while research in Iceland from 1988 to 2010 indicated incidence rates ranging from 3.65 to 9.63 [12]. These findings provide insights into mortality rates and severity distribution among patients with ARDS. In a large international, multicenter, prospective cohort study performed over a period of 4 weeks in 2014, patients with ARDS accounted for 10.4% of the total sample, with the proportions of mild, moderate, and severe cases being 30%, 46.5%, and 23.4%, respectively [13]. The mortality rates, according to severity, were 34.9% for mild, 40.3% for moderate, and 46.1% for severe. Another study conducted in China from March 2016 to February 2018 across 18 intensive care units (ICUs) reported that 3.6% of a total of 18,793 patients were diagnosed with ARDS [14]. Among these patients, 9.7% had mild ARDS, 47.4% exhibited moderate ARDS, and a substantial 42.9% were classified as having severe ARDS, with a reported mortality rate of 46.3%. Systematic research conducted since 2010 has indicated overall in-hospital, ICU, 28/30-day, and 60-day mortality rates of 45%, 38%, 30%, and 32%, respectively [15]. Multiple studies have highlighted the correlation between ARDS severity, high mortality, and prolonged ventilation duration.
1.2. ARDS Pathophysiology
Under normal physiological conditions, lung fluid balance is maintained through fluid clearance. However, dysfunction of the alveolar barrier can impair alveolar fluid clearance, a prominent hallmark of patients with ARDS, leading to hypoxemia and pulmonary bilateral edema [16,17]. Additionally, ARDS is characterized by an increased production of pro-inflammatory factors and an elevated expression of adhesion molecules, which play a crucial role in the recruitment of leukocytes. Specifically, activated neutrophils contribute to tissue damage by releasing cytotoxic agents, including granular enzymes, pro-inflammatory cytokines, bioactive lipids, and reactive oxygen species (ROS). Typical ARDS symptoms include dyspnea, an increased respiratory rate, tachycardia, and cyanosis. The pattern of injury observed in patients with ARDS is not uniform, and clinical symptoms can vary according to the severity or stage [18]. ARDS can be categorized into discrete stages, each with temporal evolution, pathophysiological alterations, histological features, and clinical implications. These stages encompass three simultaneous phases: exudative, proliferative, and fibrotic [19,20].
The acute phase, referred to as the exudative stage, typically spans from hours to the first week after the injury onset. During this stage, a cascading inflammatory response is activated, leading to an influx of neutrophils [21], a phenomenon that results in the disruption of the alveolar epithelial and endothelial barriers, and consequently, protein-rich edema in the interstitium and alveolar spaces. Hyaline membranes, composed of dead cells, surfactants, and plasma proteins, are also characteristic of diffuse alveolar damage [22]. The subsequent proliferative stage involves an organization and repair process that includes the proliferation of fibroblasts, primarily within the interstitium. In the late proliferative stage, most hyaline membranes are resorbed, although some remnants may persist along the alveolar septa [23]. Early fibrotic changes and thickening of the alveolar capillaries can also be observed. Finally, the fibrotic stage is marked by an increased deposition of collagen and enlarged air spaces, although not all patients progress to this stage. Established fibrosis reduces lung compliance, resulting in increased breathing effort, which is associated with poor lung function and a high risk of mortality [19].
To gain a deeper understanding of the intricate pathophysiology of ARDS and to develop novel therapeutic strategies, researchers have extensively utilized controlled experimental models to mimic clinical syndromes. These models serve as invaluable tools for exploring the mechanisms underlying ARDS and evaluating potential interventions. The workshop report published in 2011 by the American Thoracic Society (ATS) proposed the following four features of ARDS for preclinical models: histological evidence of tissue injury, alterations in the alveolar-capillary barrier, inflammatory response, and physiological dysfunction [24]. Histological evidence of tissue injury includes the infiltration of neutrophils in the alveolus or interstitium, the presence of hyaline membranes, and the accumulation of proteinaceous debris in the alveolus, along with thickening of the alveolar wall. Alveolar-capillary barrier disruption is reflected in increased extravascular lung water content, bronchoalveolar lavage fluid (BALF) protein levels, and microvascular filtration coefficient, and the accumulation of an exogenous tracer. The inflammatory response is characterized by an increase in the number of inflammatory cells in the BALF, lung myeloperoxidase (MPO) activity, BALF protein concentration, and pro-inflammatory cytokines in the lung or BALF. Lastly, physiological dysfunction comprises hypoxemia and an increased alveolar-arterial oxygen difference. To ensure ARDS in preclinical models, at least three of these four criteria should be fulfilled.
ARDS models have been generated through both direct and indirect methods to recapitulate human ARDS. The direct approach involves the stimulation of conditions such as pneumonia and acid aspiration, while the indirect approach involves the stimulation of pancreatitis and non-pulmonary sepsis. In direct models, ARDS is induced through the intranasal or intratracheal administration of agents such as lipopolysaccharides (LPS), HCl, bleomycin, bacteria, and viruses. Hyperoxia is also a direct cause of ARDS. In contrast, indirect lung injury can be induced via the intravenous injection of LPS, cecal ligation and puncture (CLP) to induce sepsis, hemorrhagic shock, mesenteric ischemia, and reperfusion.
1.2.1. LPS-Induced Sepsis Model
The most frequently utilized ARDS model involves the instillation of LPS via either the intratracheal or intranasal routes. Research has also explored variations based on species, age, sex, and LPS concentrations. In the LPS-induced ARDS model, several key observations include a reduced PaO2/FiO2 ratio, increased total protein concentration in the BALF, infiltration of inflammatory cells, disruption of alveolar barrier function, and excessive generation of ROS and oxidative stress [25,26,27]. These changes are often accompanied by local hemorrhage and edema [28]. However, the limitations of this model include the fact that alveolar and interstitial edema is mild, hyaline membrane formation is absent, and the decrease in PaO2 is not sustained for an extended period [29]. These limitations can be mitigated by employing a two-hit approach involving LPS stimulation, where both intratracheal LPS and intravenous LPS are administered [29]. This two-hit model involves the intraperitoneal injection of a small dose of LPS (1 mg/kg), followed by tracheal instillation with a moderate dose of LPS (5 mg/kg). In this model, the decrease in PaO2 is sustained for more than 72 h and induces more pronounced hyaline membrane formation and edema. Additionally, several studies have reported that the intraperitoneal injection of LPS can lead to lung injury [30,31].
1.2.2. Acid Aspiration Model
In the clinical setting, the aspiration of gastric contents is a major contributor to ARDS. Given the role of pH in lung injury, hydrochloric acid (HCl) has been employed to mimic human ARDS. The orotracheal and intratracheal instillation of HCl leads to reduced oxygenation, increased respiratory elastance, and pulmonary inflammation [32]. Furthermore, HCl aspiration induces histological changes, lung edema, and inflammatory responses in rabbits [33].
1.2.3. Oleic Acid Injection Model
The intravenous injection of oleic acid induces ARDS characterized by intra-alveolar edema and an increased infiltration of inflammatory cells [34,35]. An advantage of the oleic acid method is the rapid and reversible development of sparse inflammatory lung injury [36]. In research involving pigs, the administration of oleic acid resulted in a high mortality rate and extensive histological changes, as well as elevated levels of IL6 and IL8 [37]. Additionally, the intratracheal injection of oleic acid, employed in a mouse ARDS model, resulted in elevated neutrophil and protein levels in the BALF, the activation of leukocytes, and the production of inflammatory mediators [38].
1.2.4. CLP-Induced Sepsis Model
CLP is a method commonly utilized to induce sepsis in models [39]. In brief, in this method, the cecum is ligated, punctured with a needle, and compressed to expel a small amount of feces [39]. The severity of the injury can vary depending on factors such as the percentage of ceca ligated, the number of punctures, and the size of the needle used. Puncturing the cecum, which contains bacteria, leads to bacterial peritonitis and the translocation of bacteria into the bloodstream, ultimately resulting in septic shock. CLP mice exhibit high mortality and histopathological changes associated with ARDS, including the destruction of the alveolar structure, thickening of the pulmonary septa, hemorrhaged lung tissue, and the infiltration of inflammatory cells [40,41].
1.3. Treatment
The primary goal of managing ARDS is to improve blood oxygen levels. Despite administering high levels of inspired oxygen, oxygen saturation often remains inadequately low, underscoring the severity of impaired gas exchange or the failure to respond to conventional respiratory therapy in patients with ARDS. Furthermore, due to the diversity of ARDS causes and the complexity of the syndrome, there is currently no single therapy tailored specifically to ARDS. Additionally, a decline in the quality of life is frequently observed even after recovering from ARDS.
Various professional societies, including the Faculty of Intensive Care Medicine and Intensive Care Society [42], The French Society of Intensive Card Medicine (Société de Réanimation de Langue Française) [43], The ATS, the European Society of Intensive Care Medicine [44], the Korean Society of Critical Care Medicine and Korean Academy of Tuberculosis and Lung Diseases of South Korea [45], the Society of Critical Care Medicine, and the World Health Organization, offer recommendations for managing ARDS, taking into account the patient’s condition. These guidelines have been thoroughly documented in review papers [46,47] and propose various management strategies for patients with ARDS; these strategies can be categorized into two main groups: (1) ventilation strategies, including non-invasive ventilation, low tidal volume ventilation (LTVV), high positive end-expiratory pressure (PEEP) strategies, recruitment maneuvers, and high-frequency oscillatory ventilation, and (2) non-ventilation strategies, including interventions such as neuromuscular blockade, inhaled vasodilators, corticosteroids, and other pharmacological agents [47,48].
Mechanical lung ventilation is a standard therapeutic approach used to enhance oxygenation in patients with ARDS. However, it can also lead to ventilation-induced lung injury (VILI) [49,50]. VILI manifests as clinical symptoms such as shallow breathing, respiratory distress, and cyanosis, as well as chest X-ray findings indicating bilateral lung infiltrates, reduced blood oxygen levels (hypoxemia), and impaired lung elasticity, among other pulmonary functional impairments. Minimizing VILI through mechanical ventilation and effectively managing refractory hypoxemia are critical aspects of supportive ARDS management.
VILI is characterized by four primary mechanisms: volutrauma, barotrauma, atelectrauma, and biotrauma [49]. Volutrauma is closely linked to the use of high tidal volumes during mechanical ventilation. When mechanical ventilation is applied with a typical tidal volume without considering collapsed lung regions, it can result in the overdistension of lung cells, ultimately leading to increased alveolar-capillary permeability and alveolar destruction, culminating in pulmonary edema. Barotrauma arises from high airway pressure during positive pressure ventilation, causing lung damage through overinflation and the rupture of lung cells. The repeated opening and closing of lung cells contributes to increased lung size and shear stress forces, resulting in lung injury and surfactant dysfunction, known as atelectrauma. Biotrauma refers to the biochemical processes that occur when mechanical forces cause the collapse of lung cell membranes, triggering an inflammatory response. This can lead to systemic inflammatory response syndrome and multiple organ failure, ultimately resulting in death. Importantly, these four mechanisms are interconnected and can mutually influence each other. Various lung-protective ventilation methods are employed to minimize VILI.
1.3.1. Low Tidal Volume Ventilation
Patients with ARDS often exhibit reduced lung volume due to edema and inflammation, colloquially referred to as the “baby lung”. Consequently, traditional tidal volume ventilation (10 mL/kg or more) can lead to lung overdistension in patients with ARDS. LTVV is a technique that involves reducing the tidal volume, a strategy commonly used in individuals with healthy lungs to prevent lung tissue overdistension [17]. In 1998, Amato et al. first reported the potential clinical benefits of LTVV in patients with ARDS [51]. An extensive ARDS Network study involving 861 patients demonstrated that LTVV (6 mL/kg of predicted body weight) reduced mortality from 39.8% to 31% compared to traditional tidal volume ventilation (12 mL/kg of predicted body weight) and also increased the number of ventilator-free days [52]. Recent research suggests that the effects of mechanical ventilation can be enhanced with different tidal volumes (depending on lung compliance), with patients exhibiting low respiratory system compliance being more vulnerable to damage at higher driving pressures [53]. Furthermore, patients with COVID-19 receiving LTVV have shown reduced 28-day mortality [54].
1.3.2. Positive End-Expiratory Pressure
PEEP involves maintaining a pressure higher than atmospheric pressure at the end of expiration, which helps prevent atelectrauma and corrects hypoxemia resulting from alveolar hypoventilation [55]. Physiologically, PEEP increases functional residual capacity, recruits collapsed alveoli, and redistributes fluid to the interstitial space to improve oxygenation [56]. These physiological effects are expected to reduce VILI. However, it has been reported to have side effects, including a decrease in cardiac output and the potential for barotrauma. Studies on the effectiveness of high levels of PEEP in patients with ARDS have yielded inconsistent results. Some meta-analyses suggest that higher PEEP can enhance oxygenation during the first and third days of ventilation [57]. However, other meta-analyses have indicated that there is no significant difference in the 28-day mortality between high- and low-PEEP groups [58,59]. Additional studies are needed to accurately determine the impact and outcomes of PEEP application in patients with ARDS.
1.3.3. Lung Recruitment Maneuvers (LRMs)
LRMs are strategies designed to transiently increase the driving pressure to facilitate the recruitment of collapsed alveoli. This recruitment process improves oxygenation, promotes uniform volume distribution in the lungs, and mitigates the risk of overdistension [60]. Sustained inflation is employed at pressures ranging from 35 to 50 cm H2O for 20 to 40 s, with continuous monitoring for any adverse effects [61,62]. The use of LRMs in the treatment of ARDS remains a subject of debate. A meta-analysis that included data from 10 trials involving 1658 patients indicated that the implementation of LRMs in patients with ARDS led to reduced ICU mortality [63]. However, this study had limitations, primarily due to co-interventions with other mechanical ventilation strategies. Conversely, a more recent study with 10 trials involving 3025 patients suggested that LRMs did not affect the mortality of patients with ARDS, only reducing the duration of hospital stay [64]. Further research is necessary to clarify the precise impact and potential benefits of the isolated application of LRMs on the overall management and outcomes of patients with ARDS.
1.3.4. Prone Position
The prone position is commonly employed to facilitate breathing in ventilated patients. It involves turning the patient from the supine (back) position to the prone (face down) position to improve oxygenation. ARDS patients in the supine position are affected by the weight of the heart and abdominal organs, leading to increased pleural pressure in the dorsal lung regions, resulting in lung collapse. In such cases, the leveraging of the prone position can help redistribute blood and airflow more evenly [65,66]. The Prone Positioning in Severe ARDS (PROSEVA) study in 2013 demonstrated that employing the prone position during ventilation reduced both the 28- and 90-day mortality rates [67]. A meta-analysis of eight randomized controlled trials (RCTs) involving 2129 patients conducted by Munshi et al. suggested that employing the prone position for more than 12 h daily is likely to reduce mortality among patients with severe ARDS [68]. Furthermore, the PaO2/FiO2 ratio was significantly higher in the prone position group on day 4. A recent meta-analysis also indicated that prone positioning during venovenous extracorporeal membrane oxygenation improved survival [69,70]. Nevertheless, PEEP in the prone position is approached with caution due to potential adverse effects, including endotracheal tube obstruction, pressure sores, and facial edema [71].
1.3.5. Corticosteroids
Corticosteroids are potent anti-inflammatory and immunomodulatory drugs commonly employed to treat various inflammatory diseases, including asthma, allergic rhinitis, chronic obstructive pulmonary disease (COPD), and pneumonia [72]. The use of corticosteroids in patients with ARDS has yielded varying results. A meta-analysis of corticosteroid use in ARDS encompassing seven RCTs involving 851 patients indicated that corticosteroids reduced mortality and the duration of mechanical ventilation while increasing the number of ventilator-free days [73]. Furthermore, another meta-analysis demonstrated that patients with ARDS, both with COVID-19 and non-COVID-19 cases, exhibited reduced mortality and decreased mechanical ventilation duration when treated with corticosteroids [74]. However, a separate meta-analysis found that corticosteroids reduced mortality in non-COVID-19 patients with ARDS but had no effect in the COVID-19 subgroup [75]. It is important to exercise caution when using corticosteroids, as they may be associated with a higher mortality rate or the development of hyperglycemia [73,76].
2. Oxidative Stress and Lung Injury
2.1. Generation of ROS in ARDS
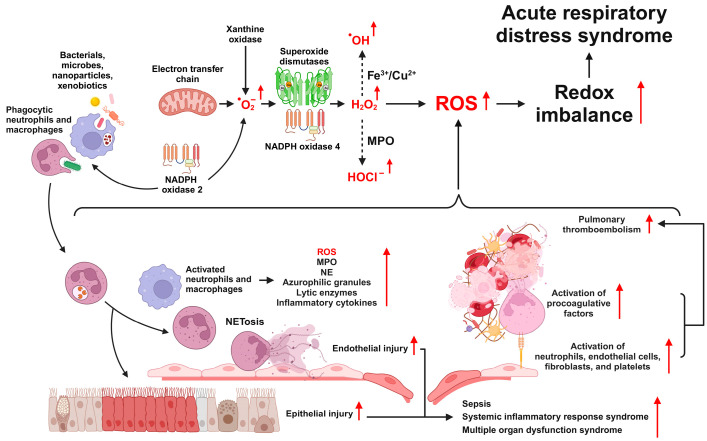
The overproduction and accumulation of ROS in ARDS can be attributed to several key factors, including the accumulation of leukocytes, elevated levels of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (NOXs) and xanthine oxidase (XO), and the use of high-concentration oxygen during mechanical ventilation. A defining characteristic of ARDS is the excessive infiltration of neutrophils and macrophages into the lung tissue [47,77]. Leukocytes play an essential role in the host defense against pathogens and contribute to the initiation and amplification of inflammatory responses by migrating to sites of infection and inflammation, engaging in phagocytic activity, and producing inflammatory mediators [78]. Neutrophils, monocytes, and macrophages employ NOX2 to generate MPO and ROS during their phagocytic actions (Figure 1). Furthermore, the presence of ROS is closely linked to the prognosis of sepsis, a primary cause of ARDS [79]. Contemporary research focuses on understanding the mechanisms underlying ARDS and exploring treatments using various preclinical models. Numerous studies have documented ROS generation in ARDS models and have shown that reduced ROS production can alleviate the lung damage associated with ARDS (refer to Table 1 for details).
Figure 1.
Overview of the roles of reactive oxygen species (ROS) in the pathophysiology of ARDS. ROS originating from various intrinsic and extrinsic sources leads to oxidative stress, cell-mediated inflammation, and damage to epithelial and endothelial cells. These processes are driven by the activation of multiple signaling pathways, ultimately contributing to the onset and progression of ARDS.
Table 1.
ROS production in preclinical models of ARDS.
| Candidate | Inducer | Conc. | Time | Animal | Organ | Method | Pathway | Ref |
|---|---|---|---|---|---|---|---|---|
| Neferine | LPS (i.p.) | 20 mg/kg | 6 h | C57BL/6 | Lung | DCFH-DA | NFκB | [80] |
| Abscisic acid | LPS (i.t.) | 4 mg/kg | 2 day | C57BL/6 | Lung | DCFH-DA | PPARγ | [81] |
| Berberine | LPS (i.p.) | 20 mg/kg | 6 h | C57BL/6 | Lung | DCFH-DA | NFκB | [82] |
| Carnosine | LPS (i.t.) | 1 mg/kg | 24 h | ICR | Lung | In vivo imaging | [83] | |
| Fraxin | LPS (i.p.) | 20 mg/kg | 6 h | C57BL/6 | Lung | DCFH-DA | NFκB, MAPK | [84] |
| MH | LPS (i.n.) | 5 mg/kg | 25 h | C57BL/6 | BALF | DCFH-DA | p38 MAPK, NFκB | [85] |
| EA | LPS (i.p.) | 5 mg/kg | 3 day | C57BL/6 | Lung | DHE | [31] | |
| Fasudil, NipR1, T-SSP | LPS (i.t.) | 2 mg/kg | 6 h | C57BL/6 | Lung | EPR | NLRP3 | [86] |
| Benziodarone | LPS (i.t.) | 1 mg/kg | 24 h | ICR | Lung | IHC(8-OHdG) | α7nAchR | [87] |
| eNAMPT-neutralizing antibody | LPS/VILI (i.v.) | 25 μg/kg/100% O2 (12 h) | Minipig | Lung | EPR | NFκB, Akt/mTORC2 | [88] | |
| Aspirin | Hyperoxia | 99% O2(72 h) | FVB/NJ | BALF | DCFH-DA | NFκB | [89] |
i.p., intraperitoneal; i.t., intratracheal; i.n., intranasal; i.v., intravenous; DCFH-DA, 2′,7′-dichlorofluorescein diacetate; DHE, dihydroethidium; EPR, electron spin resonance; IHC, immunohistochemistry; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; PPARγ, peroxisome proliferator-activated receptor γ; α7nAchR, α7 nicotinic acetylcholine receptor; MH, methyl p-hydroxycinnamate; EA, electroacupuncture; NipR1, nitration inhibitory peptide for RhoA; T-SSP, antioxidant Tiron; eNAMPT, extracellular nicotinamide phosphoribosyl transferase.
LPS treatment increases the uncoupling of eNOS and mitochondrial ROS production, resulting in the activation of NLR family pyrin domain containing 3 protein (NLRP3) inflammasome in human lung microvascular endothelial cells [9]. The inhibition of eNOS uncoupling can reduce ROS production and NLRP3 inflammasome levels. Targeting mitochondrial ROS using antioxidants can alleviate lung injury in the ARDS model. The mitochondrial redistribution of uncoupled eNOS has been implicated in the mitochondrial ROS-dependent activation of the NLRP3 inflammasome during sepsis.
Xanthine oxidoreductase (XOR) directly transfers electrons to O2, generating ROS, including O2·− and H2O2 [90]. Notably, O2·− reacts with NO, contributing to the formation of reactive nitrogen species such as peroxynitrite [91]. Grum et al. [92] showed that there was a significant increase in plasma XO activity (approximately 90-fold higher) in the group of patients with ARDS, accompanied by sepsis or pneumonia, compared to patients without ARDS. Additionally, plasma levels of hypoxanthine were approximately twice as high in the ARDS group compared to those in the non-ARDS group. A recent study reported that the myeloid-specific deficiency of XOR suppressed LPS-induced macrophage-mediated inflammation and ROS production while improving mitochondrial respiration in an ARDS model [93]. Febuxostat, a potent XO inhibitor, demonstrated dose-dependent protection against LPS-induced lung inflammation in rats, resulting in decreased levels of tumor necrosis factor alpha (TNFα) and malondialdehyde (MDA), as well as increased superoxide dismutase (SOD) activity in the lung tissue of rats [94]. The intratracheal administration of LPS significantly reduced the inflammatory response in myeloid-specific XOR knockout mice [93]. In addition, treatment with the ROS scavenger N-acetylcysteine (NAC) and XOR knockout significantly reduced NLRP3 and IL1β expression in purified BAL macrophage culture. The inhibition of NOXs also demonstrates positive effects against LPS-induced lung injury. Wang et al. [95] showed that the pharmacological inhibition of NOXs suppressed LPS-induced neutrophil ROS production and septic lung injuries in guinea pigs. Additionally, a recent study reported that increased lipid oxidation, MPO activity, and inflammatory responses were attenuated and antioxidant enzyme activity was restored through the inhibition of NOXs in a murine lung inflammation model [96].
Supplementation with oxygen is a potential treatment option for patients with ARDS [97]. However, prolonged exposure to high oxygen levels (O2 > 65%) can lead to an increase in ROS, inducing oxidative stress, worsening respiratory symptoms, and potentially causing lung injury [27]. Consistently, Kim et al. [26] showed that ROS generation significantly increased in type II alveolar epithelial cells and a model of the excessive hyperoxia-induced murine lung inflammation. Pretreatment with cimetidine, a cytochrome P450 (CYP) inhibitor, has been shown to attenuate hyperoxia-induced lung injury in lambs [98]. However, hyperoxic conditions induce cell death and ROS production in BEAS-2B cells [99]. In addition, hyperoxic conditions downregulated CYP1B1 in BEAS-2B cells, suggesting that CYP1B1 does not contribute to an increase in ROS. The siRNA-induced downregulation of CYP1B1 rescued cytotoxicity, whereas the overexpression of CYP1B1 promoted hyperoxic cytotoxicity.
2.2. Types and Functions of ROS
ROS are generated when O2 gains electrons. Under normal conditions, a stable equilibrium exists between ROS production and elimination. ROS include H2O2, •OH, 1O2, and O2•−. O2•− is generated through a single electron transfer to O2 and subsequently dismutates into H2O2, which is either spontaneously generated in the presence of water or catalyzed by SOD [100]. Notably, although ROS are viewed as a unified entity, different ROS types have distinct targets and activities. In cells, O2− and H2O2 are the two most abundant ROS subspecies, differing significantly in chemical properties and, consequently, behavior and function. O2•− is highly reactive and contributes to oxidative stress and cellular damage characterized by protein and amino acid oxidation, DNA damage, and lipid peroxidation. In contrast, H2O2, a relatively stable molecule, serves as a crucial cellular signaling factor. However, excessive production can lead to cytotoxicity.
2.3. Sources of ROS Production
ROS are primarily generated during tightly regulated enzymatic processes involving endothelial nitric oxide (NO) synthase, the mitochondrial respiratory chain, various NOX isoforms, XOs, and CYP enzymes [90,101]. Among these, NOXs appear to be the predominant source of ROS in the vasculature.
2.3.1. NAPDH Oxidase
NOXs are enzymes that oxidize NADH or NADPH to form NAD+ or NADP+. They catalyze the reduction of oxygen to produce O2•− and are known to activate other ROS sources, such as endothelial NO synthase (eNOS). Initially, NOX was discovered in phagocytic immune cells; however, it was later found in non-phagocytic cells. Activated NOX generates ROS, leading to endothelial cell dysfunction. The NOX family comprises seven isoforms: NOX1, NOX2, NOX3, NOX4, NOX5, dual oxidase 1 (Duox1), and Duox2. NOX2 and NOX4 play crucial roles in ROS production. In a spinal cord injury model, ROS had a harmful impact, contributing to oxidative stress, which is reversed by the deletion of NOX2 or inhibition of NOX4 to reduce ROS production and oxidative stress markers [102].
NOX2, in particular, comprises four cytoplasmic components, including p47phox, p67phox, p40phox, and Rac family small GTPase 2, along with transmembrane proteins p22phox and gp91phox [103,104]. Upon the initiation of phagocytosis, the cytosolic subunit Rac2, bound to guanosine diphosphate, becomes activated by a Rac guanine nucleotide exchange factor, allowing it to bind to GTP [105]. Simultaneously, it facilitates the transition of the transmembrane heterodimer p22phox and gp91phox to the phagosomal membrane and induces association with the trimeric complex composed of p40phox, p47phox, and p67phox [106]. Through the phosphorylation of p47phox and p67phox, a structural alteration and stabilization mediated by GTP-Rac2, p22phox, and gp91phox take place, inducing the overproduction of O2•− and MPO, which exists in the form of a ferric (Fe III) heme enzyme in lysosomes of phagocytic cells and chlorinates H2O2 to highly reactive hypochlorous acid (HOCl−) [103,106]. This, in turn, contributes to the sequential generation of ROS such as H2O2, •OH, and HOCl− [107].
2.3.2. Uncoupled Endothelial NO Synthase
Under normal circumstances, eNOS generates NO. eNOS transfers the electrons donated by NADPH to the amino-terminal oxygenase domain of heme via a series of cofactors located in the carboxy-terminal reductase domain. These cofactors include flavin adenine dinucleotide and flavin mononucleotide, which facilitate the electron transfer process necessary for the enzymatic conversion of l-arginine into NO. However, under conditions of oxidative stress, it shifts its production toward O2•− instead of NO; this phenomenon is known as eNOS uncoupling. eNOS uncoupling can occur under several conditions, including cofactor tetrahydrobiopterin (BH4) deficiency, a shortage of the eNOS substrate l-arginine, the accumulation of the NOS inhibitor asymmetric dimethylarginine (ADMA), and increased eNOS S-glutathionylation [108]. BH4 is highly susceptible to oxidative reactions and can be oxidized to BH2 in a highly oxidative environment. Additionally, the peroxynitrite formed by the reaction between O2 and NO can oxidize BH4. As a result, BH4 depletion destabilizes NOS and induces its uncoupling, which, in turn, affects eNOS activity. Under conditions of reduced l-arginine availability, eNOS becomes less effective at converting it into NO, and this shift in activity can lead to eNOS uncoupling. ADMA is an endogenous inhibitor of eNOS. When ADMA levels are elevated, it competes with l-arginine to bind to eNOS, thereby reducing the availability of l-arginine for NO production.
2.3.3. Cytochrome P450
CYP enzymes, a diverse group of heme monooxygenases, have been the focus of extensive research because of their central roles in the metabolism of drugs and xenobiotic compounds. Furthermore, they play crucial roles in the synthesis of sterols, fatty acids, eicosanoids, vitamins, and various other biochemical compounds, including ROS. Notably, upon modification by CYP enzymes, certain substrates give rise to reactive intermediates or products that can be implicated in the onset and progression of various diseases.
2.3.4. Xanthine Oxidase
XOR plays a pivotal role in the final stages of purine catabolism, facilitating the oxidation of hypoxanthine to xanthine or subsequently xanthine to uric acid [109]. It exists in two interchangeable forms (depending on the types of substrate): (1) XO utilizes oxygen as a substrate, generating both O2•− and H2O2, while (2) xanthine dehydrogenase is an NAD+-mediated type that utilizes NAD+ as a cofactor and does not generate ROS [110]. Importantly, oxygen concentration plays a critical role in determining whether H2O2 or O2•− is generated [111]. In one specific study, XO treatment increased O2•− and H2O2 levels in the XO inhibitor allopurinol treatment, preventing an increase in mitochondrial ROS levels in a model of cocaine-induced diastolic dysfunction [109].
2.4. ROS Signaling Pathways in ARDS
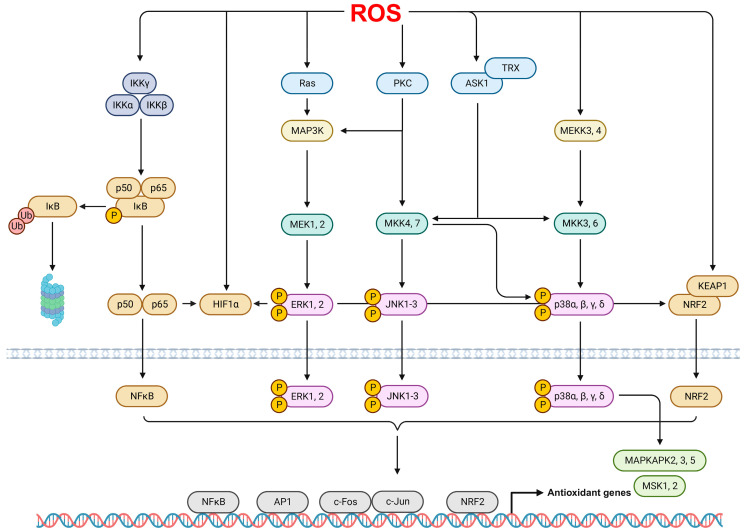
In ARDS, ROS can be induced and activated through the activation of various signaling pathways, such as mitogen-activated protein kinases (MAPKs), protein kinase C (PKC), nuclear factor kappa B (NFκB), hypoxia-inducible factor 1 (HIF1), and activator protein-1 (AP-1), as shown in Figure 2.
Figure 2.
ROS-mediated signaling pathways. The signaling pathways involving ROS include the activation of NFκB and MAPK pathways. Specifically, ROS can activate NFκB through the phosphorylation of IκB and induce the phosphorylation of the MAPK pathway, which includes JNK, ERK, and p38 MAPK. Consequently, these activations promote the nuclear translocation of transcriptional factors such as NFκB, AP-1, c-Fos, c-Jun, and NRF2. These factors contribute to the regulation of genes associated with oxidant defenses.
MAPKs are serine-threonine protein kinases involved in various processes, such as cell growth, proliferation, and differentiation. They are categorized into subgroups, including extracellular signal-related kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 MAPKs [112,113]. The ERK pathway regulates diverse cellular functions and is activated by various stimuli, such as growth hormones, oxidative stress, and inflammatory responses mediated by the phosphorylation of MAP/ERK Kinase (MEK) through MAPK kinase kinases (MAP3K) Raf [114]. Liu et al. [115] revealed that LPS stimulation augmented oxidative stress, inflammation, and neutrophil NETosis through the phosphorylation of Raf, MEK, and ERK in a murine lung inflammation model and neutrophils from patients with ARDS. Similarly, the elevated phosphorylation of ERK and JNK mediated excessive ROS production in models of hyperoxia-induced in vitro and in vivo ARDS [26].
JNK, also known as stress-activated protein kinase, is activated by physical or chemical stresses, cytokines, and ROS via the phosphorylation of upstream components such as MEK4, MEK7, and apoptosis signal-regulated kinase 1 (ASK1) [114,116]. ASK1 is inactivated upon binding to thioredoxin (TRX) in a resting state; however, when the thiol group of TRX is oxidized by H2O2 or other oxidants, it dissociates from ASK1, leading to the activation of JNK MAPKs [116,117]. Consistent with these observations, Sidramagowda et al. [118] showed that an increase in the phosphorylation of JNK and eukaryotic initiation factor 2α induced endoplasmic reticulum stress-mediated cell death in a murine model of hyperoxia-induced lung injury.
The p38 MAPKs encompass isoforms such as p38α, p38β, p38γ, and p38δ, and they are activated by a variety of stress responses, including inflammation, oxidative stress, heat, and physical shock [114,119]. Intermediaries of the p38 signaling pathway are phosphorylated by a range of stimuli, such as MEK3, MEK6, MEKK3, MEKK4, ASK1, transforming growth factor β-activated kinase 1, and dual-leucine-zipper-bearing kinase 1, allowing these molecules to crosstalk with various signaling pathways and responses [119]. A recent study showed that LPS-induced ROS and inflammatory cytokine production was significantly increased via the p38 MAPK and NFκB pathways in a murine ARDS model and RAW 264.7 macrophages [120]. Similarly, p38 MAPKs mediated increases in endothelial permeability following H2O2 exposure in bovine lung microvascular endothelial cells [121]. Additionally, Yuan et al. [122] demonstrated that MAP3K2 and MAP3K3 induced elevated ROS production through the phosphorylation of the NOX2 constituent p47phox in ARDS models.
PKC comprises at least 12 isoforms, including PKCα, PKCβI, and PKCδ, and it possesses structural features sensitive to oxidative modification [123,124]. It regulates various cellular responses, such as movement, proliferation, and apoptosis. Notably, PKC contains a zinc-binding, cysteine-rich motif at the N-terminus regulatory domain that responds sensitively to oxidants such as H2O2, thereby becoming activated [113]. Bourdonnay et al. [125] showed that pathways such as PKCδ, p21-activated protein kinase-class I, and PI3K/Akt1 were associated with p40phox-mediated ROS production in rat alveolar macrophages (AMs) stimulated with IgG-opsonized Klebsiella pneumonia. Similarly, the inhibition of PKC attenuated hypochlorous acid-induced pulmonary artery pressure and vascular permeability in a rabbit lung injury model [126]. Additionally, increased sepsis-attributable mortality in mice deficient in nuclear factor erythroid 2-related factor 2 (NRF2) and gp91phox was shown to be associated with LPS-induced ROS production (mediated by PKC activation) in murine AMs [127].
NFκB is a redox-sensitive transcription factor that regulates the expression of various genes associated with inflammation and immune responses [128]. It is composed of homodimers or heterodimers of five different subunits, including RelA/p65, RelB, cRel, p105/p50, and p100/p52, which contain the Rel homology domain essential for DNA binding, dimerization, and nuclear translocation [129,130]. Additionally, the Rel components also include a C-terminal transcription activation domain [130]. In the resting state, NFκB is retained in the cytoplasm as an inactive non-DNA binding form, regulated by IκB proteins that contain C-terminal ankyrin repeats, which inhibit DNA binding [128]. The activity of typical IκB proteins such as IκBα, IκBβ, and IκBε is regulated through phosphorylation via upstream IκB kinases (IKK) and subsequent ubiquitination by E3 ubiquitin ligases [113,128]. After partial processing by the 26S proteasome, the NFκB dimers are translocated from the cytoplasm to the nucleus, where they bind to promoters and activate target genes [113,129]. Exogenous ROS stimuli such as H2O2 typically regulate the activation of NFκB signaling pathways through the phosphorylation of tyrosine residues, particularly Tyr42, on IκBα [131]. Ghobadi et al. [132] showed that NFκB potentially mediates elevated serum ROS production while decreasing antioxidant capacity in patients with COPD. Concordantly, a recent study showed that LPS-induced NO and inducible NO synthase (iNOS) were regulated by the phosphorylation of NFκB p65 in a murine respiratory inflammation model [133]. Similarly, particulate matter-elicited oxidative stress, intracellular ROS levels, and related inflammatory responses and apoptosis were increased via NFκB and MAPK signaling pathways in [134]. Additionally, Lin et al. [135] revealed that PPARγ, which has antioxidative and anti-inflammatory functions, attenuated the NFκB-mediated recruitment of neutrophils to the lung, ROS production, and inflammatory gene expression in a murine lung inflammation model after LPS stimulation.
HIF1 is a conserved family member of the heterodimeric basic helix-loop-helix transcription factors; it is composed of oxygen-sensitive α subunits, including HIF1α, HIF2α, and HIF3α, and three constitutively expressed β subunits, including HIF1β, HIF2β, and HIF3β [136]. HIF1 accumulates in response to low oxygen levels, various cytokines, and inflammatory mediators [137]. HIF1α is a major regulator of macrophage M1 polarization and the expression of glycolytic genes such as glucose transporter 1, hexokinases, fructose-2,6-biphosphatase 3, and lactate dehydrogenase A [138,139]. Succinate, which is a Krebs cycle metabolite whose production is increased in response to enhanced glycolysis, inhibits the prolyl hydroxylases (PHDs) involved in the polyubiquitination and proteasomal degradation of HIF1α, thereby leading to the stabilization and accumulation of HIF1α and the production of inflammatory cytokines such as IL1β [139,140,141]. Additionally, increased ROS production and accumulation lead to the stabilization of HIF1α through reduced PHD activity in JunD-deficient cells [142]. A recent study showed that NFκB and HIF1α enhanced ovalbumin (OVA)-induced ROS generation, airway resistance, and mucus production in an allergic airway disease model [143]. Similarly, Sun et al. [144] showed a decrease in the activity of antioxidative enzymes including catalase, glutathione peroxidase, and SOD mediated by HIF1α and NFκB in models of LPS-induced murine lung injury.
AP-1 is a dimer comprising members of the JUN (c-Jun, JunB, and JunD), FOS (c-Fos, FosB, and Fra-1/2), ATF (ATF2/3/4/5/6B/7, B-ATF, B-ATF2/3, and JDP1/2), and MAF (MafA, MafB, c-Maf, Nrl, and MafF/G/K) families, which contain a basic leucine zipper (bZIP) domain necessary for DNA interaction and the formation of homodimers and heterodimers [145]. Further, AP-1 dimers collaborate with transcription factors such as transcription coactivators CREB binding protein/p300 and NFκB p65, which are absent from a bZIP domain, to contribute to gene expression [146]. AP-1 is activated in response to cytokines, hormones, growth factors, and ROS and plays an essential role in immunoglobulin production, cell proliferation, differentiation, and apoptosis [146]. Notably, ROS induce the expression of AP-1 and AP-1-dependent genes, contributing to relevant signal transduction related to redox cycling [147,148]. Additionally, AP-1 and ROS are involved in the pathogenesis of lung inflammation. Kim et al. [149] showed that the pharmacological inhibition of AP-1 and NFκB, through ROS scavenging, attenuated OVA-induced lung inflammation and airway hyperresponsiveness in a murine allergic asthma model. Similarly, activated AP-1, via the phosphorylation of c-Jun, has been shown to interact with ROS, leading to an increase in leukocyte infiltration and cyclooxygenase 2 (COX2) expression in a leptin-induced lung inflammation model [150].
3. Therapeutic Approaches Targeting ROS in ARDS
In ARDS, the permeability of the alveolar-capillary barrier increases, causing the edema fluid containing a large amount of protein to flow into the lung parenchyma, leading to epithelial cell dysfunction and reduced lung compliance and gas exchange [151,152]. Recently, in a murine lung injury model, Jiang et al. [153] showed that NOX4 activation and ROS production inhibit the expression of endothelial cell tight junction proteins zonula occludens-1 and occludin, leading to endothelial cell barrier dysfunction. Such damage and dysfunction with respect to the endothelium and epithelial cells lead to pulmonary edema and augmented inflammatory factors and oxidative stress, thereby amplifying the inflammatory response by inducing the activation and chemotaxis of leukocytes and lymphocytes [154]. Pulmonary endothelial and epithelial cells, particularly macrophages and neutrophils that exhibit phagocytosis, are the major factors in the production of large amounts of ROS mediated by NOX [155,156]. In addition, ROS, MPO, neutrophil elastase, and azurophilic granules released via NETosis, a process of extracellular release from activated neutrophils, also contribute to proteolytic and oxidative tissue damage and inflammatory responses [157,158]. From this perspective, strategies to suppress the excessive production of ROS and related signaling mechanisms in cells and tissues which are mainly involved in the pathology of ARDS will have a positive effect on the treatment of ARDS.
3.1. Role of the NRF2 Pathway in ARDS
NRF2 is a transcriptional regulator of genes with antioxidant and anti-inflammatory effects that has emerged as a major target in the treatment of ARDS. As a master regulator, NRF2 crosstalks with multiple signaling pathways, controlling the activity of various antioxidant enzymes and oxidizing enzymes such as NOX, NO synthase, XOR, and CYP [159,160]. Upon sensing oxidative stress, NRF2 translocates from the cytoplasm to the nucleus; it is activated by upstream signaling molecules such as Akt, AMP-activated protein kinase (AMPK), NFκB, and glycogen synthase kinase 3β (GSK3β) [160].
The phosphorylation of Akt induces the nuclear translocation of NRF2, leading to the up-regulation of heme oxygenase-1 (HO-1) and NAD(P)H quinone oxidoreductase 1 through the PI3K/Akt pathway. This process reduces oxidative stress and inflammation mediated by forkhead box protein O1-NLRP3, reducing NLRP3 inflammasome activity in the lungs [161]. The phosphorylation of AMPK increases the production of NRF2-induced antioxidant enzymes, thereby alleviating inflammatory lung injury by suppressing inflammatory responses and oxidative stress mediated by the NLRP3 inflammasome [162]. Additionally, NRF2 activation results in the suppression of inflammation and oxidative damage; this is mediated by the inhibition of IKK/IκB phosphorylation and the nuclear translocation of the p65 subunit of the redox-sensitive transcription factor NFκB [162]. Upon enhancing NRF2 activation and repressing NFκB pathways, inflammatory responses were suppressed in ischemic reperfusion-induced lung injury [163] and auranofin-stimulated AMs [164].
Kelch-like ECH-associated protein 1 (Keap1) features a broad-complex, tramtrack and bric-a-brac domain (BTB) that engages with the Cul3-Rbx1-E3 ligase complex, governing Keap1 homodimerization. Additionally, it possesses a double glycine repeat domain (DGR) that binds to the ETGE and DLG motifs within the NRF2-erythroid-derived CNC homology domain (Neh) 2 of NRF2, orchestrating the ubiquitination and degradation of NRF2 [165,166,167]. Keap1 primarily modulates the stabilization and transcriptional activity of NRF2 through its BTB and DGR domains. Normally, NRF2 undergoes swift ubiquitination by Keap1, leading to its subsequent degradation by the proteasome. However, when exposed to various stresses such as ROS, interactions with Keap1 are disrupted, reducing proteasomal degradation and enhancing nuclear translocation [166]. GSK3β is involved in an altered Keap1-independent NRF2 degradation mechanism. It phosphorylates motifs within the Neh6 domain of NRF2, initiating proteasomal degradation mediated by the dimeric β-transducin repeat-containing protein [166,168]. Only a few NRF2 molecules that are not ubiquitinated and degraded translocate to the nucleus, activating the expression of antioxidant-related factors such as HO-1, glutamate-cysteine ligase modifier subunit, glutamate-cysteine ligase catalytic subunit, glutathione peroxidase (GPx), and SOD3 [169,170].
Recent studies have shown that single-nucleotide polymorphisms (SNPs) in NRF2 correlate with the severity of ARDS. In one specific study, the hierarchical clustering of 72 inbred strains of mice identified a significant association between NRF2 haplotypes and hyperoxic lung injury phenotypes [171]. Additionally, the −617(C/A) SNP (rs6721961) within the proximal promoter of NRF2 is correlated with an increased risk of ARDS [172]. Similarly, NRF2 rs6721961 exhibited a nominal correlation with the severity of ARDS outcomes in Caucasian subjects with systemic inflammatory response syndrome [173]. Consistent with these findings, the augmented nuclear translocation of NRF2, mediated by the phosphorylation of AKT [161], AMPK [174], NFκB [175,176], and GSK3β [162], has been shown to result in the suppression of ROS production and various inflammatory responses, including inflammatory lung damage, elevated inflammatory gene expression, and the activation of the NLRP3 inflammasome, in murine lung injury models. Additionally, Cen et al. [177] demonstrated that NRF2 ameliorates mitochondrial dysfunction, endothelial disruption, the apoptosis of endothelial cells, and lung inflammation in mice. More recently, Wei et al. [178] revealed that NRF2 provides protection against LPS-induced ARDS in mice. NRF2 mitigates LPS-induced histopathological symptoms, suppresses inflammatory gene expression, and notably promotes macrophage M2 polarization through the activation of PPARγ while inhibiting the phosphorylation of NFκB p65 [178]. In summary, these observations reinforce the status of NRF2 as a potentially effective target for the prevention and treatment of ARDS, as it suppresses inflammatory responses, mitigates oxidative stress, and regulates diverse immune responses associated with various immune cells, such as macrophages and endothelial cells.
3.2. Antioxidant Therapies
Maintaining redox homeostasis and curbing excessive ROS production throughout the development, progression, and exacerbation of ARDS presents a promising avenue for alleviating and treating clinical symptoms. This can be achieved by regulating multiple signaling pathways implicated in ARDS pathogenesis. From this standpoint, antioxidant enzymes like SOD, glutathione (GSH), and various pharmacological and nonenzymatic antioxidants have been explored for their therapeutic potential in ARDS.
SOD catalyzes the conversion of O2•− into H2O2 and is categorized into cytosolic copper-zinc SOD (CuZnSOD), mitochondrial manganese SOD (MnSOD), and extracellular SOD (ECSOD), found in fibroblasts and endothelial cells [160]. While investigating therapeutic approaches to ARDS, researchers have focused on SOD’s ability to reduce and terminate intracellular ROS production [179,180,181]. Notably, in one study, the intratracheal delivery of recombinant human CuZnSOD yielded a reduction in the levels of acute inflammation markers in tracheal aspirate fluid, coupled with increased antioxidant activity and concentration in preterm neonates with respiratory distress syndrome [182]. Research has also explored the use of conjugated antioxidant enzymes with polyethylene glycol (PEG) or lecithin, revealing enhanced delivery to cells, protection against oxidative stress, and a reduction in the severity of ARDS [183,184]. A synthetic Mn-containing SOD mimetic, as shown by Ndengele et al. [185], suppressed LPS-induced O2•− and inflammatory cytokine production in murine AMs. Additionally, commercialized SOD mimetics, such as manganese(III)tetrakis(1-methyl-4-pyridyl)porphyrin, demonstrated the alleviation of oxidative and nitrative stress-mediated MPO activity and vascular permeability in a murine lung inflammation model following LPS stimulation [186]. Similarly, the synthetic salen-manganese complex EUK-8, exhibiting SOD and catalase activities, attenuated clinical symptoms of ARDS induced by LPS in a porcine model, as revealed by Gonzalez et al. [187]. Another SOD and catalase mimetic, EUK-134, encapsulated by PEG, exhibited enhanced protection against pulmonary edema in a murine lung inflammation model compared to its counterpart without EUK following LPS treatment [188]. Furthermore, a synthetic SOD mimetic, MnTBAP, repressed inflammatory gene expression, O2•− production, lung tissue damage, and necrosis in a murine lung inflammation model, as demonstrated by Suresh et al. [189].
GSH plays a pivotal role in scavenging intracellular H2O2, with GPx being the major enzyme responsible for this reducing mechanism in the redox cycle [190,191]. GPx catalyzes the oxidation of glutathione using H2O2 or lipid hydroperoxides (LOOH), with reduced GSH serving as an electron donor [192]. Glutathione disulfide (GSSG), formed during the reaction, is subsequently reduced back to GSH by glutathione reductase, utilizing the NADPH generated through the pentose phosphate pathway as an electron donor [160,193]. GPx, predominantly distributed in the cytoplasm and mitochondria, contains a selenium atom at its active site (usually in the form of selenocysteine) [194]. Additionally, free GSH functions as a water-soluble antioxidant by directly interacting with radical intermediates in non-enzymatic catalytic reactions [194]. The GSH-mediated O2•− scavenging induces a radical propagation reaction involving several steps, leading to the formation of thiyl radicals (GS·) and H2O2 [194,195]. In a murine lung injury model, GSH attenuated LPS-induced cellular apoptosis, mitochondrial dysfunction, and oxidative stress while restoring reduced levels of SOD2 protein [196]. Kim et al. [197] demonstrated that enhanced GPx activity, mediated by selenium treatment, reduced lung injuries and lipid peroxidation in paraquat-intoxicated rats. Furthermore, the administration of an organoselenium compound, Ebselen, enhanced antioxidant activity and suppressed inflammatory responses, including MPO activity, leukocyte infiltration, and lipid peroxidation, in a murine model of carrageenan-induced lung inflammation [198]. In a study by Britt et al. [199], pharmacological TRX reductase-1 aurothioglucose alleviated lung injury and mortality while increasing GSH activity in a murine ARDS model following LPS and hyperoxia exposure. Interestingly, Moute et al. [200] demonstrated that a synthetic GPx-containing selenium mimetic, BXT-51072, and BXT-51077 suppressed neutrophil-mediated inflammatory responses and endothelial alterations.
Synthetic and pharmacological antioxidants play a crucial role in preventing ARDS development by inhibiting intracellular ROS production and associated oxidases. Notable antioxidants, such as apocynin, GKT137831, tempol, sivelestat, and NAC, have been developed for this purpose. Apocynin, a NOX inhibitor, demonstrated the attenuation of acute hypoxia-induced gp91phox expression and O2•− production in porcine lung arteries, thereby mitigating the progression of ARDS [201]. Muzaffar et al. [202] showed that apocynin and iloprost inhibited XOR-induced gp91phox expression and O2•− formation in porcine pulmonary artery endothelial cells. The NOX1/4 dual inhibitor GKT137831 ameliorated lung injuries and suppressed the expression of inflammation- and autophagy-related genes in a murine ischemic lung injury model [203]. In recent studies, treatment with the commercialized free radical and NO scavenger TEMPOL resulted in reduced intracellular ROS production and increased SOD and catalase activity in the human pulmonary epithelial cell line Calu-6 [204]. Sivelestat sodium, a NOX inhibitor, suppressed pathological symptoms of ARDS, intracellular ROS, and MDA production while increasing SOD and GPx levels in A model of K. pneumonia-induced murine lung injury [205]. Additionally, sivelestat sodium promoted HO-1 production via the translocation of NRF2 to the nucleus in human pulmonary microvascular endothelial cells [205].
NAC, in its acetylated form of cysteine, possesses antioxidant capabilities by virtue of its sulfhydryl functional group (-SH) [206]. It acts as a critical cysteine supplier in the synthesis of glutathione, a precursor that elevates glutathione concentration within the cell [206,207]. NAC also forms complexes that facilitate the excretion of various toxic heavy metals and transition metals, such as Cd2+, Hg2+, and Pb2+, as well as Cu2+ and Fe3+, through the free thiols that bind with redox metal ions [208]. Clinical studies have highlighted the efficacy of NAC in preventing and treating inflammatory lung diseases such as COPD, ALI, and ARDS. Tse et al. [209] demonstrated that administered NAC improved small airway functions and alleviated exacerbation frequency in patients with COPD. In experimental settings, NAC treatment reduced lung injury, neutrophil infiltration, oxidized GSH levels, and breath H2O2 levels in rats following intratracheal IL1α administration [210]. Kolomaznik et al. [211] observed that administered NAC markedly improved ventilatory parameters while suppressing leukocyte migration, edema, oxidative stress, and inflammation in a murine lung injury model requiring mechanical ventilation following LPS stimulation. Additionally, Ortolani et al. [212] showed that NAC supplementation holds therapeutic potential for ARDS by enhancing GSH levels while reducing MDA and ethane concentration in the epithelial lining fluid of patients with ARDS.
Nonenzymatic antioxidants such as vitamins and flavonoids have been explored as treatments for ARDS by inhibiting intracellular ROS production and associated oxidases. Vitamins, serving as coenzymes, metabolic activators, cellular components, and ROS scavengers, play essential roles in combating oxidative stress [213]. Notably, vitamins C (ascorbic acid) and E (α-tocopherol) have garnered attention for their radical scavenging properties, activation of antioxidant enzymes, and regeneration of antioxidants under oxidative stress conditions [213,214]. Vitamin C, a water-soluble antioxidant, exists in forms such as l-ascorbic acid and l-dehydroascorbic acid [215]. It effectively eliminates active oxygen species, including reactive NO and H2O2. Studies have demonstrated the beneficial effects of vitamin C supplementation in various ARDS models [215]. Dioxygenase utilizes ascorbic acid as a co-substrate for substrate reduction [216]. Fisher et al. [217] showed that intraperitoneally delivered vitamin C ameliorated LPS-induced pulmonary inflammation and procoagulant activity in a murine septic lung injury model. Similarly, vitamin C supplementation attenuated sepsis-associated coagulopathy and inflammatory responses, enhanced epithelial barrier function, and improved alveolar fluid clearance in a murine abdominal sepsis-associated ARDS model [218]. Kearns et al. [219] revealed that vitamin C supplementation suppressed ischemic-reperfusion-induced MPO activity, lung neutrophil recruitment, and microvascular leakage in a murine ARDS model. Additionally, Patel et al. [220] demonstrated that vitamin C reduced oxidative and nitrosative stress, leukocyte infiltration, and HMGB1 protein-mediated macrophage dysfunction while improving lung integrity in the murine hyperoxia-induced ARDS model.
The most abundant and potent form of vitamin E, a liposoluble antioxidant consisting of eight isoforms, is α-tocopherol [221]. α-tocopherol’s chroman head group exhibits major antioxidant activity, preventing chain oxidation reactions and suppressing damage to lipid membranes [160]. The chroman head group of α-tocopherol demonstrates significant antioxidant activity by donating its hydrogen to the peroxyl radical, forming an α-tocopheroxyl radical. This process prevents chain oxidation reactions, thereby suppressing the oxidation of cell membranes and protecting lipid membranes from damage [222]. Vitamin E can be regenerated and reused by other antioxidants such as vitamin C [223]. Studies have explored the benefits of vitamin E isoforms in various contexts. One specific study showed that supplementation with the vitamin E isoform γ-tocopherol decreased LPS-elicited sputum eosinophils and mucins and neutrophil recruitment in the airways in a double-blind, placebo-controlled study for volunteers with mild asthma [224]. Similarly, γ-tocopherol has been shown to alleviate the LPS-induced infiltration of inflammatory cells, mucin production, and inflammatory gene expression in a murine lung inflammation model [225]. Rocksén et al. [226] demonstrated that administering vitamin E suppressed lung edema, lung injuries, neutrophil recruitment to airspaces and the interstitium, and transendothelial migration in a model of LPS-induced murine lung injury. Additionally, vitamin E reduced plasma nitrotyrosine and conjugated diene levels, increased airway pressure and pulmonary permeability index, and improved the PaO2/FiO2 ratio in a lung inflammation model [227].
α-Lipoic acid (α-LA), a naturally synthesized short-chain fatty acid with sulfhydryl groups, and its reduced form, i.e., dihydrolipoic acid, are known as universal antioxidants that function in both hydrophobic membranes and hydrophilic cytosol [228]. α-LA is involved in metal chelation, free radical quenching, and the recycling of other antioxidants such as vitamin C, E, and glutathione (GSH) [229]. Studies have highlighted the antioxidant properties of α-LA in various models. Zhang et al. [230] showed that α-LA repressed LPS-induced NFκB activation and related inflammatory gene expression in mice hearts, aortae, and lungs. Additionally, intraperitoneally delivered α-LA significantly enhanced GSH production and catalase and GPx activities while repressing serum MDA production in a model of oleic acid-induced murine lung injury [231]. α-LA treatment decreased pathological ARDS symptoms such as mononuclear cell infiltration, vascular permeability, and edema in lung tissues [231]. Recently, Shoji et al. [232] demonstrated that an α-LA derivative, the dihydrolipoyl histidinate zinc complex, attenuated the pathology scores of lung injury and suppressed inflammatory cell infiltration, gene expression, and NFκB p65 concentration in the BALF of a murine ARDS model following LPS stimulation.
Flavonoids, a class of polyphenols, exhibit anti-inflammatory, antioxidant, metal ion chelation, and free radical scavenging activities, countering oxidative stress by mitigating lipid oxidation and intracellular ROS [233]. Comprising aglycone and glycoside forms, flavonoids demonstrate higher antioxidant and antimicrobial activities in the glycoside form [234]. Structurally, flavonoids consist of two phenyl rings and a heterocycle, with various attached functional groups [233]. They are grouped into subgroups such as flavones, flavonols, flavanones, flavanols, isoflavonoids, anthocyanins, and chalcones based on their heterocyclic structures, each exhibiting diverse biological activities [233]. In a study by Kuo et al. [235], luteolin pretreatment improved the histopathological symptoms of ARDS and reduced oxidative damage, lipid peroxidation, and inflammatory gene expression in a murine model of LPS-induced ARDS. Administered luteolin also enhanced alveolar fluid clearance and reduced lung edema through the promotion of cyclic guanosine monophosphate/PI3K-mediated epithelial sodium channel expression in an ARDS murine model after LPS stimulation in [236]. Takashima et al. [237] demonstrated that intratracheally delivered quercetin alleviated LPS-induced wet lung-to-body weight ratios, metalloproteinase 9 activity, and the expression of inflammatory genes such as IL1β, IL6, and TNFα via a HO-1 dependent pathway in a murine lung injury model. Similarly, quercetin has been shown to decrease endotoxin-induced NO, MPO activity, MDA levels, lung permeability, leukocyte infiltration, and the expression of inflammatory genes such as IL1β, IL6, TNFα, COX2, HMGB1, and iNOS in a murine septic lung injury model [238]. Xanthohumol attenuated oxidative stress and inflammatory gene expression in LPS-induced ARDS by upregulating the NRF2 pathway through the activation of AMPK/GSK3β while downregulating the NFκB pathways [162]. Additionally, Shen et al. [239] demonstrated that epigallocatechin-3-gallate reduced hemorrhages, alveolar edema, inflammatory cell recruitment, and the expression of inflammatory genes such as TNFα, IL1β, and IL6 via Toll-like receptor-mediated NFκB activation in a model of paraquat-induced murine lung inflammation.
4. Conclusions
ARDS results in sudden and severe lung failure; despite elevated mortality rates, the syndrome is currently managed primarily through supportive care due to the absence of specific targeted treatments. The identification of increased levels of ROS in ARDS models and the clinical correlation between mortality and oxidative stress highlights the role of ROS as a potential target for ARDS treatment. Inhibiting ROS signaling through antioxidants has emerged as a prospective therapeutic approach for patients with ARDS. Nevertheless, conducting additional research, particularly through clinical trials, is imperative for comprehensively assessing the therapeutic efficacy of antioxidants.
Author Contributions
Conceptualization, E.Y.L. and G.-D.K.; writing—review and editing, E.Y.L., S.-Y.L., H.S.S. and G.-D.K.; funding acquisition, H.S.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding Statement
This review article was supported by the Main Research Program (E0210202-03) of the Korea Food Research Institute (KFRI) and funded by the Korean Ministry of Science and ICT.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ashbaugh D.G., Bigelow D.B., Petty T.L., Levine B.E. Acute respiratory distress in adults. Lancet. 1967;2:319–323. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Fioretto J.R., Carvalho W.B. Temporal evolution of acute respiratory distress syndrome definitions. J. Pediatr. 2013;89:523–530. doi: 10.1016/j.jped.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Villar J., Kacmarek R.M. The American-European Consensus Conference definition of the acute respiratory distress syndrome is dead, long live positive end-expiratory pressure! Med. Intensiv. 2012;36:571–575. doi: 10.1016/j.medin.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Shaver C.M., Bastarache J.A. Clinical and biological heterogeneity in acute respiratory distress syndrome: Direct versus indirect lung injury. Clin. Chest Med. 2014;35:639–653. doi: 10.1016/j.ccm.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lian J., Lin J., Zakaria N., Yahaya B.H. Acute Lung Injury: Disease Modelling and the Therapeutic Potential of Stem Cells. Adv. Exp. Med. Biol. 2020;1298:149–166. doi: 10.1007/5584_2020_538. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson N.D., Frutos-Vivar F., Esteban A., Gordo F., Honrubia T., Penuelas O., Algora A., Garcia G., Bustos A., Rodriguez I. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: A prospective observational study. Crit. Care. 2007;11:R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pham T., Rubenfeld G.D. Fifty Years of Research in ARDS. The Epidemiology of Acute Respiratory Distress Syndrome. A 50th Birthday Review. Am. J. Respir. Crit. Care Med. 2017;195:860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 10.Rezoagli E., Fumagalli R., Bellani G. Definition and epidemiology of acute respiratory distress syndrome. Ann. Transl. Med. 2017;5:282. doi: 10.21037/atm.2017.06.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., Stern E.J., Hudson L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 12.Sigurdsson M.I., Sigvaldason K., Gunnarsson T.S., Moller A., Sigurdsson G.H. Acute respiratory distress syndrome: Nationwide changes in incidence, treatment and mortality over 23 years. Acta Anaesthesiol. Scand. 2013;57:37–45. doi: 10.1111/aas.12001. [DOI] [PubMed] [Google Scholar]
- 13.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., van Haren F., Larsson A., McAuley D.F., et al. Epidemiology, Patterns of Care, and Mortality for Patients with Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 14.Huang X., Zhang R., Fan G., Wu D., Lu H., Wang D., Deng W., Sun T., Xing L., Liu S., et al. Incidence and outcomes of acute respiratory distress syndrome in intensive care units of mainland China: A multicentre prospective longitudinal study. Crit. Care. 2020;24:515. doi: 10.1186/s13054-020-03112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maca J., Jor O., Holub M., Sklienka P., Bursa F., Burda M., Janout V., Sevcik P. Past and Present ARDS Mortality Rates: A Systematic Review. Respir. Care. 2017;62:113–122. doi: 10.4187/respcare.04716. [DOI] [PubMed] [Google Scholar]
- 16.Ware L.B., Matthay M.A. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 17.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y.M., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spadaro S., Park M., Turrini C., Tunstall T., Thwaites R., Mauri T., Ragazzi R., Ruggeri P., Hansel T.T., Caramori G., et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J. Inflamm. 2019;16:1. doi: 10.1186/s12950-018-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellingan G.J. The pulmonary physician in critical care * 6: The pathogenesis of ALI/ARDS. Thorax. 2002;57:540–546. doi: 10.1136/thorax.57.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomashefski J.F., Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin. Chest Med. 2000;21:435–466. doi: 10.1016/S0272-5231(05)70158-1. [DOI] [PubMed] [Google Scholar]
- 21.Yang S.C., Tsai Y.F., Pan Y.L., Hwang T.L. Understanding the role of neutrophils in acute respiratory distress syndrome. Biomed. J. 2021;44:439–446. doi: 10.1016/j.bj.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cardinal-Fernandez P., Lorente J.A., Ballen-Barragan A., Matute-Bello G. Acute Respiratory Distress Syndrome and Diffuse Alveolar Damage. New Insights on a Complex Relationship. Ann. Am. Thorac. Soc. 2017;14:844–850. doi: 10.1513/AnnalsATS.201609-728PS. [DOI] [PubMed] [Google Scholar]
- 23.Cheung O.-Y., Graziano P., Smith M.L. Practical Pulmonary Pathology: A Diagnostic Approach. Elsevier; Amsterdam, The Netherlands: 2018. Acute lung injury; pp. 125–146.e3. [Google Scholar]
- 24.Matute-Bello G., Downey G., Moore B.B., Groshong S.D., Matthay M.A., Slutsky A.S., Kuebler W.M., Acute Lung Injury in Animals Study G. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011;44:725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aeffner F., Bolon B., Davis I.C. Mouse Models of Acute Respiratory Distress Syndrome: A Review of Analytical Approaches, Pathologic Features, and Common Measurements. Toxicol. Pathol. 2015;43:1074–1092. doi: 10.1177/0192623315598399. [DOI] [PubMed] [Google Scholar]
- 26.Kim M.J., Ryu J.C., Kwon Y., Lee S., Bae Y.S., Yoon J.H., Ryu J.H. Dual oxidase 2 in lung epithelia is essential for hyperoxia-induced acute lung injury in mice. Antioxid. Redox Signal. 2014;21:1803–1818. doi: 10.1089/ars.2013.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molaei E., Molaei A., Hayes A.W., Karimi G. Resolvin D1, therapeutic target in acute respiratory distress syndrome. Eur. J. Pharmacol. 2021;911:174527. doi: 10.1016/j.ejphar.2021.174527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue X., Guidry J.J. Differential Protein Expression Profiles of Bronchoalveolar Lavage Fluid Following Lipopolysaccharide-Induced Direct and Indirect Lung Injury in Mice. Int. J. Mol. Sci. 2019;20:3401. doi: 10.3390/ijms20143401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Wei H. Lipopolysaccharide “two-hit” induced refractory hypoxemia acute respiratory distress model in rats. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009;29:470–475. doi: 10.1007/s11596-009-0416-6. [DOI] [PubMed] [Google Scholar]
- 30.An X., Sun X., Hou Y., Yang X., Chen H., Zhang P., Wu J. Protective effect of oxytocin on LPS-induced acute lung injury in mice. Sci. Rep. 2019;9:2836. doi: 10.1038/s41598-019-39349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y., Zheng L., Deng H., Feng D., Hu S., Zhu L., Xu W., Zhou W., Wang Y., Min K., et al. Electroacupuncture Alleviates LPS-Induced ARDS Through alpha7 Nicotinic Acetylcholine Receptor-Mediated Inhibition of Ferroptosis. Front. Immunol. 2022;13:832432. doi: 10.3389/fimmu.2022.832432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel B.V., Wilson M.R., Takata M. Resolution of acute lung injury and inflammation: A translational mouse model. Eur. Respir. J. 2012;39:1162–1170. doi: 10.1183/09031936.00093911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y., Peng Y., Yang J. Galantamine protects against hydrochloric acid aspirationinduced acute respiratory distress syndrome in rabbits. Trop. J. Pharm. Res. 2018;17:669–673. doi: 10.4314/tjpr.v17i4.15. [DOI] [Google Scholar]
- 34.Erdem A., Gedikli E., Yersal N., Karaismailoglu S., Muftuoglu S., Fadillioglu E., Tuncer M. Protective role of erdosteine pretreatment on oleic acid-induced acute lung injury. J. Surg. Res. 2017;213:234–242. doi: 10.1016/j.jss.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 35.Liu D., Xu F., Wan D., Leng W., Zho F. Tetramethylpyrazine attenuates oleic acid-induced acute lung injury/acute respiratory distress syndrome through the downregulation of nuclear factor-kappa B (NF-kB) activation in rats. Afr. J. Biotechnol. 2011;10:12291–12298. [Google Scholar]
- 36.Goncalves-de-Albuquerque C.F., Silva A.R., Burth P., Castro-Faria M.V., Castro-Faria-Neto H.C. Acute Respiratory Distress Syndrome: Role of Oleic Acid-Triggered Lung Injury and Inflammation. Mediat. Inflamm. 2015;2015:260465. doi: 10.1155/2015/260465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borges A.M., Ferrari R.S., Thomaz L., Ulbrich J.M., Felix E.A., Silvello D., Andrade C.F. Challenges and perspectives in porcine model of acute lung injury using oleic acid. Pulm. Pharmacol. Ther. 2019;59:101837. doi: 10.1016/j.pupt.2019.101837. [DOI] [PubMed] [Google Scholar]
- 38.Goncalves-de-Albuquerque C.F., Silva A.R., Burth P., de Moraes I.M., Oliveira F.M., Younes-Ibrahim M., dos Santos Mda C., D’Avila H., Bozza P.T., Faria Neto H.C., et al. Oleic acid induces lung injury in mice through activation of the ERK pathway. Mediat. Inflamm. 2012;2012:956509. doi: 10.1155/2012/956509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siempos I.I., Lam H.C., Ding Y., Choi M.E., Choi A.M., Ryter S.W. Cecal ligation and puncture-induced sepsis as a model to study autophagy in mice. J. Vis. Exp. 2014;84:e51066. doi: 10.3791/51066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu J., Ran X., Deng J., Zhang A., Peng G., Du J., Wen D., Jiang B., Xia F. Glycyrrhizin alleviates sepsis-induced acute respiratory distress syndrome via suppressing of HMGB1/TLR9 pathways and neutrophils extracellular traps formation. Int. Immunopharmacol. 2022;108:108730. doi: 10.1016/j.intimp.2022.108730. [DOI] [PubMed] [Google Scholar]
- 41.Minato T., Yamaguchi T., Hoshizaki M., Nirasawa S., An J., Takahashi S., Penninger J.M., Imai Y., Kuba K. ACE2-like enzyme B38-CAP suppresses abdominal sepsis and severe acute lung injury. PLoS ONE. 2022;17:e0270920. doi: 10.1371/journal.pone.0270920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffiths M.J.D., McAuley D.F., Perkins G.D., Barrett N., Blackwood B., Boyle A., Chee N., Connolly B., Dark P., Finney S., et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir. Res. 2019;6:e000420. doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papazian L., Aubron C., Brochard L., Chiche J.D., Combes A., Dreyfuss D., Forel J.M., Guerin C., Jaber S., Mekontso-Dessap A., et al. Formal guidelines: Management of acute respiratory distress syndrome. Ann. Intensive Care. 2019;9:69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., Adhikari N.K.J., Amato M.B.P., Branson R., Brower R.G., et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 45.Cho Y.J., Moon J.Y., Shin E.S., Kim J.H., Jung H., Park S.Y., Kim H.C., Sim Y.S., Rhee C.K., Lim J., et al. Clinical Practice Guideline of Acute Respiratory Distress Syndrome. Tuberc. Respir. Dis. 2016;79:214–233. doi: 10.4046/trd.2016.79.4.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujishima S. Guideline-based management of acute respiratory failure and acute respiratory distress syndrome. J. Intensive Care. 2023;11:10. doi: 10.1186/s40560-023-00658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorman E.A., O’Kane C.M., McAuley D.F. Acute respiratory distress syndrome in adults: Diagnosis, outcomes, long-term sequelae, and management. Lancet. 2022;400:1157–1170. doi: 10.1016/S0140-6736(22)01439-8. [DOI] [PubMed] [Google Scholar]
- 48.Banavasi H., Nguyen P., Osman H., Soubani A.O. Management of ARDS—What Works and What Does Not. Am. J. Med. Sci. 2021;362:13–23. doi: 10.1016/j.amjms.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ak A.K., Anjum F. StatPearls. StatPearls; Treasure Island, FL, USA: 2023. Ventilator-Induced Lung Injury (VILI) [PubMed] [Google Scholar]
- 50.Beitler J.R., Malhotra A., Thompson B.T. Ventilator-induced Lung Injury. Clin. Chest Med. 2016;37:633–646. doi: 10.1016/j.ccm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amato M.B., Barbas C.S., Medeiros D.M., Magaldi R.B., Schettino G.P., Lorenzi-Filho G., Kairalla R.A., Deheinzelin D., Munoz C., Oliveira R., et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N. Engl. J. Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 52.Acute Respiratory Distress Syndrome Network. Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 53.Xie J., Jin F., Pan C., Liu S., Liu L., Xu J., Yang Y., Qiu H. The effects of low tidal ventilation on lung strain correlate with respiratory system compliance. Crit. Care. 2017;21:23. doi: 10.1186/s13054-017-1600-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nijbroek S., Hol L., Ivanov D., Schultz M.J., Paulus F., Neto A.S., PRoVENT-COVID Collaborative Group Low tidal volume ventilation is associated with mortality in COVID-19 patients-Insights from the PRoVENT-COVID study. J. Crit. Care. 2022;70:154047. doi: 10.1016/j.jcrc.2022.154047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sahetya S.K., Goligher E.C., Brower R.G. Fifty Years of Research in ARDS. Setting Positive End-Expiratory Pressure in Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017;195:1429–1438. doi: 10.1164/rccm.201610-2035CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Villar J. The use of positive end-expiratory pressure in the management of the acute respiratory distress syndrome. Minerva Anestesiol. 2005;71:265–272. [PubMed] [Google Scholar]
- 57.Santa Cruz R., Villarejo F., Irrazabal C., Ciapponi A. High versus low positive end-expiratory pressure (PEEP) levels for mechanically ventilated adult patients with acute lung injury and acute respiratory distress syndrome. Cochrane Database Syst. Rev. 2021;2021:CD009098. doi: 10.1002/14651858.CD009098.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo L., Xie J., Huang Y., Pan C., Yang Y., Qiu H., Liu L. Higher PEEP improves outcomes in ARDS patients with clinically objective positive oxygenation response to PEEP: A systematic review and meta-analysis. BMC Anesthesiol. 2018;18:172. doi: 10.1186/s12871-018-0631-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto R., Sugimura S., Kikuyama K., Takayama C., Fujimoto J., Yamashita K., Norisue Y., Narita C. Efficacy of Higher Positive End-Expiratory Pressure Ventilation Strategy in Patients with Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Cureus. 2022;14:e26957. doi: 10.7759/cureus.26957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fan E., Wilcox M.E., Brower R.G., Stewart T.E., Mehta S., Lapinsky S.E., Meade M.O., Ferguson N.D. Recruitment maneuvers for acute lung injury: A systematic review. Am. J. Respir. Crit. Care Med. 2008;178:1156–1163. doi: 10.1164/rccm.200802-335OC. [DOI] [PubMed] [Google Scholar]
- 61.Hess D.R. Recruitment Maneuvers and PEEP Titration. Respir. Care. 2015;60:1688–1704. doi: 10.4187/respcare.04409. [DOI] [PubMed] [Google Scholar]
- 62.Lapinsky S.E., Mehta S. Bench-to-bedside review: Recruitment and recruiting maneuvers. Crit. Care. 2005;9:60–65. doi: 10.1186/cc2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hodgson C., Goligher E.C., Young M.E., Keating J.L., Holland A.E., Romero L., Bradley S.J., Tuxen D. Recruitment manoeuvres for adults with acute respiratory distress syndrome receiving mechanical ventilation. Cochrane Database Syst. Rev. 2016;11:CD006667. doi: 10.1002/14651858.CD006667.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cui Y., Cao R., Wang Y., Li G. Lung Recruitment Maneuvers for ARDS Patients: A Systematic Review and Meta-Analysis. Respiration. 2020;99:264–276. doi: 10.1159/000501045. [DOI] [PubMed] [Google Scholar]
- 65.Henderson W.R., Griesdale D.E., Dominelli P., Ronco J.J. Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome? Can. Respir. J. 2014;21:213–215. doi: 10.1155/2014/472136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Zee P., Gommers D. Recruitment Maneuvers and Higher PEEP, the So-Called Open Lung Concept, in Patients with ARDS. Crit. Care. 2019;23:73. doi: 10.1186/s13054-019-2365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guerin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 68.Munshi L., Del Sorbo L., Adhikari N.K.J., Hodgson C.L., Wunsch H., Meade M.O., Uleryk E., Mancebo J., Pesenti A., Ranieri V.M., et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017;14:S280–S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 69.Papazian L., Schmidt M., Hajage D., Combes A., Petit M., Lebreton G., Rilinger J., Giani M., Le Breton C., Duburcq T., et al. Effect of prone positioning on survival in adult patients receiving venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Intensive Care Med. 2022;48:270–280. doi: 10.1007/s00134-021-06604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Poon W.H., Ramanathan K., Ling R.R., Yang I.X., Tan C.S., Schmidt M., Shekar K. Prone positioning during venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: A systematic review and meta-analysis. Crit. Care. 2021;25:292. doi: 10.1186/s13054-021-03723-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guerin C., Albert R.K., Beitler J., Gattinoni L., Jaber S., Marini J.J., Munshi L., Papazian L., Pesenti A., Vieillard-Baron A., et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020;46:2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Janahi I.A., Rehman A., Baloch N.U.-A. Corticosteroids. InTech Open; London, UK: 2018. Corticosteroids and their use in respiratory disorders; pp. 47–57. [Google Scholar]
- 73.Mammen M.J., Aryal K., Alhazzani W., Alexander P.E. Corticosteroids for patients with acute respiratory distress syndrome: A systematic review and meta-analysis of randomized trials. Pol. Arch. Intern. Med. 2020;130:276–286. doi: 10.20452/pamw.15239. [DOI] [PubMed] [Google Scholar]
- 74.Chaudhuri D., Sasaki K., Karkar A., Sharif S., Lewis K., Mammen M.J., Alexander P., Ye Z., Lozano L.E.C., Munch M.W., et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: A systematic review and meta-analysis. Intensive Care Med. 2021;47:521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang X., Li S., Fu Y., Dang H., Liu C. Safety and efficacy of corticosteroids in ARDS patients: A systematic review and meta-analysis of RCT data. Respir. Res. 2022;23:301. doi: 10.1186/s12931-022-02186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang J.W., Jiang P., Wang W.W., Wen Z.M., Mao B., Lu H.W., Zhang L., Song Y.L., Xu J.F. The Controversy About the Effects of Different Doses of Corticosteroid Treatment on Clinical Outcomes for Acute Respiratory Distress Syndrome Patients: An Observational Study. Front. Pharmacol. 2021;12:722537. doi: 10.3389/fphar.2021.722537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frank J.A., Wray C.M., McAuley D.F., Schwendener R., Matthay M.A. Alveolar macrophages contribute to alveolar barrier dysfunction in ventilator-induced lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;291:L1191–L1198. doi: 10.1152/ajplung.00055.2006. [DOI] [PubMed] [Google Scholar]
- 78.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., et al. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bime C., Zhou T., Wang T., Slepian M.J., Garcia J.G., Hecker L. Reactive oxygen species-associated molecular signature predicts survival in patients with sepsis. Pulm. Circ. 2016;6:196–201. doi: 10.1086/685547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu X.Y., Xu H.X., Li J.K., Zhang D., Ma X.H., Huang L.N., Lu J.H., Wang X.Z. Neferine Protects Endothelial Glycocalyx via Mitochondrial ROS in Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome. Front. Physiol. 2018;9:102. doi: 10.3389/fphys.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang L., Zou H., Xiao X., Wu H., Zhu Y., Li J., Liu X., Shen Q. Abscisic acid inhibited reactive oxygen species-mediated endoplasmic reticulum stress by regulating the PPAR-gamma signaling pathway in ARDS mice. Phytother. Res. 2021;35:7027–7038. doi: 10.1002/ptr.7326. [DOI] [PubMed] [Google Scholar]
- 82.Huang L., Zhang X., Ma X., Zhang D., Li D., Feng J., Pan X., Lu J., Wang X., Liu X. Berberine alleviates endothelial glycocalyx degradation and promotes glycocalyx restoration in LPS-induced ARDS. Int. Immunopharmacol. 2018;65:96–107. doi: 10.1016/j.intimp.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka K.I., Sugizaki T., Kanda Y., Tamura F., Niino T., Kawahara M. Preventive Effects of Carnosine on Lipopolysaccharide-induced Lung Injury. Sci. Rep. 2017;7:42813. doi: 10.1038/srep42813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ma X., Liu X., Feng J., Zhang D., Huang L., Li D., Yin L., Li L., Wang X.Z. Fraxin Alleviates LPS-Induced ARDS by Downregulating Inflammatory Responses and Oxidative Damages and Reducing Pulmonary Vascular Permeability. Inflammation. 2019;42:1901–1912. doi: 10.1007/s10753-019-01052-8. [DOI] [PubMed] [Google Scholar]
- 85.Kim S.M., Min J.H., Kim J.H., Choi J., Park J.M., Lee J., Goo S.H., Oh J.H., Kim S.H., Chun W., et al. Methyl p-hydroxycinnamate exerts anti-inflammatory effects in mouse models of lipopolysaccharide-induced ARDS. Mol. Med. Rep. 2022;25:37. doi: 10.3892/mmr.2021.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H., Sun X., Lu Q., Zemskov E.A., Yegambaram M., Wu X., Wang T., Tang H., Black S.M. The mitochondrial redistribution of eNOS is involved in lipopolysaccharide induced inflammasome activation during acute lung injury. Redox Biol. 2021;41:101878. doi: 10.1016/j.redox.2021.101878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishihara T., Tanaka K.I., Takafuji A., Miura K., Mizushima T. Attenuation of LPS-Induced Lung Injury by Benziodarone via Reactive Oxygen Species Reduction. Int. J. Mol. Sci. 2023;24:10035. doi: 10.3390/ijms241210035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bermudez T., Sammani S., Song J.H., Hernon V.R., Kempf C.L., Garcia A.N., Burt J., Hufford M., Camp S.M., Cress A.E., et al. eNAMPT neutralization reduces preclinical ARDS severity via rectified NFkB and Akt/mTORC2 signaling. Sci. Rep. 2022;12:696. doi: 10.1038/s41598-021-04444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tung Y.T., Wei C.H., Yen C.C., Lee P.Y., Ware L.B., Huang H.E., Chen W., Chen C.M. Aspirin Attenuates Hyperoxia-Induced Acute Respiratory Distress Syndrome (ARDS) by Suppressing Pulmonary Inflammation via the NF-kappaB Signaling Pathway. Front. Pharmacol. 2021;12:793107. doi: 10.3389/fphar.2021.793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Battelli M.G., Polito L., Bortolotti M., Bolognesi A. Xanthine Oxidoreductase-Derived Reactive Species: Physiological and Pathological Effects. Oxidative Med. Cell. Longev. 2016;2016:3527579. doi: 10.1155/2016/3527579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harrison R. Structure and function of xanthine oxidoreductase: Where are we now? Free Radic. Biol. Med. 2002;33:774–797. doi: 10.1016/S0891-5849(02)00956-5. [DOI] [PubMed] [Google Scholar]
- 92.Grum C.M., Ragsdale R.A., Ketai L.H., Simon R.H. Plasma xanthine oxidase activity in patients with adult respiratory distress syndrome. J. Crit. Care. 1987;2:22–26. doi: 10.1016/0883-9441(87)90116-X. [DOI] [Google Scholar]
- 93.Fini M.A., Monks J.A., Li M., Gerasimovskaya E., Paucek P., Wang K., Frid M.G., Pugliese S.C., Bratton D., Yu Y.R., et al. Macrophage Xanthine Oxidoreductase Links LPS Induced Lung Inflammatory Injury to NLRP3 Inflammasome Expression and Mitochondrial Respiration. bioRxiv. 2023 doi: 10.1101/2023.07.21.550055. [DOI] [Google Scholar]
- 94.Fahmi A.N., Shehatou G.S., Shebl A.M., Salem H.A. Febuxostat protects rats against lipopolysaccharide-induced lung inflammation in a dose-dependent manner. Naunyn Schmiedebergs Arch. Pharmacol. 2016;389:269–278. doi: 10.1007/s00210-015-1202-6. [DOI] [PubMed] [Google Scholar]
- 95.Wang W., Suzuki Y., Tanigaki T., Rank D.R., Raffin T.A. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am. J. Respir. Crit. Care Med. 1994;150:1449–1452. doi: 10.1164/ajrccm.150.5.7952574. [DOI] [PubMed] [Google Scholar]
- 96.Kouki A., Ferjani W., Ghanem-Boughanmi N., Ben-Attia M., Dang P.M., Souli A., El-Benna J. The NADPH Oxidase Inhibitors Apocynin and Diphenyleneiodonium Protect Rats from LPS-Induced Pulmonary Inflammation. Antioxidants. 2023;12:770. doi: 10.3390/antiox12030770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohshimo S. Oxygen administration for patients with ARDS. J. Intensive Care. 2021;9:17. doi: 10.1186/s40560-021-00532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hazinski T.A., France M., Kennedy K.A., Hansen T.N. Cimetidine reduces hyperoxic lung injury in lambs. J. Appl. Physiol. 1989;67:2586–2592. doi: 10.1152/jappl.1989.67.6.2586. [DOI] [PubMed] [Google Scholar]
- 99.Dinu D., Chu C., Veith A., Lingappan K., Couroucli X., Jefcoate C.R., Sheibani N., Moorthy B. Mechanistic role of cytochrome P450 (CYP)1B1 in oxygen-mediated toxicity in pulmonary cells: A novel target for prevention of hyperoxic lung injury. Biochem. Biophys. Res. Commun. 2016;476:346–351. doi: 10.1016/j.bbrc.2016.05.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Capek J., Rousar T. Detection of Oxidative Stress Induced by Nanomaterials in Cells-The Roles of Reactive Oxygen Species and Glutathione. Molecules. 2021;26:4710. doi: 10.3390/molecules26164710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jiang S., Liu H., Li C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods. 2021;10:1854. doi: 10.3390/foods10081854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khayrullina G., Bermudez S., Hopkins D., Yauger Y., Byrnes K.R. Differential effects of NOX2 and NOX4 inhibition after rodent spinal cord injury. PLoS ONE. 2023;18:e0281045. doi: 10.1371/journal.pone.0281045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lam G.Y., Huang J., Brumell J.H. The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Semin. Immunopathol. 2010;32:415–430. doi: 10.1007/s00281-010-0221-0. [DOI] [PubMed] [Google Scholar]
- 104.Babior B.M. Phagocytes and oxidative stress. Am. J. Med. 2000;109:33–44. doi: 10.1016/S0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 105.Diebold B.A., Bokoch G.M. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat. Immunol. 2001;2:211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 106.Zhao T., Benard V., Bohl B.P., Bokoch G.M. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J. Clin. Investig. 2003;112:1732–1740. doi: 10.1172/JCI19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bedard K., Krause K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 108.Janaszak-Jasiecka A., Ploska A., Wieronska J.M., Dobrucki L.W., Kalinowski L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023;28:21. doi: 10.1186/s11658-023-00423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vergeade A., Mulder P., Vendeville C., Ventura-Clapier R., Thuillez C., Monteil C. Xanthine oxidase contributes to mitochondrial ROS generation in an experimental model of cocaine-induced diastolic dysfunction. J. Cardiovasc. Pharmacol. 2012;60:538–543. doi: 10.1097/FJC.0b013e318271223c. [DOI] [PubMed] [Google Scholar]
- 110.Okamoto K., Kusano T., Nishino T. Chemical nature and reaction mechanisms of the molybdenum cofactor of xanthine oxidoreductase. Curr. Pharm. Des. 2013;19:2606–2614. doi: 10.2174/1381612811319140010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kelley E.E., Khoo N.K., Hundley N.J., Malik U.Z., Freeman B.A., Tarpey M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010;48:493–498. doi: 10.1016/j.freeradbiomed.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brown M.D., Sacks D.B. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee I.T., Yang C.M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012;84:581–590. doi: 10.1016/j.bcp.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 114.Son Y., Cheong Y.K., Kim N.H., Chung H.T., Kang D.G., Pae H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Q., Zhu C.L., Li H.R., Xie J., Guo Y., Li P., Zhao Z.Z., Wang J.F., Deng X.M. Salvianolic Acid A Protects against Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting Neutrophil NETosis. Oxidative Med. Cell. Longev. 2022;2022:7411824. doi: 10.1155/2022/7411824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y., Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ. Res. 2002;90:1259–1266. doi: 10.1161/01.RES.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 117.Saitoh M., Nishitoh H., Fujii M., Takeda K., Tobiume K., Sawada Y., Kawabata M., Miyazono K., Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sidramagowda Patil S., Soundararajan R., Fukumoto J., Breitzig M., Hernandez-Cuervo H., Alleyn M., Lin M., Narala V.R., Lockey R., Kolliputi N., et al. Mitochondrial Protein Akap1 Deletion Exacerbates Endoplasmic Reticulum Stress in Mice Exposed to Hyperoxia. Front. Pharmacol. 2022;13:762840. doi: 10.3389/fphar.2022.762840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cuadrado A., Nebreda A.R. Mechanisms and functions of p38 MAPK signalling. Biochem. J. 2010;429:403–417. doi: 10.1042/BJ20100323. [DOI] [PubMed] [Google Scholar]
- 120.Park J.R., Lee H., Kim S.I., Yang S.R. The tri-peptide GHK-Cu complex ameliorates lipopolysaccharide-induced acute lung injury in mice. Oncotarget. 2016;7:58405–58417. doi: 10.18632/oncotarget.11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Usatyuk P.V., Vepa S., Watkins T., He D., Parinandi N.L., Natarajan V. Redox regulation of reactive oxygen species-induced p38 MAP kinase activation and barrier dysfunction in lung microvascular endothelial cells. Antioxid. Redox Signal. 2003;5:723–730. doi: 10.1089/152308603770380025. [DOI] [PubMed] [Google Scholar]
- 122.Yuan Q., Basit A., Liang W., Qu R., Luan Y., Ren C., Li A., Xu X., Liu X., Yang C., et al. Pazopanib ameliorates acute lung injuries via inhibition of MAP3K2 and MAP3K3. Sci. Transl. Med. 2021;13:462–469. doi: 10.1126/scitranslmed.abc2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park H.S., Kim S.R., Lee Y.C. Impact of oxidative stress on lung diseases. Respirology. 2009;14:27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- 124.Rosse C., Linch M., Kermorgant S., Cameron A.J., Boeckeler K., Parker P.J. PKC and the control of localized signal dynamics. Nat. Rev. Mol. Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- 125.Bourdonnay E., Serezani C.H., Aronoff D.M., Peters-Golden M. Regulation of alveolar macrophage p40phox: Hierarchy of activating kinases and their inhibition by PGE2. J. Leukoc. Biol. 2012;92:219–231. doi: 10.1189/jlb.1211590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hammerschmidt S., Vogel T., Jockel S., Gessner C., Seyfarth H.J., Gillissen A., Wirtz H. Protein kinase C inhibition attenuates hypochlorite-induced acute lung injury. Respir. Med. 2007;101:1205–1211. doi: 10.1016/j.rmed.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 127.Kong X., Thimmulappa R., Kombairaju P., Biswal S. NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J. Immunol. 2010;185:569–577. doi: 10.4049/jimmunol.0902315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011;21:103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hayden M.S., Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 130.Ghosh S., May M.J., Kopp E.B. NF-kappa B and Rel proteins: Evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 131.Schieven G.L., Kirihara J.M., Myers D.E., Ledbetter J.A., Uckun F.M. Reactive oxygen intermediates activate NF-kappa B in a tyrosine kinase-dependent mechanism and in combination with vanadate activate the p56lck and p59fyn tyrosine kinases in human lymphocytes. Blood. 1993;82:1212–1220. doi: 10.1182/blood.V82.4.1212.1212. [DOI] [PubMed] [Google Scholar]
- 132.Ghobadi H., Abdollahi N., Madani H., Aslani M.R. Effect of Crocin from Saffron (Crocus sativus L.) Supplementation on Oxidant/Antioxidant Markers, Exercise Capacity, and Pulmonary Function Tests in COPD Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 2022;13:884710. doi: 10.3389/fphar.2022.884710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Feng L., Yang N., Li C., Tian G., Wang J., Dong Z.B., Jia X.B., Di L.Q. Pudilan xiaoyan oral liquid alleviates LPS-induced respiratory injury through decreasing nitroxidative stress and blocking TLR4 activation along with NF-KappaB phosphorylation in mice. J. Ethnopharmacol. 2018;214:292–300. doi: 10.1016/j.jep.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 134.Fernando I.P.S., Jayawardena T.U., Kim H.S., Lee W.W., Vaas A., De Silva H.I.C., Abayaweera G.S., Nanayakkara C.M., Abeytunga D.T.U., Lee D.S., et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh) Environ. Res. 2019;172:150–158. doi: 10.1016/j.envres.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 135.Lin M.H., Chen M.C., Chen T.H., Chang H.Y., Chou T.C. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-gamma-dependent inhibition of NF-kB activation. Int. Immunopharmacol. 2015;28:270–278. doi: 10.1016/j.intimp.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 136.McGettrick A.F., O’Neill L.A.J. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020;32:524–536. doi: 10.1016/j.cmet.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 137.Bardos J.I., Ashcroft M. Negative and positive regulation of HIF-1: A complex network. Biochim. Biophys. Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 138.Cramer T., Yamanishi Y., Clausen B.E., Forster I., Pawlinski R., Mackman N., Haase V.H., Jaenisch R., Corr M., Nizet V., et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/S0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Selak M.A., Armour S.M., MacKenzie E.D., Boulahbel H., Watson D.G., Mansfield K.D., Pan Y., Simon M.C., Thompson C.B., Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 141.Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E., Tourlomousis P., Dabritz J.H.M., Gottlieb E., Latorre I., et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell. 2016;167:457–470. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gerald D., Berra E., Frapart Y.M., Chan D.A., Giaccia A.J., Mansuy D., Pouyssegur J., Yaniv M., Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 143.Lee K.S., Kim S.R., Park H.S., Park S.J., Min K.H., Lee K.Y., Choe Y.H., Hong S.H., Han H.J., Lee Y.R., et al. A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp. Mol. Med. 2007;39:756–768. doi: 10.1038/emm.2007.82. [DOI] [PubMed] [Google Scholar]
- 144.Sun H.L., Peng M.L., Lee S.S., Chen C.J., Chen W.Y., Yang M.L., Kuan Y.H. Endotoxin-induced acute lung injury in mice is protected by 5,7-dihydroxy-8-methoxyflavone via inhibition of oxidative stress and HIF-1alpha. Environ. Toxicol. 2016;31:1700–1709. doi: 10.1002/tox.22172. [DOI] [PubMed] [Google Scholar]
- 145.Atsaves V., Leventaki V., Rassidakis G.Z., Claret F.X. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers. 2019;11:1037. doi: 10.3390/cancers11071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fan F., Podar K. The Role of AP-1 Transcription Factors in Plasma Cell Biology and Multiple Myeloma Pathophysiology. Cancers. 2021;13:2326. doi: 10.3390/cancers13102326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xanthoudakis S., Miao G., Wang F., Pan Y.C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11:3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sen C.K., Packer L. Antioxidant and redox regulation of gene transcription. FASEB J. 1996;10:709–720. doi: 10.1096/fasebj.10.7.8635688. [DOI] [PubMed] [Google Scholar]
- 149.Kim S.Y., Moon K.A., Jo H.Y., Jeong S., Seon S.H., Jung E., Cho Y.S., Chun E., Lee K.Y. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol. Cell Biol. 2012;90:441–448. doi: 10.1038/icb.2011.60. [DOI] [PubMed] [Google Scholar]
- 150.Hsu P.S., Lin C.M., Chang J.F., Wu C.S., Sia K.C., Lee I.T., Huang K.Y., Lin W.N. Participation of NADPH Oxidase-Related Reactive Oxygen Species in Leptin-Promoted Pulmonary Inflammation: Regulation of cPLA2alpha and COX-2 Expression. Int. J. Mol. Sci. 2019;20:1078. doi: 10.3390/ijms20051078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Englert J.A., Bobba C., Baron R.M. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight. 2019;4:e124061. doi: 10.1172/jci.insight.124061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Castillo R.L., Carrasco Loza R., Romero-Dapueto C. Pathophysiological Approaches of Acute Respiratory Distress syndrome: Novel Bases for Study of Lung Injury. Open Respir. Med. J. 2015;9:83–91. doi: 10.2174/1874306401509010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Jiang J., Huang K., Xu S., Garcia J.G.N., Wang C., Cai H. Targeting NOX4 alleviates sepsis-induced acute lung injury via attenuation of redox-sensitive activation of CaMKII/ERK1/2/MLCK and endothelial cell barrier dysfunction. Redox Biol. 2020;36:101638. doi: 10.1016/j.redox.2020.101638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rahman I., Adcock I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 155.Matthay M.A., Ware L.B., Zimmerman G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012;122:2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Williams A.E., Jose R.J., Mercer P.F., Brealey D., Parekh D., Thickett D.R., O’Kane C., McAuley D.F., Chambers R.C. Evidence for chemokine synergy during neutrophil migration in ARDS. Thorax. 2017;72:66–73. doi: 10.1136/thoraxjnl-2016-208597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 158.Lefrancais E., Mallavia B., Zhuo H., Calfee C.S., Looney M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight. 2018;3:e98178. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luan R., Ding D., Yang J. The protective effect of natural medicines against excessive inflammation and oxidative stress in acute lung injury by regulating the Nrf2 signaling pathway. Front. Pharmacol. 2022;13:1039022. doi: 10.3389/fphar.2022.1039022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kellner M., Noonepalle S., Lu Q., Srivastava A., Zemskov E., Black S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) Adv. Exp. Med. Biol. 2017;967:105–137. doi: 10.1007/978-3-319-63245-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fu Z., Jiang Z., Guo G., Liao X., Liu M., Xiong Z. rhKGF-2 Attenuates Smoke Inhalation Lung Injury of Rats via Activating PI3K/Akt/Nrf2 and Repressing FoxO1-NLRP3 Inflammasome. Front. Pharmacol. 2021;12:641308. doi: 10.3389/fphar.2021.641308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lv H., Liu Q., Wen Z., Feng H., Deng X., Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3beta-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou L., Zhao D., An H., Zhang H., Jiang C., Yang B. Melatonin prevents lung injury induced by hepatic ischemia-reperfusion through anti-inflammatory and anti-apoptosis effects. Int. Immunopharmacol. 2015;29:462–467. doi: 10.1016/j.intimp.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 164.Wall S.B., Li R., Butler B., Burg A.R., Tse H.M., Larson-Casey J.L., Carter A.B., Wright C.J., Rogers L.K., Tipple T.E. Auranofin-Mediated NRF2 Induction Attenuates Interleukin 1 Beta Expression in Alveolar Macrophages. Antioxidants. 2021;10:632. doi: 10.3390/antiox10050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dinkova-Kostova A.T., Fahey J.W., Kostov R.V., Kensler T.W. KEAP1 and Done? Targeting the NRF2 Pathway with Sulforaphane. Trends Food Sci. Technol. 2017;69:257–269. doi: 10.1016/j.tifs.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zang H., Mathew R.O., Cui T. The Dark Side of Nrf2 in the Heart. Front. Physiol. 2020;11:722. doi: 10.3389/fphys.2020.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Silva-Islas C.A., Maldonado P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018;134:92–99. doi: 10.1016/j.phrs.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 168.Mathur A., Pandey V.K., Kakkar P. Activation of GSK3beta/beta-TrCP axis via PHLPP1 exacerbates Nrf2 degradation leading to impairment in cell survival pathway during diabetic nephropathy. Free Radic. Biol. Med. 2018;120:414–424. doi: 10.1016/j.freeradbiomed.2018.04.550. [DOI] [PubMed] [Google Scholar]
- 169.Deshmukh P., Unni S., Krishnappa G., Padmanabhan B. The Keap1-Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017;9:41–56. doi: 10.1007/s12551-016-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cho H.Y., Jedlicka A.E., Gladwell W., Marzec J., McCaw Z.R., Bienstock R.J., Kleeberger S.R. Association of Nrf2 polymorphism haplotypes with acute lung injury phenotypes in inbred strains of mice. Antioxid. Redox Signal. 2015;22:325–338. doi: 10.1089/ars.2014.5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Marzec J.M., Christie J.D., Reddy S.P., Jedlicka A.E., Vuong H., Lanken P.N., Aplenc R., Yamamoto T., Yamamoto M., Cho H.Y., et al. Functional polymorphisms in the transcription factor NRF2 in humans increase the risk of acute lung injury. FASEB J. 2007;21:2237–2246. doi: 10.1096/fj.06-7759com. [DOI] [PubMed] [Google Scholar]
- 173.O’Mahony D.S., Glavan B.J., Holden T.D., Fong C., Black R.A., Rona G., Tejera P., Christiani D.C., Wurfel M.M. Inflammation and immune-related candidate gene associations with acute lung injury susceptibility and severity: A validation study. PLoS ONE. 2012;7:e51104. doi: 10.1371/journal.pone.0051104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin F.C., Lee S.S., Li Y.C., Ho Y.C., Chen W.Y., Chen C.J., Lee M.W., Yeh K.L., Tsai S.C., Kuan Y.H. Protective Effects of Kirenol against Lipopolysaccharide-Induced Acute Lung Injury through the Modulation of the Proinflammatory NFkappaB Pathway and the AMPK2-/Nrf2-Mediated HO-1/AOE Pathway. Antioxidants. 2021;10:204. doi: 10.3390/antiox10020204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gan Q., Wang X., Cao M., Zheng S., Ma Y., Huang Q. NF-kappaB and AMPK-Nrf2 pathways support the protective effect of polysaccharides from Polygonatum cyrtonema Hua in lipopolysaccharide-induced acute lung injury. J. Ethnopharmacol. 2022;291:115153. doi: 10.1016/j.jep.2022.115153. [DOI] [PubMed] [Google Scholar]
- 176.Patangrao Renushe A., Kumar Banothu A., Kumar Bharani K., Mekala L., Mahesh Kumar J., Neeradi D., Durga Veera Hanuman D., Gadige A., Khurana A. Vincamine, an active constituent of Vinca rosea ameliorates experimentally induced acute lung injury in Swiss albino mice through modulation of Nrf-2/NF-kappaB signaling cascade. Int. Immunopharmacol. 2022;108:108773. doi: 10.1016/j.intimp.2022.108773. [DOI] [PubMed] [Google Scholar]
- 177.Cen M., Ouyang W., Zhang W., Yang L., Lin X., Dai M., Hu H., Tang H., Liu H., Xia J., et al. MitoQ protects against hyperpermeability of endothelium barrier in acute lung injury via a Nrf2-dependent mechanism. Redox Biol. 2021;41:101936. doi: 10.1016/j.redox.2021.101936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wei J., Chen G., Shi X., Zhou H., Liu M., Chen Y., Feng D., Zhang P., Wu L., Lv X. Nrf2 activation protects against intratracheal LPS induced mouse/murine acute respiratory distress syndrome by regulating macrophage polarization. Biochem. Biophys. Res. Commun. 2018;500:790–796. doi: 10.1016/j.bbrc.2018.04.161. [DOI] [PubMed] [Google Scholar]
- 179.Wang G., Song Y., Feng W., Liu L., Zhu Y., Xie X., Pan Y., Ke R., Li S., Li F., et al. Activation of AMPK attenuates LPS-induced acute lung injury by upregulation of PGC1alpha and SOD1. Exp. Ther. Med. 2016;12:1551–1555. doi: 10.3892/etm.2016.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koyama S., Kobayashi T., Kubo K., Sekiguchi M., Ueda G. Recombinant-human superoxide dismutase attenuates endotoxin-induced lung injury in awake sheep. Am. Rev. Respir. Dis. 1992;145:1404–1409. doi: 10.1164/ajrccm/145.6.1404. [DOI] [PubMed] [Google Scholar]
- 181.Davis J.M., Rosenfeld W.N., Koo H.C., Gonenne A. Pharmacologic interactions of exogenous lung surfactant and recombinant human Cu/Zn superoxide dismutase. Pediatr. Res. 1994;35:37–40. doi: 10.1203/00006450-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 182.Davis J.M., Rosenfeld W.N., Richter S.E., Parad M.R., Gewolb I.H., Spitzer A.R., Carlo W.A., Couser R.J., Price A., Flaster E., et al. Safety and pharmacokinetics of multiple doses of recombinant human CuZn superoxide dismutase administered intratracheally to premature neonates with respiratory distress syndrome. Pediatrics. 1997;100:24–30. doi: 10.1542/peds.100.1.24. [DOI] [PubMed] [Google Scholar]
- 183.Tanaka K.I., Tamura F., Sugizaki T., Kawahara M., Kuba K., Imai Y., Mizushima T. Evaluation of Lecithinized Superoxide Dismutase for the Prevention of Acute Respiratory Distress Syndrome in Animal Models. Am. J. Respir. Cell Mol. Biol. 2017;56:179–190. doi: 10.1165/rcmb.2016-0158OC. [DOI] [PubMed] [Google Scholar]
- 184.Suzuki Y., Tanigaki T., Heimer D., Wang W.Z., Ross W.G., Sussman H.H., Raffin T.A. Polyethylene glycol-conjugated superoxide dismutase attenuates septic lung injury in guinea pigs. Am. Rev. Respir. Dis. 1992;145:388–393. doi: 10.1164/ajrccm/145.2_Pt_1.388. [DOI] [PubMed] [Google Scholar]
- 185.Ndengele M.M., Muscoli C., Wang Z.Q., Doyle T.M., Matuschak G.M., Salvemini D. Superoxide potentiates NF-kappaB activation and modulates endotoxin-induced cytokine production in alveolar macrophages. Shock. 2005;23:186–193. doi: 10.1097/01.shk.0000144130.36771.d6. [DOI] [PubMed] [Google Scholar]
- 186.Cai L., Yi F., Dai Z., Huang X., Zhao Y.D., Mirza M.K., Xu J., Vogel S.M., Zhao Y.Y. Loss of caveolin-1 and adiponectin induces severe inflammatory lung injury following LPS challenge through excessive oxidative/nitrative stress. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014;306:L566–L573. doi: 10.1152/ajplung.00182.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gonzalez P.K., Zhuang J., Doctrow S.R., Malfroy B., Benson P.F., Menconi M.J., Fink M.P. EUK-8, a synthetic superoxide dismutase and catalase mimetic, ameliorates acute lung injury in endotoxemic swine. J. Pharmacol. Exp. Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- 188.Howard M.D., Greineder C.F., Hood E.D., Muzykantov V.R. Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control. Release. 2014;177:34–41. doi: 10.1016/j.jconrel.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Suresh M.V., Yu B., Lakshminrusimha S., Machado-Aranda D., Talarico N., Zeng L., Davidson B.A., Pennathur S., Raghavendran K. The protective role of MnTBAP in oxidant-mediated injury and inflammation in a rat model of lung contusion. Surgery. 2013;154:980–990. doi: 10.1016/j.surg.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Espinosa-Diez C., Miguel V., Mennerich D., Kietzmann T., Sanchez-Perez P., Cadenas S., Lamas S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015;6:183–197. doi: 10.1016/j.redox.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ross D., Norbeck K., Moldeus P. The generation and subsequent fate of glutathionyl radicals in biological systems. J. Biol. Chem. 1985;260:15028–15032. doi: 10.1016/S0021-9258(18)95697-8. [DOI] [PubMed] [Google Scholar]
- 192.Day B.J. Catalase and glutathione peroxidase mimics. Biochem. Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Amir Aslani B., Ghobadi S. Studies on oxidants and antioxidants with a brief glance at their relevance to the immune system. Life Sci. 2016;146:163–173. doi: 10.1016/j.lfs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 194.Comhair S.A., Erzurum S.C. Antioxidant responses to oxidant-mediated lung diseases. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;283:L246–L255. doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- 195.Bast A., Haenen G.R., Doelman C.J. Oxidants and antioxidants: State of the art. Am. J. Med. 1991;91:2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 196.Aggarwal S., Dimitropoulou C., Lu Q., Black S.M., Sharma S. Glutathione supplementation attenuates lipopolysaccharide-induced mitochondrial dysfunction and apoptosis in a mouse model of acute lung injury. Front. Physiol. 2012;3:161. doi: 10.3389/fphys.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kim K.S., Suh G.J., Kwon W.Y., Kwak Y.H., Lee K., Lee H.J., Jeong K.Y., Lee M.W. Antioxidant effects of selenium on lung injury in paraquat intoxicated rats. Clin. Toxicol. 2012;50:749–753. doi: 10.3109/15563650.2012.708418. [DOI] [PubMed] [Google Scholar]
- 198.Petronilho F., Florentino D., Silvestre F., Danielski L.G., Nascimento D.Z., Vieira A., Kanis L.A., Fortunato J.J., Badawy M., Barichello T., et al. Ebselen Attenuates Lung Injury in Experimental Model of Carrageenan-Induced Pleurisy in Rats. Inflammation. 2015;38:1394–1400. doi: 10.1007/s10753-015-0113-5. [DOI] [PubMed] [Google Scholar]
- 199.Britt R.D., Jr., Velten M., Locy M.L., Rogers L.K., Tipple T.E. The thioredoxin reductase-1 inhibitor aurothioglucose attenuates lung injury and improves survival in a murine model of acute respiratory distress syndrome. Antioxid. Redox Signal. 2014;20:2681–2691. doi: 10.1089/ars.2013.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Moutet M., d’Alessio P., Malette P., Devaux V., Chaudiere J. Glutathione peroxidase mimics prevent TNFalpha- and neutrophil-induced endothelial alterations. Free Radic. Biol. Med. 1998;25:270–281. doi: 10.1016/S0891-5849(98)00038-0. [DOI] [PubMed] [Google Scholar]
- 201.Muzaffar S., Shukla N., Angelini G.D., Jeremy J.Y. Acute hypoxia simultaneously induces the expression of gp91phox and endothelial nitric oxide synthase in the porcine pulmonary artery. Thorax. 2005;60:305–313. doi: 10.1136/thx.2003.018796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Muzaffar S., Shukla N., Angelini G.D., Jeremy J.Y. Superoxide auto-augments superoxide formation and upregulates gp91(phox) expression in porcine pulmonary artery endothelial cells: Inhibition by iloprost. Eur. J. Pharmacol. 2006;538:108–114. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 203.Cui Y., Wang Y., Li G., Ma W., Zhou X.S., Wang J., Liu B. The Nox1/Nox4 inhibitor attenuates acute lung injury induced by ischemia-reperfusion in mice. PLoS ONE. 2018;13:e0209444. doi: 10.1371/journal.pone.0209444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Park W.H. Tempol differently affects cellular redox changes and antioxidant enzymes in various lung-related cells. Sci. Rep. 2021;11:14869. doi: 10.1038/s41598-021-94340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang H., Zeng J., Li J., Gong H., Chen M., Li Q., Liu S., Luo S., Dong H., Xu Y., et al. Sivelestat sodium attenuates acute lung injury by inhibiting JNK/NF-kappaB and activating Nrf2/HO-1 signaling pathways. Biomol. Biomed. 2023;23:457–470. doi: 10.17305/bb.2022.8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tenorio M., Graciliano N.G., Moura F.A., Oliveira A.C.M., Goulart M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants. 2021;10:967. doi: 10.3390/antiox10060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 208.Samuni Y., Goldstein S., Dean O.M., Berk M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 2013;1830:4117–4129. doi: 10.1016/j.bbagen.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 209.Tse H.N., Raiteri L., Wong K.Y., Yee K.S., Ng L.Y., Wai K.Y., Loo C.K., Chan M.H. High-dose N-acetylcysteine in stable COPD: The 1-year, double-blind, randomized, placebo-controlled HIACE study. Chest. 2013;144:106–118. doi: 10.1378/chest.12-2357. [DOI] [PubMed] [Google Scholar]
- 210.Leff J.A., Wilke C.P., Hybertson B.M., Shanley P.F., Beehler C.J., Repine J.E. Postinsult treatment with N-acetyl-L-cysteine decreases IL-1-induced neutrophil influx and lung leak in rats. Am. J. Physiol. 1993;265:L501–L506. doi: 10.1152/ajplung.1993.265.5.L501. [DOI] [PubMed] [Google Scholar]
- 211.Kolomaznik M., Mikolka P., Hanusrichterova J., Kosutova P., Matasova K., Jr., Mokra D., Calkovska A. N-Acetylcysteine in Mechanically Ventilated Rats with Lipopolysaccharide-Induced Acute Respiratory Distress Syndrome: The Effect of Intravenous Dose on Oxidative Damage and Inflammation. Biomedicines. 2021;9:1885. doi: 10.3390/biomedicines9121885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ortolani O., Conti A., De Gaudio A.R., Masoni M., Novelli G. Protective effects of N-acetylcysteine and rutin on the lipid peroxidation of the lung epithelium during the adult respiratory distress syndrome. Shock. 2000;13:14–18. doi: 10.1097/00024382-200013010-00003. [DOI] [PubMed] [Google Scholar]
- 213.Erol N., Saglam L., Saglam Y.S., Erol H.S., Altun S., Aktas M.S., Halici M.B. The Protection Potential of Antioxidant Vitamins Against Acute Respiratory Distress Syndrome: A Rat Trial. Inflammation. 2019;42:1585–1594. doi: 10.1007/s10753-019-01020-2. [DOI] [PubMed] [Google Scholar]
- 214.Chen X., Touyz R.M., Park J.B., Schiffrin E.L. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–611. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 215.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta. 2012;1826:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Arrigoni O., De Tullio M.C. Ascorbic acid: Much more than just an antioxidant. Biochim. Biophys. Acta. 2002;1569:1–9. doi: 10.1016/S0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 217.Fisher B.J., Seropian I.M., Kraskauskas D., Thakkar J.N., Voelkel N.F., Fowler A.A., 3rd, Natarajan R. Ascorbic acid attenuates lipopolysaccharide-induced acute lung injury. Crit. Care Med. 2011;39:1454–1460. doi: 10.1097/CCM.0b013e3182120cb8. [DOI] [PubMed] [Google Scholar]
- 218.Fisher B.J., Kraskauskas D., Martin E.J., Farkas D., Wegelin J.A., Brophy D., Ward K.R., Voelkel N.F., Fowler A.A., 3rd, Natarajan R. Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;303:L20–L32. doi: 10.1152/ajplung.00300.2011. [DOI] [PubMed] [Google Scholar]
- 219.Kearns S.R., Kelly C.J., Barry M., Abdih H., Condron C., Leahy A., Bouchier-Hayes D. Vitamin C reduces ischaemia-reperfusion-induced acute lung injury. Eur. J. Vasc. Endovasc. Surg. 1999;17:533–536. doi: 10.1053/ejvs.1999.0833. [DOI] [PubMed] [Google Scholar]
- 220.Patel V., Dial K., Wu J., Gauthier A.G., Wu W., Lin M., Espey M.G., Thomas D.D., Ashby C.R., Jr., Mantell L.L. Dietary Antioxidants Significantly Attenuate Hyperoxia-Induced Acute Inflammatory Lung Injury by Enhancing Macrophage Function via Reducing the Accumulation of Airway HMGB1. Int. J. Mol. Sci. 2020;21:977. doi: 10.3390/ijms21030977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Azzi A. Tocopherols, tocotrienols and tocomonoenols: Many similar molecules but only one vitamin E. Redox Biol. 2019;26:101259. doi: 10.1016/j.redox.2019.101259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ham A.J., Liebler D.C. Vitamin E oxidation in rat liver mitochondria. Biochemistry. 1995;34:5754–5761. doi: 10.1021/bi00017a007. [DOI] [PubMed] [Google Scholar]
- 223.Chan A.C. Partners in defense, vitamin E and vitamin C. Can. J. Physiol. Pharmacol. 1993;71:725–731. doi: 10.1139/y93-109. [DOI] [PubMed] [Google Scholar]
- 224.Burbank A.J., Duran C.G., Pan Y., Burns P., Jones S., Jiang Q., Yang C., Jenkins S., Wells H., Alexis N., et al. Gamma tocopherol-enriched supplement reduces sputum eosinophilia and endotoxin-induced sputum neutrophilia in volunteers with asthma. J. Allergy Clin. Immunol. 2018;141:1231–1238.e1. doi: 10.1016/j.jaci.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wagner J.G., Birmingham N.P., Jackson-Humbles D., Jiang Q., Harkema J.R., Peden D.B. Supplementation with gamma-tocopherol attenuates endotoxin-induced airway neutrophil and mucous cell responses in rats. Free Radic. Biol. Med. 2014;68:101–109. doi: 10.1016/j.freeradbiomed.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rocksen D., Ekstrand-Hammarstrom B., Johansson L., Bucht A. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am. J. Respir. Cell Mol. Biol. 2003;28:199–207. doi: 10.1165/rcmb.4899. [DOI] [PubMed] [Google Scholar]
- 227.Morita N., Shimoda K., Traber M.G., Westphal M., Enkhbaatar P., Murakami K., Leonard S.W., Traber L.D., Traber D.L. Vitamin E attenuates acute lung injury in sheep with burn and smoke inhalation injury. Redox Rep. 2006;11:61–70. doi: 10.1179/135100006X101020. [DOI] [PubMed] [Google Scholar]
- 228.Packer L., Witt E.H., Tritschler H.J. alpha-Lipoic acid as a biological antioxidant. Free Radic. Biol. Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-R. [DOI] [PubMed] [Google Scholar]
- 229.Gunther S., Storm J., Muller S. Plasmodium falciparum: Organelle-specific acquisition of lipoic acid. Int. J. Biochem. Cell Biol. 2009;41:748–752. doi: 10.1016/j.biocel.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 230.Zhang W.J., Wei H., Hagen T., Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc. Natl. Acad. Sci. USA. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bulmus F.G., Gursu M.F., Muz M.H., Yaman I., Bulmus O., Sakin F. Protective effects of alpha-lipoic Acid on oleic Acid-induced acute lung injury in rats. Balk. Med. J. 2013;30:309–314. doi: 10.5152/balkanmedj.2013.8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shoji Y., Takeuchi H., Fukuda K., Fukunaga K., Nakamura R., Takahashi T., Wada N., Kawakubo H., Miyasho T., Hiratsuka T., et al. The alpha-lipoic acid derivative DHLHZn: A new therapeutic agent for acute lung injury in vivo. Inflamm. Res. 2017;66:803–811. doi: 10.1007/s00011-017-1059-x. [DOI] [PubMed] [Google Scholar]
- 233.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: An overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Xiao J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017;57:1874–1905. doi: 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- 235.Kuo M.Y., Liao M.F., Chen F.L., Li Y.C., Yang M.L., Lin R.H., Kuan Y.H. Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFkappaB pathways in mice with endotoxin-induced acute lung injury. Food Chem. Toxicol. 2011;49:2660–2666. doi: 10.1016/j.fct.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 236.Hou Y., Li J., Ding Y., Cui Y., Nie H. Luteolin attenuates lipopolysaccharide-induced acute lung injury/acute respiratory distress syndrome by activating alveolar epithelial sodium channels via cGMP/PI3K pathway. J. Ethnopharmacol. 2022;282:114654. doi: 10.1016/j.jep.2021.114654. [DOI] [PubMed] [Google Scholar]
- 237.Takashima K., Matsushima M., Hashimoto K., Nose H., Sato M., Hashimoto N., Hasegawa Y., Kawabe T. Protective effects of intratracheally administered quercetin on lipopolysaccharide-induced acute lung injury. Respir. Res. 2014;15:150. doi: 10.1186/s12931-014-0150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Wang L., Chen J., Wang B., Wu D., Li H., Lu H., Wu H., Chai Y. Protective effect of quercetin on lipopolysaccharide-induced acute lung injury in mice by inhibiting inflammatory cell influx. Exp. Biol. Med. 2014;239:1653–1662. doi: 10.1177/1535370214537743. [DOI] [PubMed] [Google Scholar]
- 239.Shen H., Wu N., Liu Z., Zhao H., Zhao M. Epigallocatechin-3-gallate alleviates paraquat-induced acute lung injury and inhibits upregulation of toll-like receptors. Life Sci. 2017;170:25–32. doi: 10.1016/j.lfs.2016.11.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.