Abstract
Metabolic-associated liver disease (MAFLD) affects up to 70% of overweight and more than 90% of morbidly obese people, and its pathogenesis is rather complex and multifactorial. The criteria for MAFLD include the presence of hepatic steatosis in addition to one of the following three criteria: overweight or obesity, presence of type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation. If the specific criteria are present, the diagnosis of MAFLD can be made regardless of alcohol consumption and previous liver disease. The pathophysiological mechanisms of MAFLD, including inflammation, lipotoxicity, mitochondrial disfunction, and oxidative stress, as well as the impact of intestinal gut microbiota, are constantly being elucidated. Treatment strategies that are continually emerging are based on different key points in MAFLD pathogenesis. Yet, the ideal therapeutic option has still not been found and future research is of great importance, as MAFLD represents a multisystemic disease with numerous complications.
Keywords: metabolic-associated fatty liver disease, inflammation, gut microbiota, treatment strategies
1. Introduction
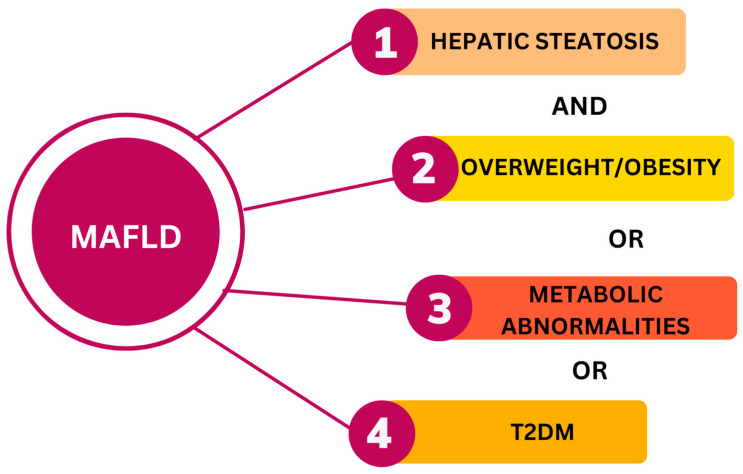
Metabolic-associated fatty liver disease (MAFLD) is the new term related to nonalcoholic fatty liver disease (NAFLD). MAFLD was suggested by experts of The International Consensus Panel of MAFLD in 2020. MAFLD emphasizes the importance of metabolic factors in the development and progression of liver disease. The criteria for MAFLD include the presence of hepatic steatosis in addition to one of the following three criteria: overweight or obesity, presence of type 2 diabetes mellitus (T2DM), or evidence of metabolic dysregulation (Figure 1). Since the association of metabolic syndrome and fatty liver is common, it has been suggested that a new term, MAFLD, is more appropriate. If the specific criteria are present, the diagnosis of MAFLD can be made regardless of alcohol consumption and previous liver disease [1,2].
Figure 1.
Diagnostic criteria for MAFLD. T2DM—type 2 diabetes mellitus. The figure was created in the Canva program (https://www.canva.com/).
Hepatic steatosis can be detected using imaging techniques: ultrasound, transient elastography (FibroScan), CT or MR scan, different scores and biomarkers (FIB-4, FLI), and liver biopsy. Overweight/obesity is defined as BMI ≥ 25 kg/m2 in Caucasians or BMI ≥ 23 kg/m2 in Asians. Metabolic dysfunction implies the presence of at least two metabolic risk abnormalities:
Waist circumference ≥ 102/88 cm in Caucasian men and women (or ≥90/80 cm in Asian men and women).
Blood pressure ≥ 130/85 mmHg or specific drug treatment.
Plasma triglycerides (TG) ≥ 150 mg/dL (≥1.70 mmol/L) or specific drug treatment.
Plasma HDL-cholesterol < 40 mg/dL (<1.0 mmol/L) for men and <50 mg/dL (<1.3 mmol/L) for women or specific drug treatment.
Prediabetes (i.e., fasting glucose levels 100 to 125 mg/dL (5.6 to 6.9 mmol/L), 2 h post-load glucose levels 140 to 199 mg/dL (7.8 to 11.0 mmol), or HbA1c 5.7% to 6.4% (39 to 47 mmol/mol)).
Homeostasis model assessment of insulin resistance (HOMA-IR) score ≥ 2.5.
Plasma high-sensitivity C-reactive protein level > 2 mg/L [1].
This spectrum of diseases affects up to 70% of overweight and more than 90% of morbidly obese people [3].
2. Pathophysiological Mechanisms of MAFLD
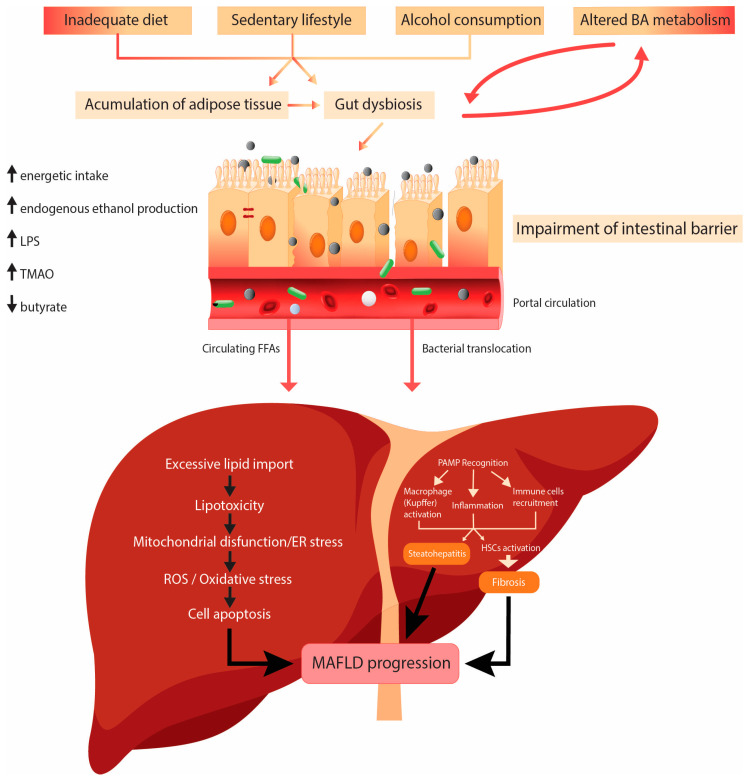
The pathogenesis of MAFLD is rather complex and multifactorial (Figure 2).
Figure 2.
Pathophysiological mechanisms of MAFLD. Abbreviations: LPS—lipopolysaccharide; TMAO—trimethylamine–N-oxide; PAMP—pathogen-associated molecular pattern; ER—endoplasmic reticulum; ROS—reactive oxygen species; HSCs—hepatic stellate cells. The figure was created in Adobe Illustrator.
The genetic variations in Patatin-like Phospholipase Domain-containing 3 (PNPLA3), Transmembrane 6 Superfamily Member 2 (TM6SF2), and Membrane Bound O-acyltransferase Domain-containing 7 (MBOAT7) play the principal roles in the development and progression of MAFLD. The PNPLA3 gene is associated with increased hepatic lipid levels and hepatic inflammation, hepatic fibrogenesis, and elevated risk of development of HCC [4]. TM6SF2 is associated with hepatic steatosis and progression of liver disease, because it increases the liver TG content and decreases VLDL secretion [5]. Variants of the MBOAT7 gene are related to alcoholic cirrhosis, but also are associated with the development and severity of MAFLD via remodeling of phospholipids [6].
Environmental factors like high-caloric diet and decreased physical activity play a role in the pathogenesis of MAFLD.
High-fat diet (HFD) means high consumption of fat, typically known as the Western diet. High fat intake plays a significant role in the pathogenesis of MAFLD, and it leads to excessive accumulation of fat in the liver in patients with a genetic predisposition. HFD is very often associated with insulin resistance, lipid disorders, and metabolic and cardiovascular diseases, components of metabolic syndrome. The hypothesis that most likely explains the theory of progression MAFLD to nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma is the double-hit hypothesis. The primary disorder contributing to steatosis is IR. IR starts hepatic de novo lipogenesis (DNL) and impaired fatty acid (FA) transport. The second hit implies mitochondrial dysfunction, endoplasmic reticulum stress, inflammation, and many other disturbances [7].
The fatty acids (FAs) in the liver originate from dietary fat, adipose tissue lipolysis, or de novo lipogenesis (DNL) from carbohydrates or other dietary fat precursors. Inside the liver, FAs are esterified into TG and assembled into very-low-density lipoprotein (VLDL) to be secreted in the circulation, oxidized in the mitochondria (β-oxidation), or stored in lipid droplets (LDs). LDs undergo lipid hydrolysis in fasting conditions to provide FAs for β-oxidation. With chronic nutrient overload and IR in MAFLD, the input of FAs to the liver exceeds their disposal via VLDL secretion or β-oxidation [8]. In patients with MAFLD, about 15% of liver FAs originate from the diet, 59% are derived from circulation, and 26% from DNL [9]. IR increases DNL in the liver [10,11]. Two main transcriptional factors are involved in DNL: sterol regulatory element binding protein 1c (SREBP1c), regulated by insulin signaling, and carbohydrate response element binding protein (ChREBP), activated by glucose uptake [12]. The results of mice studies have shown the important role of the peroxisome proliferator-activated receptors (PPARγ) in hepatic lipogenesis [13].
Lipotoxicity is defined as accumulation or transient generation of toxic lipids in LDs (>5% of liver weight), which may result in hepatocyte injury or death. The toxic lipids are the ones that cause cellular injury and cell death in MAFLD pathogenesis. The saturated fatty acids (SFAs) (palmitate, ceramides, lysophosphatidylcholine, and free cholesterol) are directly cytotoxic and can induce progressive liver injury. The monounsaturated free FAs, such as oleate and palmitoleate, may protect from lipotoxicity [14]. The mechanisms of lipotoxicity involve several cellular processes in the liver, such as endoplasmic reticulum (ER) stress, mitochondrial dysfunction, oxidative stress and production of reactive oxygen species (ROS), and intestinal dysbiosis [15].
Mitochondria, ER, and peroxisomes produce ROS in normal cellular metabolism, but 90% of cellular ROS are produced in mitochondria. An HFD increases the amount of substrate available for oxidation and the production of free radicals and damages the antioxidant defense [16]. An imbalance between the production of ROS and the antioxidant activities leads to oxidative stress. Antioxidants with main function to eliminate free radicals involve nonenzymatic molecules such as ascorbic acid (vitamin C), α-tocopherol (vitamin E), glutathione (GSH), carotenoids, and flavonoids and the antioxidant enzymes, such as superoxide dismutase, glutathione peroxidase, and catalase. Excessive ROS production causes mitochondrial damage and activation of Kupffer cells, which then produce cytokines and cause inflammation. ROS activates hepatic stellate cells (HSCs) to produce extracellular matrix, leading to fibrosis [8,16,17].
Mitochondrial dysfunction is a consequence of enormous production of ROS, but in this vicious circle, mitochondrial dysfunction is also the cause of increased production of ROS. Uncontrolled production of ROS in the mitochondria damages mitochondrial components and leads to activation of the mitochondrial quality control (MQC) system. MQC is a specific mechanism responsible for maintaining mitochondrial integrity. This system includes biogenesis, fission, fusion, and mitophagy. Enormous production of ROS can damage structural parts of mitochondria, mitochondrial membrane, DNA, and can activate apoptotic processes, leading to mitochondrial autophagy, known as mitophagy [18,19,20].
A recent study in a “human-like” MAFLD rat model, which was induced by HFD-feeding for 14 weeks in association with thermoneutral housing, showed the effect of HFD on DNA damage and mitochondrial quality control mechanisms in an MAFLD. The results of this study showed that the mtDNA copy number and expression of markers involved in mitochondrial biogenesis were significantly reduced in the HFD rats group. Also, the protein levels of MFN2 (mitofusin 2), GTPase protein, which regulates the process of mitochondria, were significantly reduced in the liver of the HFD rats, but there were no significant changes in the other protein levels of fusion-optic atrophy 1 (OPA1), or in the protein levels of fission-dynamin-related protein 1 (DRP1). These results suggest a disorder of mitochondrial dynamic in MAFLD, probably more of fission. The analysis showed that, in HFD rats, the phosphorylation/activation of AMPK on Thr172 and of unc-51-like kinase 1 (ULK1) on Ser555 were both significantly increased. A significantly increased expression of AMBRA1, binding partners, and downstream effectors of ULK1 confirmed the activation of autophagy in the HFD group. A significant decrease in the expression of key proteins involved in the mitophagy flux in the liver of the HFD rats, PTEN-induced kinase 1(PINK1), the E3 ligase (PARKIN), and microtubule-associated protein 1A/1B-light chain 3B (LC3BII), indicated an impaired autophagic flux [21].
It has been known for a long time that oxidative stress can be assessed using serum marker serum, soluble NOX2-derived peptide, and urine marker, urinary 8-iso-PGF2α. The results of the studies showed that urinary 8-iso-PGF2α and serum-soluble NOX2-derived peptide are increased in patients with nonalcoholic fatty liver. Their level is independent of obesity, diabetes, and metabolic syndrome. Levels of urinary 8-iso-PGF2α and serum-soluble NOX2-derived peptide increase with the severity of liver steatosis at ultrasound. The mentioned study indicated that the markers of oxidative stress can also be used in assessing the severity of liver steatosis [22].
Since ER is involved in both protein and lipid homeostasis, proteotoxic and lipotoxic stress are closely related, and are likely bidirectional. Disequilibrium in the lipid bilayer induces lipotoxic ER stress, while the accumulation of unfolded or misfolded proteins in the ER lumen triggers proteotoxic ER stress. The unfolded protein sensors can be directly activated by toxic lipids. The unfolded protein response (UPR) is activated via the luminal domains of three principal transmembrane sensors: inositol-requiring enzyme (IRE)-1α, protein kinase RNA-like ER kinase (PERK), and activating transcription factor (ATF)-6α. ER is involved in protein folding and trafficking and lipid biosynthesis and trafficking. That is the reason these two types of stress are interconnected. When homeostasis is disturbed and equilibrium cannot be established, ER stress-induced apoptosis occurs [23,24].
3. Gut Microbiota—In Sickness and Health
The intestinal microbiota represent a community of more than 200 prevalent bacteria, fungi, and viruses that inhabit the human gastrointestinal tract [25]. During the last decade, constantly emerging research efforts have enlightened us with knowledge of these gut inhabitants, bringing out a different perspective regarding human health. At first, the information of gut microbiota was gathered from the difficult processes of isolating and culturing different species, while later, along with the development of modern methods such as sequence analysis and shotgun metagenomics, the term microbiome emerged, referring to the collective genomes of all microorganisms inhabiting an environment. With these new methods, almost 2000 new bacterial species have been discovered, as well as their authentic functional capacity [26]. This has led to better comprehension of the gut microbiome, yet it remains not fully determined and understood. The human genome consists of about 23,000 genes, whereas the microbiome encodes over three million genes, providing unique metabolic setting and impacting host metabolism in numerous ways [27]. Therefore, the interplay between host and gut microbiota has an important role in pathophysiological mechanisms regarding development of various diseases, such as inflammatory bowel disease (IBD), T2DM, cardiovascular disease (CVD), colorectal cancer (CRC), and MAFLD, which is discussed in this review [28,29]. The diversification of the gut microbiome starts at birth and composition modifications depend on a variety of genetic, nutritional, and environmental factors. Dietary intake is considered as the main culprit for disturbance of the microbiota composition, as well as the bile acid (BA) composition, the alteration of intestinal barrier homeostasis, and activation of inflammatory pathways [30]. Numerous taxonomic studies have tried to characterize the distinctive microbiota signatures correlating with different diseases, including MAFLD [31]. This field of research set microbiota as a potentially important noninvasive biomarker of MAFLD, or main variable for MAFLD prevention or treatment through modulation of microbiota composition. It has been shown that some species are associated with MAFLD, like Proteobacteria, Enterobacteria, Escherichia, or Bacteroides, being of higher abundance in patients with steatohepatitis as compared to the matched healthy controls [32]. Also, a lower abundance of Bacteroidetes, and in particular, the genera Prevotella, has been found in the MAFLD group. Some research demonstrated that the Firmicutes/Bacteroidetes ratio is disturbed in obese patients, as well as in those with MAFLD [33]. Across the MAFLD spectrum from simple steatosis to steatohepatitis and fibrosis, some other distinctive microbial signatures have been established by metagenomic sequencing, for example, an increased abundance of Escherichia coli and Bacteriodes vulgatus in advanced fibrosis, or an independent association of the genera Ruminococcus with this stage of liver disease [31,34]. Although extensive research has been conducted regarding this issue so far, the results are rather heterogenic and sometimes conflicting, this being probably due to the underlying complex interactions among the different elements and the effect of probable confounders.
In addition to the previously corroborated pathophysiological mechanisms, there is no talk of MAFLD without mentioning the term gut–liver axis, the bidirectional communication between the intestines and the liver. Being more than mere interorgan connection, the gut–liver axis also includes the complex interplay between the immune system and the gut microbiota [35]. There are several entities stretching along the gut–liver axis, with gut microbiota as the main intersection in this interorgan communication. The role of microbiota in MAFLD development and evolution can be substantiated by several mechanisms, which are discussed in the following section.
Intact intestinal barrier plays a crucial role in the preservation of normal environment of the intestinal tract. It represents the first line of defense from unwanted passage of toxins, microorganisms, or their metabolites from gut lumen. Protection strategies include tight junctions (TJs) between adjacent enterocytes, immunoglobulins (sIgA), mucins, antimicrobial lectins, and gut microbiota, being the first soldier in line within organism defense [36]. If any of these components is compromised, the impairment of the intestinal barrier will occur, leading to increased intestinal permeability—the leaky gut syndrome. In addition to being the mere change in composition of commensal bacteria, gut dysbiosis promotes increased intestinal permeability also through changes in expression and distribution of TJs, and a decrease in the secretion of antimicrobial peptides [37,38]. Also, it has been found that gut bacteria can cause alterations of the mucus layer and in that way contribute to the increased intestinal permeability, like Akkermansia muciniphila, for example. This Gram-negative bacteria has already been linked to obesity, IR, and MAFLD, and recent studies explored its promising therapeutic potential, as it is shown that oral supplementation with it ameliorates MAFLD and its features via the gut–liver axis [39,40]. In addition to leaky gut, the alteration of vascular barrier has also been explored. These two features, along with the small intestine bacterial overgrowth (SIBO), enable translocation of bacteria or bacterial products from intestinal lumen to portal circulation, representing a sort of warranty of the ensuing step in liver damage—the induction of hepatic inflammatory response to microbial antigens [40,41].
The immune system and its components are the backbone of the gut–liver axis. The gut-associated lymphoid tissue (GALT) consists of intraepithelial lymphocytes, Peyer’s patches, the effector cells in the lamina propria, mesenteric lymph nodes, and isolated lymphoid follicles. There are also immune cells within the liver and the adipose tissue included in the functioning of the gut–liver axis; hence, all these elements normally maintain a balance between tolerance and response to bacterial antigens [42]. In MAFLD, distinct proinflammatory pathways are triggered. In the microbiota-induced inflammation it all starts with bacterial translocation, when pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs), which are small pathogen components or products, such as lipopolysaccharide (LPS), the major outer membrane part of the Gram-negative bacteria. This LPS endotoxemia and consequent recognition by Toll-like receptors (TLRs) 4 activate an immunologic cascade which is considered to be the pivotal step in the development of inflammation, which distinguishes steatosis from steatohepatitis [43,44]. Numerous studies have shown elevated serum levels of LPS in patients with steatohepatitis, compared to the ones with simple steatosis or healthy subjects, thus confirming the role of gut dysbiosis in metabolic-associated steatohepatitis (MASH) development [44,45]. Also, recent studies confirmed the important role of LPS-TLR4 signaling in MAFLD pathogenesis, where the treatment with sevelamer, a BA sequestrant, was conducted. Sevelamer was binding to LPS in the intestinal lumen, promoting its fecal excretion, leading to restoration of the TJs and reduction of portal LPS levels, thus suppressing the hepatic TLR4 signaling pathway. In addition, this treatment improved the diversity of gut microbiota by decreasing the abundance of MAFLD-promoting species [46].
Though LPS-mediated inflammation via TLR4 may be the primary driver of the immune response in MAFLD, it is not the only one. The inflammation cascade can also be triggered through TLR9, TLR5, or TLR2, whose ligands are methylated DNA, flagellins, and peptidoglycan from Gram-positive bacteria (in the order given) [47]. When stimulated, TLR4 generates intracellular signaling pathways, specifically NF-κB and MAPK, which further induce the synthesis of proinflammatory cytokines like TNF [48]. Kupffer cells are the main culprit for liver injury as they have the largest number of expressed TLR4, along with the ability to produce ROS and contribute to oxidative stress. In addition to induction of oxidative stress and inflammation, fibrogenesis is also induced in this scenario, by triggered HSCs [49].
The intestinal microbiota has a significant influence on host metabolic state, not only by facilitating harvest of nutrients from food, but also by its capacity to produce certain metabolites, thus being the possible key regulator of the host metabolic profile. These functional interactions are primarily defined through metabolism of polysaccharides, i.e., microbial fermentation of dietary indigestible fibers that produce short chain fatty acids (SCFAs)—acetate, propionate, and butyrate [50]. Emerging evidence strongly suggests that SCFAs not only have a say in MAFLD pathogenesis via hepatic glucose and lipid homeostasis but are speculated to play a key role in the gut–brain axis [51,52]. The positive effects of a high-fiber diet are already very well known. Studies have shown that butyrate represents the most potent anti-inflammatory mediator, which can also promote tight junction function and intestinal integrity. SCFAs accomplish their functions by binding to the specific G-protein-coupled receptors (GPRs) or through the activation of PPARs [53,54].
Along with playing a key role in SCFAs metabolism, it has been demonstrated that gut microbiota are strongly connected to the metabolism of BA, because of their capacity to process deconjugated BAs into secondary ones and, in that way, promote their fecal excretion [55,56]. Alterations of the BA metabolism lead to the suppressed activity of BA receptors FXR and TGR5, which further leads to decreased energy outflow and increased lipogenesis, intensified BA synthesis, and increased macrophage activity [54,57].
Except for meddling in BA metabolism, it has been known that intestinal bacteria are involved in choline metabolism. Choline, derived from diet, plays an important role in liver and brain functioning. It is transformed into lecithin which helps excretion of VLDL from liver, thus preventing hepatic lipid accumulation [58]. Gut bacteria produce enzymes that convert choline into toxic molecules, like trimethylamine (TMA), which then travel through portal circulation to the liver where they are transformed into TMA-N-oxide (TMAO). TMAO can also be derived from dietary L-carnitine. This toxic compound has been linked not only to hepatic damage but also to cardiometabolic complications and mental disorders [59,60,61,62]. Regarding liver diseases, research has shown that intestinal dysbiosis can promote steatosis and chronic inflammatory status, as seen in MASH, by reducing choline levels and increasing levels of TMAO [63]. Also, it has been demonstrated that some ethanol-producing microbial strains might be involved in the pathogenesis of MAFLD, like high-alcohol-producing Klebsiella pneumoniae [64]. Both nonalcoholic and alcoholic liver disease show increased levels of ethanol and its metabolites, acetaldehyde and acetate, which have been linked to hepatic injury by causing mitochondrial dysfunction and inflammation [65].
4. The Impact of Intestinal Microbiota on Cardiometabolic Function
Now, it is well known that MAFLD should be referred to as a multisystemic disease, as it has been linked to CVD, chronic kidney disease, and some brain disorders [66]. With MAFLD and CVD being highly prevalent, and both being associated with metabolic alterations, they are often found in coexistence. The answer to this occurrence may lie in the mutual pathophysiological pathways comprising oxidative stress, persistent low-grade inflammation, and IR [67]. Along with that, the answer is maybe hiding in the disturbed intestinal microbiota and its capacity to interfere with host metabolism, or its production of toxic metabolites [68]. Several meta-analyses and cohort studies showed that patients with MAFLD are at higher risk of major adverse cardiovascular events (MACEs), independently of the extent of coronary disease or other CV risk factors. The previously mentioned gut-microbiota-derived TMAO has been established as a risk factor of CVD, as its high levels are closely related to the occurrence of atherosclerosis, heart failure, hypertension, and other CVDs [69,70]. The mechanisms of action in the pathogenesis of atherosclerotic disease and other CV entities include accumulation of cholesterol, endothelial dysfunction, and activation of proinflammatory and prothrombotic pathways [71,72].
With regard to MAFLD undoubtedly being connected to CVD, it is with great certainty that we can now establish causal connection between these two clinical entities, with intestinal microbiota being the possible underlying common denominator. It is of crucial importance that patients with MAFLD undergo cardiovascular assessment. As numerous efforts are still being made in order to elucidate more precisely this cobweb of correlations, new promising therapeutic targets are emerging for CVD along with MAFLD.
5. Treatment Options of NAFLD/MAFLD
In the last 15 years, numerous different molecules have been tested for the treatment of NAFLD/MAFLD. Since then, the goal of NASH/NAFLD therapy has been changed, and today includes three major pharmacological targets: the reduction of liver steatosis, the reduction of inflammation, and the regression of fibrosis [73]. The first approach to NASH treatment was dietary changes together with lifestyle changes, from a sedentary to a physically active lifestyle [74]. Nowadays, it is well known that NASH is a part of complex metabolic diseases with multiplex pathophysiology pathways including complicated genetic background together with different environmental factors [75,76]. On the other hand, a wide spectrum of disease progression, from mild inflammation in fatty liver to the end-stage liver disease with cirrhosis and its complications, contributes to the complexity of pharmacological interventions requiring specific interventions for different stages of disease [77]. For these reasons, the pharmacotherapy for this heterogeneous disease includes a variety of molecules with different pharmacological targets, from drugs targeting metabolic conditions that contribute to development and progression of NASH (antidiabetic and hypolipidemic agents) to drugs used to treat liver inflammation and liver fibrosis.
Thus far, more than 20 drugs have been tested for the treatment of NASH, while many molecules are currently in the development or in preclinical trials.
In this review, we discuss the molecules in the most advanced clinical development, including hypolipidemic and antidiabetic drugs, PPAR agonists, FXR agonists, FGF analogs, and thyroid hormone receptor (THR) agonists. Table 1 shows some of the results of different clinical trials that are in different phases, active or terminated, in the treatment of NASH/MASH (Table 1) [73,78].
Table 1.
Clinical trials in NASH/MASH.
| Molecule | Mechanism of Action | Primary Objective | Results |
|---|---|---|---|
| New antidiabetic agents | |||
| Semaglutide | Agonist GLP1 | NASH resolution without worsening of fibrosis | NASH resolution No fibrosis improvement |
| Liraglutide | Agonist GLP1 | Histological resolution of NASH | NASH resolution with worsening of fibrosis |
| Dapaglifozin | Inhibitor of SGLT2 | Improvement in scored liver | NA |
| PPAR modulators | |||
| Pioglitazone | PPARγ agonist | NASH resolution without worsening of fibrosis | NA |
| Pioglitazone, vitamin E |
PPARγ agonist | Improvement in scored liver histology | Vitamin E vs. placebo: improvement Pioglitazone vs. placebo: no improvement |
| Pemafibrate | PPARα agonist | Percentage change in liver fat content measured by MRI from baseline to week 24 | No differences in liver fat content |
| Saroglitazar (oral) | Dual PPARα and PPARγ agonist | Reduce ALT from baseline | Improvement of NASH by liver biopsy after 52 weeks |
| Elafibranor(oral) PPARα/γ/δ agonists |
Dual PPARα and PPARδ agonist | NASH resolution | No improvement in NASH |
| Lanifibranor | Pan-PPAR agonist | Histological resolution of NASH | Improvement of NASH and fibrosis by liver biopsy |
| FXF agonists | CCR2/CCR5 agonist | Improvement in liver fibrosis by ≥1 stage and no worsening of steatohepatitis on liver histology | Ended trial by interymanalisys and lack of efficacy |
| PXL065 | Inhibitor of mitochondrial pyruvate carrier and acyl-CoA synthetase 4 | Hepatic fat fraction measured by MRI | Improvement in liver fat content and improve in fibrosis stage |
| Aramchol | Partial inhibitor of hepatic stearoyl-CoA desaturase | Hepatic fat fraction measured by MRI | No changes in liver fat by MRS. Improvement in liver fibrosis by ≥ 1 stage and no worsening of NASH on liver histology |
| Selonsertib | Inhibitor of ASK1 | ≥1-stage improvement in fibrosis according to the NASH CRN classification without worsening of NASH at week 48 | Study failed |
As previously mentioned, NAFLD is associated with metabolic comorbidities including T2DM, hypertriglyceridemia, hypercholesterolemia, IR, hypertension, and obesity or overweight in some patients. Hypolipidemic and antidiabetic drugs are well-studied medical agents aiming to stimulate metabolic processes that cause NAFLD and to reduce intrahepatic fat accumulation. Treatment with statins and ezetimibe aims to reduce circulating and tissue-accumulated cholesterol and is recommended in NAFLD. These molecules may improve the lipid profile in NAFLD [79]. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an important player in cholesterol homeostasis and intracellular lipogenesis, with a possible impact of circulating PCSK9 in the early stages of NASH, but not in the late stage of liver disease. Up to now, data from clinical trials with PCSK9 inhibitors reassure the safety of these molecules. However, real lifelong term data are required [80,81].
The efficacy of several antidiabetic drugs has been studied in NAFLD. Pioglitazone showed histological improvement, while the effect on liver fibrosis was not significant in both study groups: patients with T2DM and patients with IR [82,83]. Sodium-glucose co-transporter 1/2 (SGLT1/2) inhibitors and GLP-1RAs (glucagon-like peptide 1 receptor agonists) are newer antidiabetic agents that reached late-stage clinical development in NASH. Dapagliflozin is an oral SGLT2 inhibitor which impedes glucose reabsorption in proximal tubule, with consecutive glucosuria and plasma glucose reduction. Dapagliflozin is most likely to act through the reduction of visceral fat and improvement in liver tests and metabolic variables in patients with T2DM and NASH [84]. Other SGLT1/2 inhibitors are in phase 2 studies. The ELIVATE study is assessing licogliflozin alone or in combination with tropifexor, an agonist of the BA receptor FXR that may improve fibrosis [85]. Another ongoing study is evaluating a synthetic FXR agonist MET409 alone or combined with empagliflozin in patients with NASH and T2DM [86]. Semaglutide (GLP-1RAs) mimics GLP-1, a gut hormone released in response to eating and that reacts to release insulin, and also interacts with the brain to reduce appetite. The FDA (US Food and Drug Administration) has approved semaglutide in 2021 for adults with obesity or overweight with at least one condition: hypertension, T2DM, or high cholesterol.
A phase 3 clinical study with GLP-1RA (ESSENCE) evaluates the potential resolution of NASH and improvement in fibrosis in noncirrhotic patients with NAFLD [87]. A phase 2 trial evaluated the effect of liraglutide (1.8 mg daily) in patients with NASH, while preliminary data confirmed histological resolution of NASH (39% with liraglutide vs. 9% in placebo group) [88]. Modified GLP1 and GIP peptides such as cotadutide, tirzepatide, and efinopegdutide are in early clinical trials and results will be published soon [89,90].
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors of the nuclear hormone receptor family comprising three subtypes: PPARα, PPARγ, and PPAR β/δ. PPARs are mainly located in the liver, brown adipose tissue, and macrophages. PPARs activate FA oxidation, lower synthesis of triglycerides, and increase insulin sensitivity; thus, PPAR agonists modulate key metabolic, inflammatory, and fibrogenic pathways in the pathogenesis of NAFLD [91]. PPAR agonists have been approved for patients with T2DM and NASH confirmed by liver biopsy [92]. However, there are still ongoing clinical trials to assess the efficacy of PPAR agonists in patients with NASH without T2DM. Lanifibranor, the only pan-PPAR agonist, has been assessed in noncirrhotic biopsy-confirmed high-active NASH patients in a phase 2 trial with promising findings that support further assessment of lanifibranor in phase 3 trials [93]. Pemafibrate is a novel selective PPARα modulator (SPPARMα) investigated in a phase 2 trial in high-risk NAFLD patients. Preliminary results show that pemafibrate may be a promising therapeutic agent for NAFLD and a promising candidate for combination therapy with agents that reduce liver fat content [94].
Eight years ago, the FLINT study demonstrated for the first time that obeticholic acid (OCA) can improve hepatic lipid and glucose metabolism and, through its anti-inflammatory and antifibrotic activity, improve liver histopathology and fibrosis in NASH. Furthermore, it was shown that OCA increases insulin sensitivity and improves glucose homeostasis in patients with NASH and T2DM. However, it was not clear whether these effects were drug (OCA)- or target (FXR)-specific [95]. In addition to BA synthesis, this receptor is involved in glucose and lipid metabolism, and in the regulation of inflammation. Up to now, many studies have demonstrated that FXR modulation may have various implications in the treatment of NASH. Emerging data have demonstrated that FXR agonists might be quite effective in reducing different sequelae associated with NASH and even different dysfunctional processes coexisting with liver cirrhosis and its complications. However, important manifestations of FXR agonists, both steroidal and nonsteroidal, are pruritus and there is worsening of the HDL-c/LDL-c ratio, since all investigated FXR agonists demonstrated these effects in different trials in humans [96]. Novel agents like MET642 and MET409 are structurally optimized synthetic nonbile acid FXR agonists with enhanced potency. Preliminary results have shown that these molecules produce less pruritus than OCA, but complete clinical results are expected in the future. Ciloflexor is a small-molecule nonsteroidal agonist of FXR that showed efficacy in improving liver steatosis and serum bile acids in patients with NASH [97]. The preliminary results of the phase 2 clinical study demonstrated synergistic activity of ciloflexor in combination with firsocostat (acetyl-CoA carboxylase inhibitor) and semaglutide, in decrease of liver steatosis and improvement of liver biochemistry [98].
It is well known that the thyroid hormones significantly influence energy metabolism, lipid utilization, and glucose homeostasis. Numerous trials have shown an inverse correlation between serum TH levels and NAFLD, given that patients with hypothyroidism have increased risk for NAFLD and vice versa [99]. Moreover, thyroid hormones (THs) stimulate FA β-oxidation and oxidative phosphorylation in the liver. Pharmacological control of these pathways would likely impact the treatment of metabolic syndrome and MAFLD. Because of that, experimental and clinical trials have provided evidence of potential utility of the activation of T3-dependent pathways in MAFLD, and even that some metabolites of thyroid hormones (THs) could also play a role in the treatment of metabolic syndrome. THs exert their physiological effects by binding to the thyroid hormone receptors (THRs) α and β. The β isoform is the major THR expressed in the liver, while the α isoform is specifically abundant in the heart. This observation has led to the development of several THRβ-agonists and organ-selective (liver-selective) thyromimetics in the last 25 years [100]. Recently, novel thyromimetics that target liver and/or main TH receptor (THR) isoform in the liver, THR-β, have been developed to treat NAFLD. These promising agents have fewer thyrotoxic effects, such as arrhythmias and osteoporosis, due to their selectivity for the THR-β [101]. Liver-directed THR-β agonists, VK2879 and MGL-3196, have been studied for their effects on liver steatosis, inflammation, and fibrosis in NAFLD. Preliminary assessment showed improved liver steatosis and liver fibrosis in patients with NAFLD in comparison to placebo [102]. In a phase 3 trial, the THR-β agonist resmetirom improved lipid metabolism and liver biochemistry and decreased liver steatosis in patients with NAFLD, while there were no major safety concerns. A phase 3 study that includes patients with NASH and fibrosis is still ongoing and results will be available soon [103].
Currently, many new molecules with antifibrotic and anti-inflammatory effects have been studied regarding NAFLD treatment. Many of them are in the early stage of clinical development, while agents that are already in phase 2 or phase 3 of clinical trials are cenicriviroc (CVC), a small molecule antagonist that blocks chemokine 2 and 5 receptors, both with important roles in liver inflammation and fibrosis, in combination with tropifexor [104]. Balapectin is a complex carbohydrate that targets galectin-3, which plays a role in inflammatory response and fibrosis. A phase 2b/3 trial is analyzing the effect of belapectin in patients with NAFLD in the stage of compensated cirrhosis and portal hypertension, compared to placebo [105].
Endocrine fibroblast growth factor (FGF) analogs have emerged as promising agents due to their ability to act in two directions: directly on the liver, and to improve metabolic processes in the whole organism to a healthier state. The subfamily of FGFs, consisting of FGF19 and FGF21, may decrease liver steatosis and hepatocyte injury and consecutively suppress inflammation and liver fibrosis. Available data from clinical and preclinical studies with FGF19 and FGF21 analogs in NASH and underlying metabolic diseases showed improvement in liver fibrosis, inflammation, and liver steatosis, and improvement in liver biochemistry. The safety profile has been favorable so far [106,107,108,109].
6. Conclusions
The pharmacological scenario of MAFLD/NAFLD therapy will certainly change in the next future. The results of the latest clinical trials show that the three major components of MAFLD—steatosis, inflammation, and fibrosis—can be pharmacologically targeted. Although therapies on trial have acceptable tolerance and a good safety profile, a drug with the potential to ameliorate all components of MAFLD is yet to be identified. Indeed, the results of single-drug trials, most of them preliminary, showed improvement of MAFLD components in only a proportion of patients, in general lower than 50%. Combination therapy looks like a promising strategy. The rationale behind the drug combination is, on one hand, to increase the efficacy of one single drug and, on the other hand, to reduce the side effects of one drug by allowing the use of a lower dose, or by controlling the side effects of the first drug. Therefore, the best treatment will probably be defined in the next few years, not only according to different disease stages but also aiming to tailor the treatment for each patient according to the presence of comorbidities.
Abbreviations
| Acronym | Full Terminology |
| AMBRA1 | Activating molecule in Beclin1-regulated autophagy |
| AMP | Adenosine monophosphate |
| AMPK | AMP-activated protein kinase |
| ATF | Activating transcription factor |
| BA | Bile acid |
| BMI | Body mass index |
| ChREBP | Carbohydrate response element binding protein |
| CRC | Colorectal cancer |
| CVC | Cenicriviroc |
| CVD | Cardiovascular disease |
| DNA | Deoxyribonucleic acid |
| DNL | De novo lipogenesis |
| DRP1 | Fission-dynamin-related protein 1 |
| ER | Endoplasmic reticulum |
| FA | Fatty acid |
| FDA | US Food and Drug Administration |
| FGF | Fibroblast growth factor |
| FXR | Farnesoid X receptor |
| GALT | Gut-associated lymphoid tissue |
| GLP | Glucagon-like peptide |
| GLP-1RAs | Glucagon-like peptide 1 receptor agonists |
| GPRs | G-protein-coupled receptors |
| GSH | Glutathione |
| GTP | Guanosine triphosphate |
| HCC | Hepatocellular carcinoma |
| HFD | High fat diet |
| HOMA-IR | Homeostasis model assessment of insulin resistance |
| HSCs | Hepatic stellate cells |
| IBD | Inflammatory bowel disease |
| Ig | Immunoglobulin |
| IR | Insulin resistance |
| IRE | Inositol-requiring enzyme |
| LC3BII | Microtubule-associated protein 1A/1B-light chain 3B |
| LDs | Lipid droplets |
| LPS | Lipopolysaccharide |
| MACE | Major adverse cardiovascular events |
| MAFLD | Metabolic-associated liver disease |
| MASH | Metabolic-associated steatohepatitis |
| MBOAT7 | Membrane Bound O-acyltransferase Domain-containing 7 |
| MFN2 | Mitofusin 2 |
| MQC | Mitochondrial quality control |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NAFLD | Nonalcoholic fatty liver disease |
| NASH | Nonalcoholic steatohepatitis |
| NOX | NADPH oxidase |
| OCA | Obeticholic acid |
| OPA1 | Fusion-optic atrophy 1 |
| PAMPs | Pathogen-associated molecular patterns |
| PARKIN | E3 ligase |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| PERK | Protein kinase RNA-like ER kinase |
| PG | Prostaglandin |
| PINK1 | PTEN-induced kinase 1 |
| PNPLA3 | Patatin-like Phospholipase Domain-containing 3 |
| PPAR | Peroxisome proliferator-activated receptors |
| PPRs | Pattern recognition receptors |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SCFAs | Short chain fatty acids |
| SFAs | Saturated fatty acids |
| SGLT1/2 | Sodium-glucose co-transporter 1/2 |
| SIBO | Small intestine bacterial overgrowth |
| SPPARMα | Selective PPARα modulator |
| SREBP1c | Sterol regulatory element binding protein 1c |
| T2DM | Type 2 diabetes mellitus |
| TG | Triglycerides |
| TH | Thyroid hormones |
| THR | Thyroid hormone receptor |
| THR | Thyroid hormone receptors |
| TJs | Tight junctions |
| TLRs | Toll-like receptors |
| TM6SF2 | Transmembrane 6 Superfamily Member 2 |
| TMA | Trimethylamine |
| TMAO | Trimethylamine—oxide |
| ULK1 | Unc-51-like kinase 1 |
| UPR | Unfolded protein response |
| VLDL | Very-low-density lipoprotein |
Author Contributions
Conceptualization, B.F. and D.M.; writing—original draft preparation, M.M.-H., S.L., S.K. (Suncica Kapor) and S.K. (Slobodan Kapor); writing—review and editing, D.P., A.S. and A.D. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Eslam M., Newsome P.N., Sarin S.K., Anstee Q.M., Targher G., Romero-Gomez M., Zelber-Sagi S., Wong V.W.-S., Dufour J.-F., Schattenberg J.M., et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M., Sanyal A.J., George J., International Consensus Panel MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology. 2020;158:1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Boerwinkle E., Cohen J.C., Hobbs H.H. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jonas W., Schürmann A. Genetic and epigenetic factors determining NAFLD risk. Mol. Metab. 2020;50:101111. doi: 10.1016/j.molmet.2020.101111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teo K., Abeysekera K.W.M., Adams L., Aigner E., Anstee Q.M., Banales J.M., Banerjee R., Basu P., Berg T., Bhatnagar P., et al. rs641738C>T near MBOAT7 is associated with liver fat, ALT and fibrosis in NAFLD: A meta-analysis. J. Hepatol. 2021;74:20–30. doi: 10.1016/j.jhep.2020.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian C.Y., Zhai Z.Z., Li Z.F., Wang L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020;330:109199. doi: 10.1016/j.cbi.2020.109199. [DOI] [PubMed] [Google Scholar]
- 8.Nassir F. NAFLD: Mechanisms, Treatments, and Biomarkers. Biomolecules. 2022;12:824. doi: 10.3390/biom12060824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith G.I., Shankaran M., Yoshino M., Schweitzer G.G., Chondronikola M., Beals J.W., Okunade A.L., Patterson B.W., Nyangau E., Field T., et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J. Clin. Investig. 2020;130:1453–1460. doi: 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace M., Metallo C.M. Tracing insights into de novo lipogenesis in liver and adipose tissues. Semin. Cell Dev. Biol. 2020;108:65–71. doi: 10.1016/j.semcdb.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Khan R.S., Bril F., Cusi K., Newsome P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70:711–724. doi: 10.1002/hep.30429. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Nakajima T., Gonzalez F.J., Tanaka N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020;21:2061. doi: 10.3390/ijms21062061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso G., Cassader M., Paschetta E., Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology. 2018;155:282–302. doi: 10.1053/j.gastro.2018.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Rada P., González-Rodríguez Á., García-Monzón C., Valverde Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020;11:802. doi: 10.1038/s41419-020-03003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Chen Z., Tian R., She Z., Cai J., Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020;152:116–141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 18.Ramanathan R., Ali A.H., Ibdah J.A. Mitochondrial Dysfunction Plays Central Role in Nonalcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022;23:7280. doi: 10.3390/ijms23137280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashrafi G., Schwarz T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasun P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020;1866:165838. doi: 10.1016/j.bbadis.2020.165838. [DOI] [PubMed] [Google Scholar]
- 21.Cioffi F., Giacco A., Petito G., de Matteis R., Senese R., Lombardi A., de Lange P., Moreno M., Goglia F., Lanni A., et al. Altered Mitochondrial Quality Control in Rats with Metabolic Dysfunction-Associated Fatty Liver Disease (MAFLD) Induced by High-Fat Feeding. Genes. 2022;13:315. doi: 10.3390/genes13020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Ben M., Polimeni L., Carnevale R., Bartimoccia S., Nocella C., Baratta F., Loffredo L., Pignatelli P., Violi F., Angelico F. NOX2-generated oxidative stress is associated with severity of ultrasound liver steatosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2014;14:81. doi: 10.1186/1471-230X-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma X., McKeen T., Zhang J., Ding W.X. Role and Mechanisms of Mitophagy in Liver Diseases. Cells. 2020;9:837. doi: 10.3390/cells9040837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song M.J., Malhi H. The unfolded protein response and hepatic lipid metabolism in non alcoholic fatty liver disease. Pharmacol. Ther. 2019;203:107401. doi: 10.1016/j.pharmthera.2019.107401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henkel A.S. Unfolded Protein Response Sensors in Hepatic Lipid Metabolism and Nonalcoholic Fatty Liver Disease. Semin. Liver Dis. 2018;38:320–332. doi: 10.1055/s-0038-1670677. [DOI] [PubMed] [Google Scholar]
- 26.Noureddin M., Sanyal A.J. Pathogenesis of NASH: The Impact of Multiple Pathways. Curr. Hepatol. Rep. 2018;17:350–360. doi: 10.1007/s11901-018-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bessone F., Razori M.V., Roma M.G. Molecular pathways of nonalcoholic fatty liver disease development and progression. Cell. Mol. Life Sci. 2019;76:99–128. doi: 10.1007/s00018-018-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hills R.D., Pontefract B.A., Mishcon H.R., Black C.A., Sutton S.C., Theberge C.R. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019;11:1613. doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida A., Mitchell A.L., Boland M., Forster S.C., Gloor G.B., Tarkowska A., Lawley T.D., Finn R.D. A new genomic blueprint of the human gut microbiota. Nature. 2019;568:499–504. doi: 10.1038/s41586-019-0965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valdes A.M., Walter J., Segal E., Spector T.D. Role of the gut microbiota in nutrition and health. BMJ. 2018;361:k2179. doi: 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Y., Pedersen O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021;19:55–71. doi: 10.1038/s41579-020-0433-9. [DOI] [PubMed] [Google Scholar]
- 32.Singh R.K., Chang H.W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H., et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albillos A., de Gottardi A., Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020;72:558–577. doi: 10.1016/j.jhep.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Aron-Wisnewsky J., Vigliotti C., Witjes J., Le P., Holleboom A.G., Verheij J., Nieuwdorp M., Clément K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020;17:279–297. doi: 10.1038/s41575-020-0269-9. [DOI] [PubMed] [Google Scholar]
- 35.Tripathi A., Debelius J., Brenner D.A., Karin M., Loomba R., Schnabl B., Knight R. The gut-liver axis and the intersection with the microbiome. Nat. Rev. Gastroenterol. Hepatol. 2018;15:397–411. doi: 10.1038/s41575-018-0011-z. Erratum in Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolodziejczyk A.A., Zheng D., Shibolet O., Elinav E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019;11:e9302. doi: 10.15252/emmm.201809302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Magne F., Gotteland M., Gauthier L., Zazueta A., Pesoa S., Navarrete P., Balamurugan R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients. 2020;12:1474. doi: 10.3390/nu12051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loomba R., Seguritan V., Li W., Long T., Klitgord N., Bhatt A., Dulai P.S., Caussy C., Bettencourt R., Highlander S.K., et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25:1054–1062. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Allam-Ndoul B., Castonguay-Paradis S., Veilleux A. Gut Microbiota and Intestinal Trans-Epithelial Permeability. Int. J. Mol. Sci. 2020;21:6402. doi: 10.3390/ijms21176402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chopyk D.M., Grakoui A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology. 2020;159:849–863. doi: 10.1053/j.gastro.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Depommier C., Everard A., Druart C., Plovier H., Van Hul M., Vieira-Silva S., Falony G., Raes J., Maiter D., Delzenne N.M., et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: A proof-of-concept exploratory study. Nat. Med. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao Y., Kuang Z., Li C., Guo S., Xu Y., Zhao D., Hu Y., Song B., Jiang Z., Ge Z., et al. Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. 2021;13:1–19. doi: 10.1080/19490976.2021.1927633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kazankov K., Jørgensen S.M.D., Thomsen K.L., Møller H.J., Vilstrup H., George J., Schuppan D., Grønbæk H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019;16:145–159. doi: 10.1038/s41575-018-0082-x. [DOI] [PubMed] [Google Scholar]
- 44.Zhao J., Zhang S., Liu Y., He X., Qu M., Xu G., Wang H., Huang M., Pan J., Liu Z., et al. Single-cell RNA sequencing reveals the heterogeneity of liver-resident immune cells in human. Cell Discov. 2020;6:22. doi: 10.1038/s41421-020-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nati M., Haddad D., Birkenfeld A.L., Koch C.A., Chavakis T., Chatzigeorgiou A. The role of immune cells in metabolism-related liver inflammation and development of non-alcoholic steatohepatitis (NASH) Rev. Endocr. Metab. Disord. 2016;17:29–39. doi: 10.1007/s11154-016-9339-2. [DOI] [PubMed] [Google Scholar]
- 46.Ji Y., Yin Y., Li Z., Zhang W. Gut Microbiota-Derived Components and Metabolites in the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2019;11:1712. doi: 10.3390/nu11081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hegazy M.A., Mogawer S.M., Alnaggar A.R.L.R., Ghoniem O.A., Abdel Samie R.M. Serum LPS and CD163 Biomarkers Confirming the Role of Gut Dysbiosis in Overweight Patients with NASH. Diabetes Metab. Syndr. Obes. 2020;13:3861–3872. doi: 10.2147/DMSO.S249949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuji Y., Kaji K., Kitade M., Kaya D., Kitagawa K., Ozutsumi T., Fujinaga Y., Takaya H., Kawaratani H., Namisaki T., et al. Bile Acid Sequestrant, Sevelamer Ameliorates Hepatic Fibrosis with Reduced Overload of Endogenous Lipopolysaccharide in Experimental Nonalcoholic Steatohepatitis. Microorganisms. 2020;8:925. doi: 10.3390/microorganisms8060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miura K., Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:7381–7391. doi: 10.3748/wjg.v20.i23.7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manne V., Handa P., Kowdley K.V. Pathophysiology of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis. Clin. Liver Dis. 2018;22:23–37. doi: 10.1016/j.cld.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Yang F., Li H., Li Y., Hao Y., Wang C., Jia P., Chen X., Ma S., Xiao Z. Crosstalk between hepatic stellate cells and surrounding cells in hepatic fibrosis. Int. Immunopharmacol. 2021;99:108051. doi: 10.1016/j.intimp.2021.108051. [DOI] [PubMed] [Google Scholar]
- 52.Koh A., De Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 53.Morrison D.J., Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016;7:189–200. doi: 10.1080/19490976.2015.1134082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalile B., Van Oudenhove L., Vervliet B., Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019;16:461–478. doi: 10.1038/s41575-019-0157-3. [DOI] [PubMed] [Google Scholar]
- 55.Chen J., Vitetta L. The Role of Butyrate in Attenuating Pathobiont-Induced Hyperinflammation. Immune Netw. 2020;20:e15. doi: 10.4110/in.2020.20.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen J., Vitetta L. Gut Microbiota Metabolites in NAFLD Pathogenesis and Therapeutic Implications. Int. J. Mol. Sci. 2020;21:5214. doi: 10.3390/ijms21155214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramírez-Pérez O., Cruz-Ramón V., Chinchilla-López P., Méndez-Sánchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017;16((Suppl. S1)):S15–S20. doi: 10.5604/01.3001.0010.5672. [DOI] [PubMed] [Google Scholar]
- 58.Schoeler M., Caesar R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019;20:461–472. doi: 10.1007/s11154-019-09512-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gottlieb A., Canbay A. Why Bile Acids Are So Important in Non-Alcoholic Fatty Liver Disease (NAFLD) Progression. Cells. 2019;8:1358. doi: 10.3390/cells8111358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan X., Liu Y., Long J., Chen S., Liao G., Wu S., Li C., Wang L., Ling W., Zhu H. Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2019;63:e1900257. doi: 10.1002/mnfr.201900257. [DOI] [PubMed] [Google Scholar]
- 61.Vogt N.M., Romano K.A., Darst B.F., Engelman C.D., Johnson S.C., Carlsson C.M., Asthana S., Blennow K., Zetterberg H., Bendlin B.B., et al. The gut microbiota-derived metabolite trimethylamine N-oxide is elevated in Alzheimer’s disease. Alzheimer’s Res Ther. 2018;10:124. doi: 10.1186/s13195-018-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trøseid M., Ueland T., Hov J.R., Svardal A., Gregersen I., Dahl C.P., Aakhus S., Gude E., Bjørndal B., Halvorsen B., et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015;277:717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 63.Zhen J., Zhou Z., He M., Han H.X., Lv E.H., Wen P.B., Liu X., Wang Y.-T., Cai X.-C., Tian J.-Q., et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front. Endocrinol. 2023;14:1085041. doi: 10.3389/fendo.2023.1085041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chidambaram S.B., Rathipriya A.G., Mahalakshmi A.M., Sharma S., Hediyal T.A., Ray B., Sunanda T., Rungratanawanich W., Kashyap R.S., Qoronfleh M.W., et al. The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells. 2022;11:1239. doi: 10.3390/cells11071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou D., Zhang J., Xiao C., Mo C., Ding B.S. Trimethylamine-N-oxide (TMAO) mediates the crosstalk between the gut microbiota and hepatic vascular niche to alleviate liver fibrosis in nonalcoholic steatohepatitis. Front. Immunol. 2022;13:964477. doi: 10.3389/fimmu.2022.964477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yuan J., Chen C., Cui J., Lu J., Yan C., Wei X., Zhao X., Li N., Li S., Xue G., et al. Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell Metab. 2019;30:675–688.e7. doi: 10.1016/j.cmet.2019.08.018. Erratum in Cell Metab. 2019, 30, 1172. [DOI] [PubMed] [Google Scholar]
- 67.Chen X., Zhang Z., Li H., Zhao J., Wei X., Lin W., Zhao X., Jiang A., Yuan J. Endogenous ethanol produced by intestinal bacteria induces mitochondrial dysfunction in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2020;35:2009–2019. doi: 10.1111/jgh.15027. [DOI] [PubMed] [Google Scholar]
- 68.Rosato V., Masarone M., Dallio M., Federico A., Aglitti A., Persico M. NAFLD and Extra-Hepatic Comorbidities: Current Evidence on a Multi-Organ Metabolic Syndrome. Int. J. Environ. Res. Public Health. 2019;16:3415. doi: 10.3390/ijerph16183415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witkowski M., Weeks T.L., Hazen S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Duttaroy A.K. Role of Gut Microbiota and Their Metabolites on Atherosclerosis, Hypertension and Human Blood Platelet Function: A Review. Nutrients. 2021;13:144. doi: 10.3390/nu13010144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drożdż K., Nabrdalik K., Hajzler W., Kwiendacz H., Gumprecht J., Lip G.Y.H. Metabolic-Associated Fatty Liver Disease (MAFLD), Diabetes, and Cardiovascular Disease: Associations with Fructose Metabolism and Gut Microbiota. Nutrients. 2021;14:103. doi: 10.3390/nu14010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lv S., Wang Y., Zhang W., Shang H. Trimethylamine oxide: A potential target for heart failure therapy. Heart. 2022;108:917–922. doi: 10.1136/heartjnl-2021-320054. [DOI] [PubMed] [Google Scholar]
- 73.Zhou W., Cheng Y., Zhu P., Nasser M.I., Zhang X., Zhao M. Implication of gut microbiota in cardiovascular diseases. Oxid. Med. Cell. Longev. 2020;2020:5394096. doi: 10.1155/2020/5394096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sangro P., de la Torre Aláez M., Sangro B., D’Avola D. Metabolic dysfunction-associated fatty liver disease (MAFLD): An update of the recent advances in pharmacological treatment. J. Physiol. Biochem. 2023 doi: 10.1007/s13105-023-00954-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Younossi Z.M., Corey K.E., Lim J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology. 2021;160:912–918. doi: 10.1053/j.gastro.2020.11.051. [DOI] [PubMed] [Google Scholar]
- 76.European Association for the Study of the Liver (EASL) European Association for the Study of Diabetes (EASD) European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Anstee Q.M., Seth D., Day C.P. Genetic Factors That Affect Risk of Alcoholic and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2016;150:1728–1744.e7. doi: 10.1053/j.gastro.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 78.Wong R.J., Aguilar M., Cheung R., Perumpail R.B., Harrison S.A., Younossi Z.M., Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 79.Cho Y., Rhee H., Kim Y., Lee M., Lee B.W., Seok Kang E., Cha B.-S., Choi J.-Y., Lee Y.-H. Ezetimibe combination therapy with statin for non-alcoholic fatty liver disease: An open-label randomized controlled trial (ESSENTIAL study) BMC Med. 2022;20:93. doi: 10.1186/s12916-022-02288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Perla F.M., Prelati M., Lavorato M., Visicchio D., Anania C. The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease. Children. 2017;4:46. doi: 10.3390/children4060046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Momtazi-Borojeni A.A., Banach M., Ruscica M., Sahebkar A. The role of PCSK9 in NAFLD/NASH and therapeutic implications of PCSK9 inhibition. Expert Rev. Clin. Pharmacol. 2022;15:1199–1208. doi: 10.1080/17512433.2022.2132229. [DOI] [PubMed] [Google Scholar]
- 82.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C., Tio F., Hardies J., Darland R.C., Musi N., et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern. Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 83.Sanyal A.J., Chalasani N., Kowdley K.V., McCullough A., Diehl A.M., Bass N.M., Neuschwander-Tetri B.A., Lavine J.E., Tonascia J., Unalp A., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N. Engl. J. Med. 2010;362:1675–1685. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tobita H., Sato S., Miyake T., Ishihara S., Kinoshita Y. Effects of Dapagliflozin on Body Composition and Liver Tests in Patients with Nonalcoholic Steatohepatitis Associated with Type 2 Diabetes Mellitus: A Prospective, Open-label, Uncontrolled Study. Curr. Ther. Res. Clin. Exp. 2017;87:13–19. doi: 10.1016/j.curtheres.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harrison S.A., Manghi F.P., Smith W.B., Alpenidze D., Aizenberg D., Klarenbeek N., Chen C.-Y., Zuckerman E., Ravussin E., Charatcharoenwitthaya P., et al. Licogliflozin for nonalcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a study. Nat. Med. 2022;28:1432–1438. doi: 10.1038/s41591-022-01861-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pokharel A., Kc S., Thapa P., Karki N., Shrestha R., Jaishi B., Paudel M.S. The Effect of Empagliflozin on Liver Fat in Type 2 Diabetes Mellitus Patients with Non-Alcoholic Fatty Liver Disease. Cureus. 2021;13:e16687. doi: 10.7759/cureus.16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Newsome P.N., Buchholtz K., Cusi K., Linder M., Okanoue T., Ratziu V., Sanyal A.J., Sejling A.-S., Harrison S.A. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021;384:1113–1124. doi: 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 88.Armstrong M.J., Gaunt P., Aithal G.P., Barton D., Hull D., Parker R., Hazlehurst J.M., Guo K., LEAN trial team. Abouda G., et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): A multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679–690. doi: 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 89.Nahra R., Wang T., Gadde K.M., Oscarsson J., Stumvoll M., Jermutus L., Hirshberg B., Ambery P. Effects of Cotadutide on Metabolic and Hepatic Parameters in Adults with Overweight or Obesity and Type 2 Diabetes: A 54-Week Randomized Phase 2b Study. Diabetes Care. 2021;44:1433–1442. doi: 10.2337/dc20-2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Targher G., Mantovani A., Byrne C.D. Mechanisms and possible hepatoprotective effects of glucagon-like peptide-1 receptor agonists and other incretin receptor agonists in non-alcoholic fatty liver disease. Lancet Gastroenterol. Hepatol. 2023;8:179–191. doi: 10.1016/S2468-1253(22)00338-7. [DOI] [PubMed] [Google Scholar]
- 91.Tyagi S., Gupta P., Saini A.S., Kaushal C., Sharma S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011;2:236–240. doi: 10.4103/2231-4040.90879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 93.Francque S.M., Bedossa P., Ratziu V., Anstee Q.M., Bugianesi E., Sanyal A.J., Loomba R., Harrison S.A., Balabanska R., Mateva L., et al. A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH. N. Engl. J. Med. 2021;385:1547–1558. doi: 10.1056/NEJMoa2036205. [DOI] [PubMed] [Google Scholar]
- 94.Nakajima A., Eguchi Y., Yoneda M., Imajo K., Tamaki N., Suganami H., Nojima T., Tanigawa R., Iizuka M., Iida Y., et al. Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2021;54:1263–1277. doi: 10.1111/apt.16596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F., Chalasani N., Dasarathy S., Diehl A.M., Hameed B., et al. NASH Clinical Research Network. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. Erratum in Lancet 2015, 385, 946; Erratum in Lancet 2016, 387, 1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kremoser C. FXR agonists for NASH: How are they different and what difference do they make? J. Hepatol. 2021;75:12–15. doi: 10.1016/j.jhep.2021.03.020. [DOI] [PubMed] [Google Scholar]
- 97.Patel K., Harrison S.A., Elkhashab M., Trotter J.F., Herring R., Rojter S.E., Kayali Z., Wong V.W.-S., Greenbloom S., Jayakumar S., et al. Cilofexor, a Nonsteroidal FXR Agonist, in Patients with Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial. Hepatology. 2020;72:58–71. doi: 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 98.Alkhouri N., Herring R., Kabler H., Kayali Z., Hassanein T., Kohli A., Huss R.S., Zhu Y., Billin A.N., Damgaard L.H., et al. Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial. J. Hepatol. 2022;77:607–618. doi: 10.1016/j.jhep.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 99.Sinha R.A., Bruinstroop E., Singh B.K., Yen P.M. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid. 2019;29:1173–1191. doi: 10.1089/thy.2018.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kowalik M.A., Columbano A., Perra A. Thyroid Hormones, Thyromimetics and Their Metabolites in the Treatment of Liver Disease. Front. Endocrinol. 2018;9:382. doi: 10.3389/fendo.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sinha R.A., Bruinstroop E., Singh B.K., Yen P.M. Thyroid Hormones and Thyromimetics: A New Approach to Nonalcoholic Steatohepatitis. Hepatology. 2020;72:770–771. doi: 10.1002/hep.31204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lian B., Loomba R., Neutel J., Margaritescu C., Homer K., Luk A., Mancini M., Ji S., Barker G., Severance R., et al. VK2809, a novel liver-directed thyroid receptor agonist, produces durable reductions in liver fat in patients with non-alcoholic fatty liver disease: Results of 4-week follow-up assessment from a 12-week phase 2 randomized, placebo-controlled trial. J. Hepatol. 2020;73:S53. doi: 10.1016/S0168-8278(20)30652-8. [DOI] [Google Scholar]
- 103.Harrison S., Taub R., Neff G., Moussa S., Alkhouri N., Bashir M. Primary data analyses of MAESTRO-NAFLD-1 a 52 week double-blind placebo-controlled phase 3 clinical trial of resmetirom in patients with NAFLD. J. Hepatol. 2022;77:S14. doi: 10.1016/S0168-8278(22)00445-7. [DOI] [Google Scholar]
- 104.Pedrosa M., Seyedkazemi S., Francque S., Sanyal A., Rinella M., Charlton M., Loomba R., Ratziu V., Kochuparampil J., Fischer L., et al. A randomized, double-blind, multicenter, phase 2b study to evaluate the safety and efficacy of a combination of tropifexor and cenicriviroc in patients with nonalcoholic steatohepatitis and liver fibrosis: Study design of the TANDEM trial. Contemp. Clin. Trials. 2020;88:105889. doi: 10.1016/j.cct.2019.105889. [DOI] [PubMed] [Google Scholar]
- 105.Chalasani N., Abdelmalek M.F., Garcia-Tsao G., Vuppalanchi R., Alkhouri N., Rinella M., Noureddin M., Pyko M., Shiffman M., Sanyal A., et al. Belapectin (GR-MD-02) Study Investigators. Effects of Belapectin, an Inhibitor of Galectin-3, in Patients with Nonalcoholic Steatohepatitis with Cirrhosis and Portal Hypertension. Gastroenterology. 2020;158:1334–1345.e5. doi: 10.1053/j.gastro.2019.11.296. [DOI] [PubMed] [Google Scholar]
- 106.Harrison S.A., Wong V.W., Okanoue T., Bzowej N., Vuppalanchi R., Younes Z., Kohli A., Sarin S., Caldwell S.H., Alkhouri N., et al. STELLAR-3 and STELLAR-4 Investigators. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J. Hepatol. 2020;73:26–39. doi: 10.1016/j.jhep.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 107.Tillman E.J., Rolph T. FGF21: An Emerging Therapeutic Target for Non-Alcoholic Steatohepatitis and Related Metabolic Diseases. Front. Endocrinol. 2020;11:601290. doi: 10.3389/fendo.2020.601290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Talukdar S., Kharitonenkov A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 2021;46:101152. doi: 10.1016/j.molmet.2020.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harrison S.A., Ruane P.J., Freilich B.L., Neff G., Patil R., Behling C.A., Hu C., Fong E., de Temple B., Tillman E.J., et al. Efruxifermin in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled, phase 2a trial. Nat. Med. 2021;27:1262–1271. doi: 10.1038/s41591-021-01425-3. [DOI] [PubMed] [Google Scholar]