Abstract
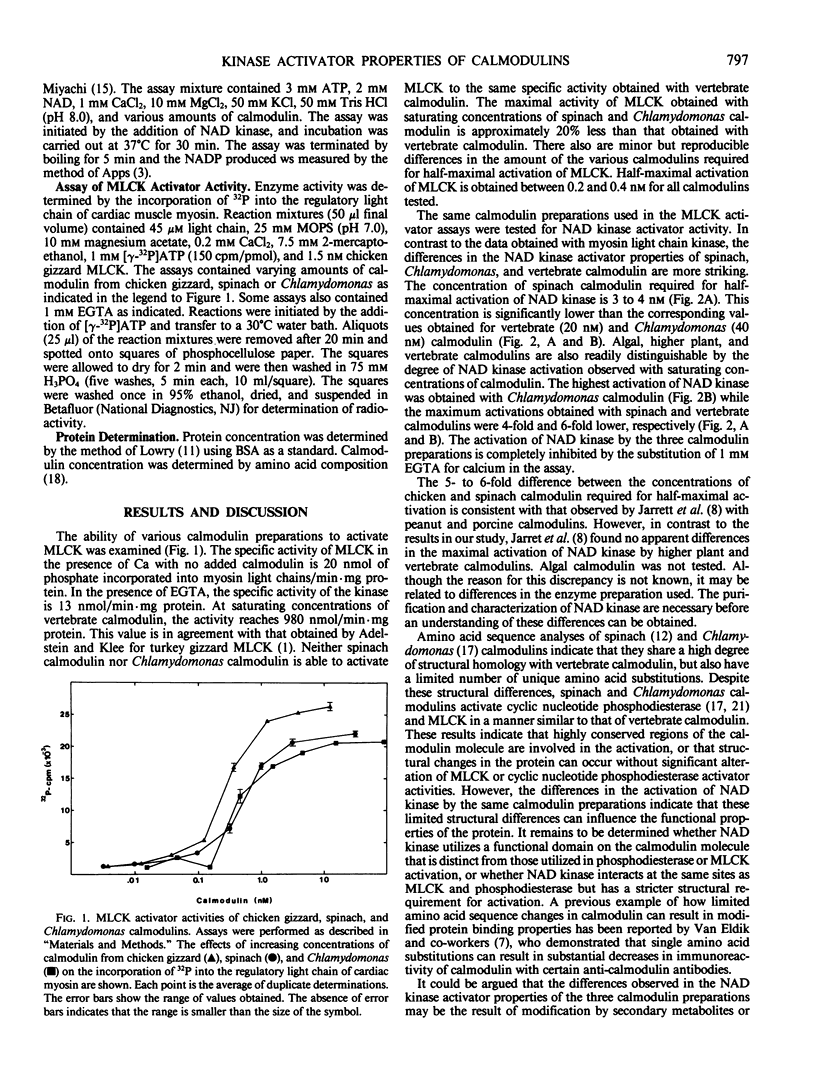
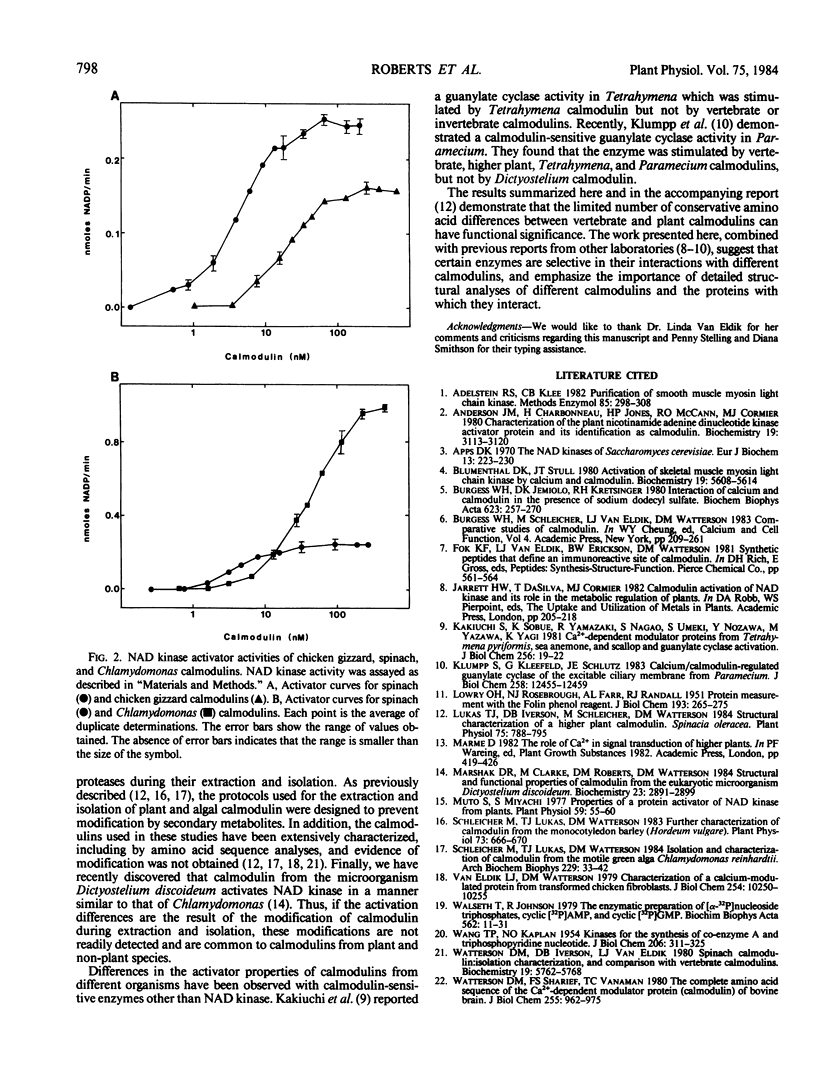
In the preceding paper (Lukas, Iverson, Schleicher, Watterson 1984 Plant Physiol 75: 788-795), we reported that the amino acid sequence of spinach calmodulin has at least 13 amino acid sequence differences from vertebrate calmodulin. In the present study, we investigated the effect of these amino acid sequence substitutions on the enzyme activator properties of vertebrate and plant calmodulins. Calmodulins from spinach and the green alga Chlamydomonas reinhardtii activate chicken gizzard myosin light chain kinase in a manner similar but not identical to chicken calmodulin. In contrast, these calmodulins have very different NAD kinase activator properties. The concentration required for half-maximal activation of pea seedling NAD kinase by spinach calmodulin (3-4 nanomolar) is lower than the corresponding concentrations of chicken (20 nanomolar) and Chlamydomonas (40 nanomolar) calmodulins. However, the maximum level of activation obtained with Chlamydomonas calmodulin is 4- to 6-fold higher than spinach or chicken calmodulin. These data indicate that the limited structural heterogeneity among calmodulins have differential effects on their biochemical activities.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelstein R. S., Klee C. B. Purification of smooth muscle myosin light-chain kinase. Methods Enzymol. 1982;85(Pt B):298–308. doi: 10.1016/0076-6879(82)85029-5. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Charbonneau H., Jones H. P., McCann R. O., Cormier M. J. Characterization of the plant nicotinamide adenine dinucleotide kinase activator protein and its identification as calmodulin. Biochemistry. 1980 Jun 24;19(13):3113–3120. doi: 10.1021/bi00554a043. [DOI] [PubMed] [Google Scholar]
- Apps D. K. The NAD kinases of Saccharomyces cerevisiae. Eur J Biochem. 1970 Apr;13(2):223–230. doi: 10.1111/j.1432-1033.1970.tb00921.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Activation of skeletal muscle myosin light chain kinase by calcium(2+) and calmodulin. Biochemistry. 1980 Nov 25;19(24):5608–5614. doi: 10.1021/bi00565a023. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Jemiolo D. K., Kretsinger R. H. Interaction of calcium and calmodulin in the presence of sodium dodecyl sulfate. Biochim Biophys Acta. 1980 Jun 26;623(2):257–270. doi: 10.1016/0005-2795(80)90254-8. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Nagao S., Umeki S., Nozawa Y., Yazawa M., Yagi K. Ca2+-dependent modulator proteins from Tetrahymena pyriformis, sea anemone, and scallop and guanylate cyclase activation. J Biol Chem. 1981 Jan 10;256(1):19–22. [PubMed] [Google Scholar]
- Klumpp S., Kleefeld G., Schultz J. E. Calcium/calmodulin-regulated guanylate cyclase of the excitable ciliary membrane from Paramecium. Dissociation of calmodulin by La3+: calmodulin specificity and properties of the reconstituted guanylate cyclase. J Biol Chem. 1983 Oct 25;258(20):12455–12459. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lukas T. J., Iverson D. B., Schleicher M., Watterson D. M. Structural characterization of a higher plant calmodulin : spinacia oleracea. Plant Physiol. 1984 Jul;75(3):788–795. doi: 10.1104/pp.75.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak D. R., Clarke M., Roberts D. M., Watterson D. M. Structural and functional properties of calmodulin from the eukaryotic microorganism Dictyostelium discoideum. Biochemistry. 1984 Jun 19;23(13):2891–2899. doi: 10.1021/bi00308a007. [DOI] [PubMed] [Google Scholar]
- Muto S., Miyachi S. Properties of a Protein Activator of NAD Kinase from Plants. Plant Physiol. 1977 Jan;59(1):55–60. doi: 10.1104/pp.59.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher M., Lukas T. J., Watterson D. M. Further Characterization of Calmodulin from the Monocotyledon Barley (Hordeum vulgare). Plant Physiol. 1983 Nov;73(3):666–670. doi: 10.1104/pp.73.3.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher M., Lukas T. J., Watterson D. M. Isolation and characterization of calmodulin from the motile green alga Chlamydomonas reinhardtii. Arch Biochem Biophys. 1984 Feb 15;229(1):33–42. doi: 10.1016/0003-9861(84)90127-9. [DOI] [PubMed] [Google Scholar]
- Van Eldik L. J., Watterson D. M. Characterization of a calcium-modulated protein from transformed chicken fibroblasts. J Biol Chem. 1979 Oct 25;254(20):10250–10255. [PubMed] [Google Scholar]
- WANG T. P., KAPLAN N. O. Kinases for the synthesis of coenzyme A and triphosphopyridine nucleotide. J Biol Chem. 1954 Jan;206(1):311–325. [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Spinach calmodulin: isolation, characterization, and comparison with vertebrate calmodulins. Biochemistry. 1980 Dec 9;19(25):5762–5768. doi: 10.1021/bi00566a015. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]


