Abstract
Simple Summary
Despite the benign nature of these tumors, spinal meningiomas can cause significant neurological damage via compression of the spinal cord. In this study, we found that neurological function improves in a significant proportion of patients after surgery. Preoperative Frankel grade was a significant predictor of postoperative neurological worsening. Cross-section area measurements on MRI scans are not associated with early postoperative outcomes.
Abstract
Background: Due to the slow-growing nature of spinal meningiomas, they are mostly asymptomatic for a long time, and become symptomatic after the compression of the spinal cord or nerve roots. The aim of this study was to identify predictors for a poor clinical outcome after the surgical resection of spinal meningiomas and thereby to allow a preoperative identification of high-risk spinal meningiomas. Methods: Data acquisition was conducted as a single-center retrospective analysis. From 1 January 2004 to 31 December 2019, 121 patients who underwent surgical resection of a spinal meningioma were reviewed. Clinical and radiological data (such as tumor size, location, occupation ratio of the spinal canal, and the degree of spinal cord compression) were assessed. The functional clinical findings of the patients were recorded using the Karnofsky Performance Score, modified McCormick scale, and Frankel scale preoperatively, at discharge, and 3–6 months after surgery. Results: The mean patient age was 66 ± 13 years. A total of 104 (86%) patients were female and 17 (14%) were male. The thoracic spine (68%) was the most common location, followed by the cervical (29%) and lumbar (3%) spine. Preoperatively, 11.7% of patients were categorized as McCormick 1, 35.8% as 2, 39.2% as 3, 11.7% as 4, and 1.7% as 5. The neurological function of the patients with a functional deficit prior to surgery improved in 46% of the patients, remained unchanged in 52%, and worsened in 2% at discharge. At early follow-up, the proportions were 54%, 28%, and 5%, respectively. Preoperative Frankel scale was a significant predictor of a postoperative deterioration. Patients with Frankel score A to C preoperatively had a 9.2 times higher chance of clinical deterioration postoperatively (OR = 9.16). We found that the Frankel scale weakly correlated with the degree of spinal cord compression. In this study, other radiological parameters, such as the degree of cord compression and spinal canal occupation ratio, did not show a significant effect on the outcome. Conclusions: Surgery of intraspinal meningiomas can be considered safe. Neurological function improves in a large proportion of patients after surgery. However, a relevant preoperative deficit according to the Frankel scale (grade A–C) was a significant predictor of a postoperative neurological deterioration.
Keywords: meningioma, outcome, spine, surgery
1. Introduction
Spinal meningiomas (SMs) are the most common intradural extramedullary tumors, accounting for 25–45% of all primary intradural spinal tumors and 7.9–12% of all meningiomas [1,2,3]. They occur with an age-adjusted incidence of 0.33 per 100,000 inhabitants [4]. The incidence increases with age, and most meningiomas occur from the fifth decade of life [5]. In SMs, the female-to-male ratio is approximately 5:1, compared to a 2:1 ratio in intracranial meningiomas [6,7,8].
They are typically solitary, well-circumscribed neoplasms not invading other adjacent tissues [9]. Histologically, the majority of SMs are CNS grade 1, according to the classification of the World Health Organization [10]. However, they can also appear as CNS grade 2 and grade 3, which are associated with a more aggressive growth and a high risk of recurrence after resection [10].
Surgical resection is the first choice of treatment and is predominantly performed via a posterior spinal approach and hemilaminectomies. Although the postoperative course may be heterogeneous, most patients show satisfactory results with neurological improvement after surgery. Their mortality is low, and it is mostly associated with older age and severe comorbidities [11].
However, despite the benign nature of these tumors, they can cause significant neurological damage via compression of the spinal cord. Furthermore, for a small subgroup of patients, the postoperative outcome is unfavorable and associated with new neurological deficits. Therefore, the present study aimed to identify preoperative risk factors for an unfavorable outcome and, thereby, to identify high-risk spinal meningiomas.
2. Patients and Methods
The medical records and MRIs of 135 consecutive patients undergoing surgical resection of SM in our department between January 2004 and December 2021 were retrospectively reviewed. The inclusion and exclusion criteria were defined prior to the start of the study.
The inclusion criteria were (1) age > 18 years, (2) surgical treatment of SM, (3) availability of preoperative MRI (T1-weighted with contrast agent and T2-weighted sequences), and (4) clinical outcome data until at least 3 months after surgery.
The exclusion criteria were (1) patients with only one postoperative follow-up after the initial surgery and (2) incomplete medical documentation.
A total of 14 patients were excluded according to the inclusion and exclusion criteria due to incomplete documentation of medical data. Thus, 121 patients were included in the complete data processing.
Baseline characteristics, including sex, age, patient history, and Charlson comorbidity index, were assessed [12].
Based on neuroradiological images, tumor location was divided by spinal level into four categories: cervical, thoracic, lumbar, and sacral. Further, we classified SM by attachment to the dura mater into ventral, dorsal, lateral, dorsolateral, and ventrolateral.
In addition, intraoperative data, such as the operative approach, duration of the operation, and intraoperative blood loss, were documented. Histopathological data, such as tumor grade according to the WHO, histological tumor subtype, and Ki-67 index, were taken from the neuropathological records.
The functional clinical findings of the patients were recorded using the Karnofsky Performance Score (KPS), modified McCormick scale (Table 1), and Frankel scale (Table 2) at admission to the hospital, discharge from the hospital, and the first postoperative and last visit to our clinic [13,14,15].
Table 1.
Modified McCormick scale.
| Grade | Description |
|---|---|
| I | Intact neurologically, normal ambulation, minimal dysesthesia |
| II | Mild motor or sensory deficit, functional independence |
| III | Moderate deficit, limitation of function, independent with external aid |
| IV | Severe motor or sensory deficit, limited function, dependent |
| V | Paraplegia or quadriplegia, even with flickering movement |
Table 2.
Frankel scale.
| Grade | Description | |
|---|---|---|
| A | Complete | No motor or sensory function below level of lesion |
| B | Sensory only | No motor function, but some sensation preserved below lesion |
| C | Motor useless | Some motor function without practical application |
| D | Motor useful | Useful motor function below level of lesion |
| E | Recovery | Normal motor or sensory function, may have reflex abnormalities |
Early follow-up is defined as a follow-up between 3 and 6 months after surgery, whereas late follow-up represents the patient’s last visit to the outpatient clinic.
3. Radiologic Assessment
A quantitative assessment of the tumor was performed using MR images. Tumor volume was estimated using sagittal and axial T1-weighted MRI sequences with the following formula:
| Volume (mm3) = (π × height × width × depth)/6 |
Height (H) was measured on sagittal images, whereas width (W) and depth (D) were measured on axial images. All measurements were recorded in millimeters.
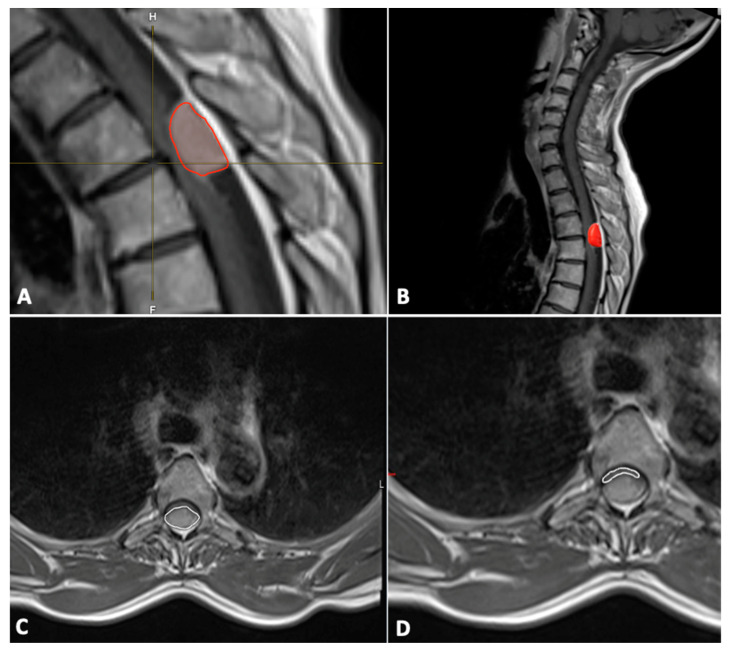
In addition, the tumor volume (mm3) was also segmented manually using Brainlab Elements® version 3.1.0 (Brainlab AG, Munich, Germany) using axial and sagittal contrast-enhanced T1-weighted images (Figure 1).
Figure 1.
Tumor (red frame in the image) volume measurements with Brainlab Elements® version 3.1.0 (A,B); cross-section of spinal meningioma (C) and spinal cord (D).
The cross-sectional area (mm2) of the spinal canal, spinal cord, and tumor were measured on the axial T1-weighted contrast-enhanced MR image at the level of maximal tumor occupancy of the spinal canal (Figure 1).
The occupation ratio of the tumor in the spinal canal was calculated using the following formula:
| Occupancy ratio (%) = tumor area (mm2)/spinal canal area (mm2) × 100 |
The degree of compression of the spinal cord by the tumor was calculated on the axial T2-weighted MR images using the following formula:
| Spinal cord compression ratio (%) = 100 − (area of spinal cord at maximum compression/[spinal cord area above + spinal cord area below]/2) × 100 |
4. Statistical Analysis
Data analysis was performed using the statistics software SPSS (Version 16.0, SPSS Inc., Chicago, IL, USA). Categorical data are represented by absolute and relative counts.
Differences in normally distributed numerical variables between two independent groups were tested using Student’s t-test. The Mann–Whitney U-test was chosen to compare continuous variables as the data were mainly not normally distributed.
Logistic regression was used to assess the influence of multiple factors on treatment outcome. Results with p < 0.05 were considered statistically significant.
5. Results
A total of 121 patients were included in this study. The mean age of all patients was 66 ± 13 years (range, 29–88 years), and 86% of 121 patients were females. The detailed patient characteristics are provided in Table 3.
Table 3.
Baseline demographics and clinical characteristics.
| Variable | N (%) |
|---|---|
| Gender | |
| Male | 17 (14%) |
| Female | 104 (86%) |
| Mean age ± SD, yrs | 66 ± 13 |
| Spinal level | |
| Cervical | 36 (29.7%) |
| Thoracic | 82 (67.7%) |
| Lumbar | 3 (2.5%) |
| Clinical symptoms | |
| Ataxia | 67 (55.4%) |
| Hyperreflexia | 40 (33.1%) |
| Babinski | 20 (16.5%) |
| Sensory deficit | 83 (68.6%) |
| Motor deficit | 58 (47.9%) |
| Pain | 63 (52.1%) |
| Bladder–bowel disturbances | 9 (7.4%) |
| Myelopathy | 77 (63.6%) |
| Syrinx | 5 (4.1%) |
| CNS WHO Grade | |
| 1 | 113 (93.4%) |
| 2 | 5 (4.1%) |
| Approach | |
| Anterior | 1 (0.8%) |
| Posterior | 117 (96.6%) |
| Lateral | 3 (2.5%) |
| Complications | |
| Epidural hematoma | 3 (2.4%) |
| CSF fistula | 2 (1.6%) |
| Meningitis | 1 (0.8%) |
| Embolism | 1 (0.8%) |
5.1. Symptoms
The duration of the symptoms prior to the first presentation varied. Five patients (4.1%) showed acute symptoms within the 2 weeks prior to the diagnosis of SM. In total, 32 patients (26.4%) had medium-term symptoms ranging from 3 to 8 weeks, and 78 (64.5%) had longer-standing symptoms for more than 8 weeks.
The largest number of patients presented with sensory deficits as the leading symptom (present in 68.6% [n = 82] of the patients), followed by ataxia (in 55.4% [n = 65] of the patients) and pain (in 52.1% [n = 62] of the patients). Motor deficits were present in 47.9% (n = 56) of the patients, whereas hyperreflexia was present in 33.1% (n = 39). A positive Babinski reflex was documented in 16.5% (n = 20) of the patients, and 7.4% (n = 9) had bladder or rectum disorders.
In total, 19 percent of the patients had a monosymptomatic initial disease manifestation, whereas 78.5% (n = 95) of the patients had two or more symptoms at initial presentation. Three patients had no symptoms, and all three had SM as an incidental finding. In these three patients, the decision on surgical treatment was made based on tumor growth during follow-up.
5.2. Location and Dural Attachment
Among the patients, about two-thirds of the SMs (67.2%, n = 82) were located in the thoracic spine, followed by the cervical spine (29.8%, n = 36) and lumbar spine (2.5%, n = 3).
A dorsal or posterior dural attachment was the most common in the examined group. It was present in 39.4% (n = 48) of the patients. The attachment was located anteriorly in 31.2% (n = 38) of the patients and located laterally in 19.6% (n = 24). In 5.7% (n = 7) of the patients, the dural attachment was anterolateral, and it was posterolateral in the remaining 4.1%.
Multiple meningiomas were present in 4.1% (n = 5) of the patients.
5.3. Surgical Treatment, Complications, and Recurrence
In 96% of the patients, the tumor was resected via a posterior approach, followed by a lateral approach in 2.4% and an anterior approach in 1.6%. The mean operation time was 183 ± 68 (range: 92–430 min) minutes. The mean intraoperative blood loss was 402.8 ± 205 mL.
Perioperative complications occurred in 5.6% of the patients. The most common postoperative complications were epidural hematoma (three patients), followed by CSF leak (in two patients). All five patients underwent a surgical revision, and no further complications occurred thereafter. The mortality rate was 0%.
Four patients were operated on for a recurrent tumor; i.e., recurrence was present in 3.3% (n = 4) of the patients. The mean time until recurrence was 52 months.
Two patients with recurrence presented with a calcified tumor and one patient with SM recurrence presented with multiple spinal and intracranial meningiomas, whereas in the fourth recurrent case, a partial resection was initially performed, and the regrowth of the tumor was recorded at follow-up.
In 93.4% (n = 113) of the patients, the tumor tissue was pathohistologically classified as a CNS WHO Grade 1 meningioma, while 4.1% (n = 5) of the patients had a CNS WHO Grade 2. The most common histological subtype of CNS WHO Grade 1 meningiomas was psammomatous meningioma (52.1%), followed by transitional (20.7%) meningothelial (16.5%).
The average Ki-67 index in our cohort was 3.7 (range: 2–10%). Among all WHO CNS Grade 2 spinal meningiomas, the Ki-67 index was 10%.
The patient data and clinical parameters are summarized in Table 3.
5.4. Radiological Measurements
The mean tumor volume was 1032.1 mm3, with a minimum value of 78.4 mm3 and a maximum of 3126.5 mm3. Additional measurements are provided in Table 4. Myelopathy was present in 63.6% (n = 77) of the patients, whereas syringomyelia was visible on MRI scans in 4.1% (n = 5).
Table 4.
Tumor volumetry.
| Parameter | Mean | Min–Max |
|---|---|---|
| Volume (mm3) | 1032.1 | 78.4–3126.5 |
| Spinal canal cross-sectional area (mm2) | 232.2 | 80.2–505.4 |
| Cross-sectional area of spinal cord (mm2) | 44.1 | 7.2–95.7 |
| Cross-sectional area of tumor (mm2) | 112.3 | 11.2–332.1 |
| Occupation ratio of the tumor (%) | 47.9 | 7.7–88.7 |
| Degree of compression of the spinal cord (%) | 29.4 | 4.8–83.5 |
5.5. Analysis of Preoperative Functional Status and Postoperative Outcome
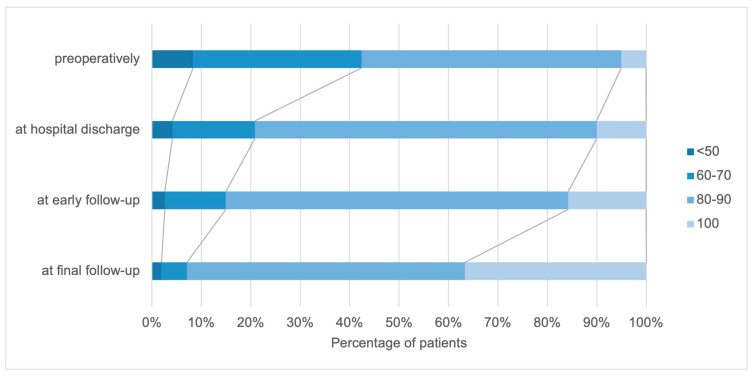
In the examined group, KPS values between 40% and 100% were found at the time of admission when collecting the KPS (Figure 2). The mean KPS was 76 ± 13. More than 50% of the patients had a KPS of 80 or higher upon admission to the hospital, meaning they were able to work and function normally.
Figure 2.
Distribution of patients according to the (KPS) preoperatively, at hospital discharge, and at early and final follow–up.
Neurological improvement was recorded in a considerable proportion of patients directly after the operation. Comparing the preoperative KPS and the KPS at the time of hospital discharge, the number of patients without symptoms and with mild functional impairment increased: there was a 5% increase in the proportion in the KPS 100% group and a 16% increase in the KPS 80–90% group. The functional performance continued to improve in the short-term as well as the long-term follow-up. At the last visit, 92 patients had a satisfactory KPS score, with KPS scores of 100 in 36.2% of the patients compared to the preoperative scores. Deterioration in the KPS score of the patients’ functional status was noted in five patients.
A similar trend was observed in the analysis of the McCormick and Frankel scales. The preoperative examinations resulted in an average McCormick scale value of 2.6. The mean McCormick scale value at hospital discharge was 2.09. Preoperatively, 11.7% of the patients were categorized as McCormick 1, 35.8% as McCormick 2, 39.2% as McCormick 3, 11.7% as McCormick 4, and 1.7% as McCormick 5. Postoperatively, 22.5% of the patients were categorized as McCormick 1, 52.5% as McCormick 2, 19.7% as McCormick 3, 5% as McCormick 4, and 0.8% as McCormick 5.
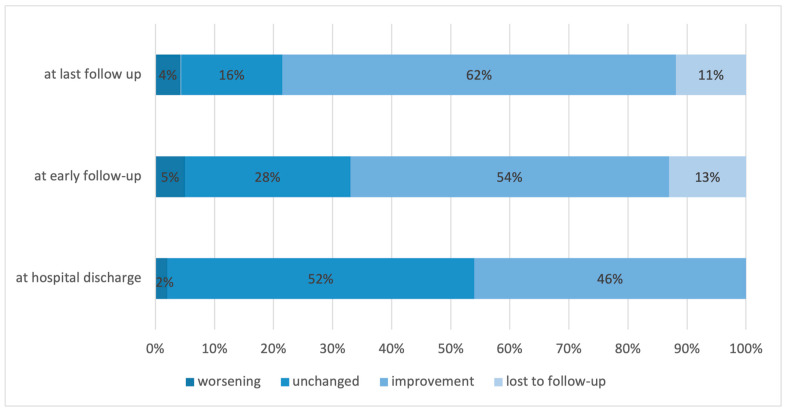
Neurological function improved in 46.2%, remained unchanged in 52.4%, and worsened in 2.4%. At the early follow-up, the proportions were 54.3%, 28.3%, and 5.2%, respectively (Figure 3).
Figure 3.
Distribution of patients based on the change in their neurological function at hospital discharge, at early and final follow-up.
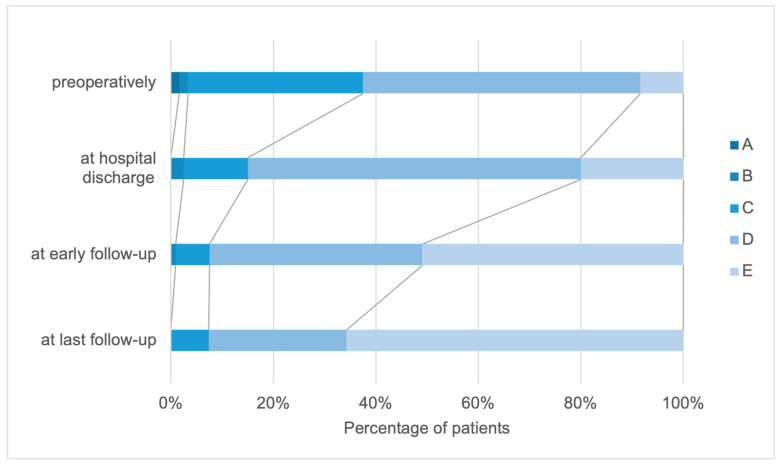
Preoperatively, 1.7% of the patients were categorized as Frankel grades A and B, whereas 34.2% of the patients were Frankel grade C. Frankel grade D was the most common preoperatively and 54.2% of the patients received this grade, whereas 8.3% of the patients were categorized as Frankel grade E. When comparing the different follow-up intervals, a continuous improvement was observed in all Frankel grades.
In the entire examination interval from before the operation to the last follow-up, an improvement in the patient’s functional status was determined in 75.5% of the patients. Comparing the Frankel scale values preoperatively and at the last visit, 59.4% of the patients improved by one grade, whereas 16.1% improved by two grades.
In 22.3% of the patients, the Frankel scale remained unchanged throughout the examination interval, whereas the functional status according to the Frankel scale deteriorated in 2.83% of the patients (Figure 4).
Figure 4.
Distribution of patients according to the Frankel scale (A–E) preoperatively, at hospital discharge, and at early and final follow-up.
5.6. Correlation between Radiological Parameters and Functional Outcomes
We found significant differences in the tumor volume (p < 0.001), tumor cross-section (p < 0.001), and occupancy ratio (p = 0.03) according to the preoperative McCormick scale (Table 5).
Table 5.
Correlation between tumor volumetry and McCormick scale.
| Median (IQR) McCormick | p * | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Tumor volume (mm3) t2 | 359.4 | 801.1 | 959.8 | 1863.1 | 1500.01 | <0.001 † |
| (227.2–834.4) | (514.5–1275.4) | (666.6–1379.3) | (878–2374) | (1226–1774) | ||
| Cross-section (tumor) t2 (mm2) | 47 | 46 | 81.25 | 109 | 133 | <0.001 ‡ |
| (39–13) | (26–81) | (75.5–124.8) | (108–147) | (136.3–202.8) | ||
| Cross-section (spinal canal) t2 (mm2) | 184 | 217 | 228 | 297 | 271 | 0.007 § |
| (170–249) | (191.7–253.8) | (189.5–264.8) | (234.5–345.5) | (270–272) | ||
| Cross-section (cord) t2 (mm2) | 45 | 43 | 40 | 36 | 49 | 0.72 |
| (35–62) | (36–53.5) | (28.8–54.3) | (30–60.8) | (37–61) | ||
| Occupation ratio | 40.18 | 48 | 48 | 61.17 | 41.11 | 0.03 ≈ |
| (29.3–48.2) | (41.1–53.6) | (37.5–62.8) | (49.5–65.6) | (33.3–48.9) | ||
| Degree of cord compression | 17.6 | 22.64 | 30.15 | 24.1 | 25.9 | 0.13 |
| (14.5–24.1) | (12.3–34.8) | (16.2–46.5) | (19.3–54.7) | (15.8–36.1) | ||
IQR—interquartile range; * Kruskal–Wallis test (post hoc Conover). † at the p < 0.05 level, there are significant differences (1) vs. (2), (3), (4), (5); (4) vs. (2), (3). ‡ at the p < 0.05 level, there are significant differences (1) vs. (2), (3), (4); (4) vs. (2), (3). § at the p < 0.05 level, there are significant differences (4) vs. (1), (2), (3). ≈ at the p < 0.05 level, there are significant differences (1) vs. (3), (4); (2) vs. (4).
There were significant differences in the tumor volume (p = 0.007), tumor cross-section (p = 0.03), and occupancy ratio (p = 0.03) associated with different Frankel grades, but there were no significant differences in the spinal cord cross-section and the degree of cord compression (Table 6).
Table 6.
Correlation between tumor volumetry and Frankel grade.
| Median (IQR) Frankel Scale | p * | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | ||
| Tumor volume (mm3) t2 | 1500 | 1595.8 | 1005.5 | 884.8 | 294.7 | 0.007 † |
| (1226–1774) | (688.1–2503.6) | (693.4–1829.2) | (523.2–1300.6) | (170–834) | ||
| Cross-section (tumor) t2 (mm2) | 111.5 | 172 | 109 | 109 | 68.5 | 0.03 ‡ |
| (90–133) | (143–201) | (81.5–146.5) | (77–137.3) | (49–92) | ||
| Cross-section (spinal canal) t2 (mm2) | 271 | 279,5 | 238 | 220 | 199.5 | 0.13 |
| (270–272) | (262–297) | (194–288.5) | (190.5–257) | (170–275) | ||
| Cross-section (cord) t2 mm2 | 49 (37–61) | 55.5 (46–65) | 38 (30–53) | 41 (31–54) | 46 (35–62) | 0.49 |
| Occupation ratio | 41.1 | 61.1 | 49.8 | 48 | 35.7 | 0.03 ‡ |
| (33.3–48.9) | (54.5–67.7) | (39.7–60.8) | (40.8–57.4) | (28.3–46.9) | ||
| Degree of cord compression | 25.9 | 36.2 | 28.8 | 22.9 | 15.6 | 0.13 |
| (15.9–36) | (24.6–47.8) | (17.8–49.1) | (13.9–46.3) | (14.5–23.1) | ||
IQR—interquartile range; * Kruskal–Wallis test (post hoc Conover). † At the p < 0.05 level, there are significant differences (E) vs. (A), (C), (D). ‡ At the p < 0.05 level, there are significant differences (E) vs. (B), (C), (D).
We used Spearman’s correlation coefficient to assess the association of the Frankel grade with the degree of cord compression, occupation ratio, surgical duration, and blood loss. We observed that there was a significant but weak negative relationship between the Frankel grade and the degree of cord compression (Rho = −0.228), operative time (Rho = −0.249), and blood loss (Rho = −0.222), and that there was no significant correlation with the occupation ratio (Table 7).
Table 7.
Analysis according to Spearman’s correlation coefficient.
| Frankel Scale | Degree of Cord Compression | Occupation Ratio | Operative Time | |
|---|---|---|---|---|
| Frankel scale | 1 | |||
| Degree of cord compression | −0.228 (0.01) | 1 | ||
| Occupation ratio | −0.173 (0.07) | 0.371 (<0.001) | 1 | |
| Operative time | −0.249 (0.006) | 0.211 (0.03) | 0.043 (0.65) | 1 |
| Blood loss | −0.222 (0.02) | 0.140 (0.14) | 0.083 (0.38) | 0.448 (<0.001) |
Multivariate logistic regression showed that a Frankel grade is a significant predictor for a worsening of neurological function. Patients with a worse Frankel scale (grade A–C) result when admitted to the hospital had a 9.16 times higher probability of a worsening of clinical symptoms (OR = 9.16), whereas tumor localization in the lower part of the thoracic spine was protective compared to the upper cervical part (OR = 0.28) (Table 8).
Table 8.
Multivariate logistic regression analysis for worsening of neurological function.
| Parameter | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| Frankel Scale | 9.16 | 3.69–22.7 | <0.001 |
| Lower thoracic spine | 0.28 * | 0.10–0.75 | 0.01 |
| KPS | 1.07 | 1.02–1.13 | 0.009 |
| Operative time | 1.02 | 1.003–1.04 | 0.02 |
* p < 0.05. The factors that were statistically significant in a univariate analysis (i.e., x, y, z….) were used for a multivariate model. Nagelkerke R square. CI—confidence interval.
6. Discussion
The present study aimed to analyze the potential preoperative clinical and imaging factors associated with a postoperative neurological deterioration. Overall, our results show that the surgery of spinal meningiomas is safe, and that a significant proportion of patients experience an improvement in their functional performance before discharge. Further functional improvement was observed in the short-term and long-term follow-up. Only a small number of patients suffered from a worsening of functional performance after surgery. Our study shows that the Frankel scale may be a good tool for assessing the risk of a postoperative worsening in patients with spinal meningiomas. Furthermore, although we observed a correlation between the degree of spinal cord compression by the tumor and preoperative deficits, cross-sectional area measurements were not associated with the early postoperative outcome.
El-Hajj et al. reported in a systematic review of 49 studies that the adjusted complication rate for the surgical resection of spinal meningiomas is 7.4% [16]. This is slightly higher than our results of 5.6%. The most common complication in the published review was CSF leakage, while in our study, epidural hematomas were the most common complication, followed by CSF leaks.
In addition, the authors of the systematic review reported that the cumulative complication rate of patients older than 70 years is 16.8%, which exceeds the complication rate of the overall cohort of spinal meningioma patients [16]. In our study, we could not associate age with a higher complication rate, which is similar to previous studies [8,17]. In contrast, Schwanke et al. stated that the majority of complications in their cohort occurred in older patients [18].
Furthermore, some studies reported that an anterior dural attachment was associated with a complication rate of 26.3%, with cervical spine tumors being associated with the majority of the complications. We did not find an association between dural attachment location and complication rate or poor outcome, but, in accordance with the aforementioned study outcome, the complication rate was better in patients with thoracic spinal meningiomas in comparison to those with cervical tumors. Other factors that are associated with higher complication rates are obesity, reoperation, a lack of surgeon experience and presence of calcification, and length of operation [19,20]. From the factors tested in our cohort, we found the operative time to be associated with a worsening of the functional score, which may relate to the size and complexity of the tumor. The inverse correlation between the degree of cord compression or occupation ratio and the duration of surgery found in our study supports this statement.
Experimental studies have shown that functional recovery after acute spinal cord compression depends on both the magnitude of the compression force and its duration [21,22]. Another clinical study confirmed that in acute spinal cord compression, regardless of the cause, the prognosis for recovery depends primarily on two factors: the severity of the neurological deficit and the duration of the deficit before decompression [23]. Our results showed that 84% of the patients had a better or unchanged McCormick scale result postoperatively. When comparing the measurement intervals, we found that the functional status of the patients continuously improved during the follow-up. These data demonstrate that the neurological function may improve even a long time after the decompression of the spinal cord, and stress the importance of long-term multimodal rehabilitative treatment in such cases.
Moreover, regular follow-up may be needed, not only as part of the oncological routine but also in order to monitor the patient’s progress and recovery of the neurological status.
In addition, our study showed that a worse preoperative Frankel grade can be taken as a predictor of postoperative deterioration and that patients with a worse preoperative Frankel score were up to nine times more likely to have a postoperative deterioration of functional status.
Few studies have examined tumor volume and its impact on the severity of preoperative symptoms [8,24,25]. Our study showed significant differences in the tumor volume, tumor cross-section, occupancy ratio, and preoperative McCormick scale.
Our data are consistent with the published results from Jesse and colleagues who also demonstrated that the degree of spinal cord compression is associated with preoperative McCormick scale and the presence of sensory deficits [25].
The recurrence rate in our study was 3.3%. Similar reoperation rates have been reported in other large series [26,27,28]. Maiuri et al. reported that arachnoid invasion and higher Ki-67 could be considered significant risk factors for recurrence [29]. In our cohort, recurrence occurred in patients with calcification, multiple meningiomas, and partial resection. Given that the average time to recurrence was 5 years after surgery, long-term follow-up is needed [16].
Yamamuro et al. and Nakamura et al. found dural invasion in 76% and 35% of cases, respectively [30,31]. These results suggest that the late development of tumor recurrence is due to residual tumor cells between the inner and outer dura. In addition, the presence of a dural tail is significantly associated with a higher recurrence rate. Various other factors are associated with an increased rate of recurrence. Cohen-Gadol et al. observed recurrences in younger patients with cervical meningiomas, whereas Klekamp and Samii reported that plaque formation, infiltrating meningiomas, and arachnoid scars have a significantly higher association with increased recurrence rates [26,32]. Older patients showed a lower recurrence rate [15].
7. Limitations
First, this was a retrospective single-center study, which may not completely reflect the natural course of the disease. Although our patient sample was large and contained long-term follow-up data concerning functional outcomes, the number of patients with functional worsening was low, which must be taken into account when interpreting the results.
8. Conclusions
According to our data, the surgery of spinal meningiomas is safe, with satisfactory results and minimal morbidity. Neurological function improved in a significant proportion of patients after surgery. Preoperative Frankel grade was a significant predictor of postoperative neurological worsening. Cross-section area measurements on MRI scans were not associated with early postoperative outcomes.
Author Contributions
Conceptualization: N.K.; methodology: D.J. and D.K.; data acquisition: D.J. statistical analysis: D.J.; data interpretation: D.J.; writing—original draft preparation: D.J.; writing—review and editing: D.J., N.K., A.O., M.A.B., C.J.S. and F.R.; supervision: N.K. and F.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
According to the local laws of Rhineland Palatinate, Germany (Landeskrankenhausgesetz §37), no formal approval or informed consent is necessary for this kind of retrospective analysis. The patient data were de-identified before analysis.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available at the Department of Neurosurgery at the University Medical Mainz and can be requested from the director (Prof. Florian Ringel). Each request should be based on a scientific hypothesis and reviewed by a (local) ethical committee. Any request must be made in writing. Data will be saved for ten years after publishing (according to GCP-guidelines).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kshettry V.R., Hsieh J.K., Ostrom Q.T., Kruchko C., Benzel E.C., Barnholtz-Sloan J.S. Descriptive Epidemiology of Spinal Meningiomas in the United States. Spine. 2015;40:E886–E889. doi: 10.1097/BRS.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 2.Ogasawara C., Philbrick B.D., Adamson D.C. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines. 2021;9:319. doi: 10.3390/biomedicines9030319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnautovic K., Arnautovic A. Extramedullary intradural spinal tumors: A review of modern diagnostic and treatment options and a report of a series. Bosn. J. Basic. Med. Sci. 2009;9((Suppl. 1)):S40–S45. doi: 10.17305/bjbms.2009.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duong L.M., McCarthy B.J., McLendon R.E., Dolecek T.A., Kruchko C., Douglas L.L., Ajani U.A. Descriptive epidemiology of malignant and nonmalignant primary spinal cord, spinal meninges, and cauda equina tumors, United States, 2004–2007. Cancer. 2012;118:4220–4227. doi: 10.1002/cncr.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed N., Ferini G., Hossain M., Barua K.K., Hossain M.N., Umana G.E., Shlobin N.A., Scalia G., Palmisciano P., Tomasi O.S., et al. Evaluation of Surgical Cleavage Plane by Preoperative Magnetic Resonance Imaging Findings in Adult Intracranial Meningiomas. Life. 2022;12:473. doi: 10.3390/life12040473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bondy M., Ligon B.L. Epidemiology and etiology of intracranial meningiomas: A review. J. Neurooncol. 1996;29:197–205. doi: 10.1007/BF00165649. [DOI] [PubMed] [Google Scholar]
- 7.Sandalcioglu I.E., Hunold A., Müller O., Bassiouni H., Stolke D., Asgari S. Spinal meningiomas: Critical review of 131 surgically treated patients. Eur. Spine J. 2008;17:1035–1041. doi: 10.1007/s00586-008-0685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pettersson-Segerlind J., Fletcher-Sandersjöö A., Tatter C., Burström G., Persson O., Förander P., Mathiesen T., Bartek J., Jr., Edström E., Elmi-Terander A. Long-Term Follow-Up and Predictors of Functional Outcome after Surgery for Spinal Meningiomas: A Population-Based Cohort Study. Cancers. 2021;13:3244. doi: 10.3390/cancers13133244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delfini R., Fazzolari B., Colistra D. Spinal Meningiomas. In: Arnautović K.I., Gokaslan Z.L., editors. Spinal Cord Tumors. Springer International Publishing; Cham, Switzerland: 2019. pp. 147–159. [Google Scholar]
- 10.Louis D.N., Perry A., Wesseling P., Brat D.J., Cree I.A., Figarella-Branger D., Hawkins C., Ng H.K., Pfister S.M., Reifenberger G., et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwee L.E., Harhangi B.S., Ponne G.A., Kros J.M., Dirven C.M.F., Dammers R. Spinal meningiomas: Treatment outcome and long-term follow-up. Clin. Neurol. Neurosurg. 2020;198:106238. doi: 10.1016/j.clineuro.2020.106238. [DOI] [PubMed] [Google Scholar]
- 12.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Karnofsky D., Burchenal J. Evaluation of Chemotherpeutic Agents. Columbia University; New York, NY, USA: 1949. p. 19. [Google Scholar]
- 14.Manzano G., Green B.A., Vanni S., Levi A.D. Contemporary management of adult intramedullary spinal tumors-pathology and neurological outcomes related to surgical resection. Spinal Cord. 2008;46:540–546. doi: 10.1038/sc.2008.51. [DOI] [PubMed] [Google Scholar]
- 15.Frankel H.L., Hancock D.O., Hyslop G., Melzak J., Michaelis L.S., Ungar G.H., Vernon J.D., Walsh J.J. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 16.El-Hajj V.G., Pettersson-Segerlind J., Fletcher-Sandersjöö A., Edström E., Elmi-Terander A. Current Knowledge on Spinal Meningiomas-Surgical Treatment, Complications, and Outcomes: A Systematic Review and Meta-Analysis (Part 2) Cancers. 2022;14:6221. doi: 10.3390/cancers14246221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambekar S., Sharma M., Kukreja S., Nanda A. Complications and outcomes of surgery for spinal meningioma: A Nationwide Inpatient Sample analysis from 2003 to 2010. Clin. Neurol. Neurosurg. 2014;118:65–68. doi: 10.1016/j.clineuro.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Schwake M., Adeli A., Sporns P., Ewelt C., Schmitz T., Sicking J., Hess K., Cäcilia Spille D., Paulus W., Stummer W., et al. Spinal meningiomas—Risks and potential of an increasing age at the time of surgery. J. Clin. Neurosci. 2018;57:86–92. doi: 10.1016/j.jocn.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 19.Wach J., Banat M., Schuss P., Güresir E., Vatter H., Scorzin J. Age at Diagnosis and Baseline Myelomalacia Sign Predict Functional Outcome After Spinal Meningioma Surgery. Front. Surg. 2021;8:682930. doi: 10.3389/fsurg.2021.682930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilinc F., Setzer M., Marquardt G., Keil F., Dubinski D., Bruder M., Seifert V., Behmanesh B. Functional outcome and morbidity after microsurgical resection of spinal meningiomas. Neurosurg. Focus. 2021;50:E20. doi: 10.3171/2021.2.FOCUS201116. [DOI] [PubMed] [Google Scholar]
- 21.Tarlov I.M., Herz E. Spinal cord compression studies. IV. Outlook with complete paralysis in man. AMA Arch. Neurol. Psychiatry. 1954;72:43–59. doi: 10.1001/archneurpsyc.1954.02330010045003. [DOI] [PubMed] [Google Scholar]
- 22.Tarlov I.M. Spinal cord compression studies. III. Time limits for recovery after gradual compression in dogs. AMA Arch. Neurol. Psychiatry. 1954;71:588–597. doi: 10.1001/archneurpsyc.1954.02320410050004. [DOI] [PubMed] [Google Scholar]
- 23.Johnston R.A. The management of acute spinal cord compression. J. Neurol. Neurosurg. Psychiatry. 1993;56:1046–1054. doi: 10.1136/jnnp.56.10.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baro V., Moiraghi A., Carlucci V., Paun L., Anglani M., Ermani M., Saladino A., Chioffi F., d’Avella D., Landi A., et al. Spinal Meningiomas: Influence of Cord Compression and Radiological Features on Preoperative Functional Status and Outcome. Cancers. 2021;13:4183. doi: 10.3390/cancers13164183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jesse C.M., Alvarez Abut P., Wermelinger J., Raabe A., Schar R.T., Seidel K. Functional Outcome in Spinal Meningioma Surgery and Use of Intraoperative Neurophysiological Monitoring. Cancers. 2022;14:3989. doi: 10.3390/cancers14163989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen-Gadol A.A., Zikel O.M., Koch C.A., Scheithauer B.W., Krauss W.E. Spinal meningiomas in patients younger than 50 years of age: A 21-year experience. J. Neurosurg. 2003;98((Suppl. 3)):258–263. doi: 10.3171/spi.2003.98.3.0258. [DOI] [PubMed] [Google Scholar]
- 27.Hohenberger C., Gugg C., Schmidt N.O., Zeman F., Schebesch K.M. Functional outcome after surgical treatment of spinal meningioma. J. Clin. Neurosci. 2020;77:62–66. doi: 10.1016/j.jocn.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 28.Hua L., Zhu H., Deng J., Tian M., Jiang X., Tang H., Luan S., Wakimoto H., Xie Q., Gong Y. Clinical and prognostic features of spinal meningioma: A thorough analysis from a single neurosurgical center. J. Neurooncol. 2018;140:639–647. doi: 10.1007/s11060-018-2993-3. [DOI] [PubMed] [Google Scholar]
- 29.Maiuri F., De Caro M.L., de Divitiis O., Vergara P., Mariniello G. Spinal meningiomas: Age-related features. Clin. Neurol. Neurosurg. 2011;113:34–38. doi: 10.1016/j.clineuro.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M., Tsuji O., Fujiyoshi K., Hosogane N., Watanabe K., Tsuji T., Ishii K., Toyama Y., Chiba K., Matsumoto M. Long-term surgical outcomes of spinal meningiomas. Spine. 2012;37:E617–E623. doi: 10.1097/BRS.0b013e31824167f1. [DOI] [PubMed] [Google Scholar]
- 31.Yamamuro K., Seichi A., Kimura A., Kikkawa I., Kojima M., Inoue H., Hoshino Y. Histological investigation of resected dura mater attached to spinal meningioma. Spine. 2012;37:E1398–E1401. doi: 10.1097/BRS.0b013e318268c419. [DOI] [PubMed] [Google Scholar]
- 32.Klekamp J., Samii M. Surgical results for spinal meningiomas. Surg. Neurol. 1999;52:552–562. doi: 10.1016/S0090-3019(99)00153-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at the Department of Neurosurgery at the University Medical Mainz and can be requested from the director (Prof. Florian Ringel). Each request should be based on a scientific hypothesis and reviewed by a (local) ethical committee. Any request must be made in writing. Data will be saved for ten years after publishing (according to GCP-guidelines).