Abstract
The phosphoenolpyruvate (PEP)-dependent phosphotransferase system (PTS) utilizes high-energy phosphate present in PEP to drive the uptake of several different carbohydrates in bacteria. In order to examine the role of the PTS in the physiology of Listeria monocytogenes, we identified the ptsH and ptsI genes encoding the HPr and enzyme I proteins, respectively, of the PTS. Nucleotide sequence analysis indicated that the predicted proteins are nearly 70% similar to HPr and enzyme I proteins from other organisms. Purified L. monocytogenes HPr overexpressed in Escherichia coli was also capable of complementing an HPr defect in heterologous extracts of Staphylococcus aureus ptsH mutants. Additional studies of the transcriptional organization and control indicated that the ptsH and ptsI genes are organized into a transcription unit that is under the control of a consensus-like promoter and that expression of these genes is mediated by glucose availability and pH or by by-products of glucose metabolism.
Listeria monocytogenes is a ubiquitous gram-positive organism that can be found in several environments, including soil, plants, animals, and water. Under certain conditions, however, this organism can cause serious food-borne illnesses with manifestations ranging from malaise and flu-like symptoms to severe meningitis and fulminating bacteremia in immunocompromised individuals (13). While many studies have focused on the arsenal of virulence factors carried by this organism and the growth patterns of the organism in food matrices, the mechanisms through which L. monocytogenes transports and metabolizes nutrients have only recently been studied (6, 15, 17).
We have begun to examine glucose uptake in L. monocytogenes by using biochemical experiments to identify the nature of the uptake systems (6, 17). In our experiments, we observed that glucose uptake persisted in the presence of bacteriocins which collapse the proton motive force gradient of resting cells. The residual glucose uptake and phosphorylation were subsequently found to be dependent on phosphoenolpyruvate (PEP), suggesting that L. monocytogenes possesses two distinct systems for glucose uptake, a high-affinity PEP-dependent phosphotransferase system (PTS) and a low-affinity proton motive force-mediated system (17). In separate studies, biochemical evidence obtained by Mitchell et al. (15) also demonstrated that there is a fructose-specific PTS whose components appear to be inducible.
The PTS has been well-studied in enteric bacteria, lactic acid bacteria, and the gram-positive model organism Bacillus subtilis (18, 22–25) and consists of two nonspecific energy-coupling proteins, HPr and enzyme I. An additional set of three activities distributed among one or more proteins (enzyme II protein complex) provides the substrate specificity for transport. Unlike the protein found in Escherichia coli, HPr plays an active role in coordinating the expression of carbon catabolism genes in B. subtilis (20). While the precise mechanisms through which HPr exerts this regulatory effect are not known, two highly conserved residues in HPr, His-15 and Ser-46, have been shown to be necessary for it to function and have been shown to serve as sites for phosphorylation (8, 24).
In order to further study the mechanisms of sugar transport in L. monocytogenes, we isolated the genes encoding the HPr (ptsH) and enzyme I (ptsI) components of the L. monocytogenes PTS. We report here that the overexpressed and purified ptsH product (HPr) can complement PTS activity in Staphylococcus aureus extracts prepared from ptsH mutants, demonstrating that this protein is capable of functioning in a heterologous system. The results of studies of the expression of ptsH and ptsI in L. monocytogenes further suggest that these genes form an operon transcribed by the primary form of RNA polymerase, whose transcription is subject to regulation by glucose and pH or by metabolites of glucose.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were grown in Luria broth (LB) at 37°C. S. aureus and L. monocytogenes were grown in tryptic soy broth (Difco Laboratories, Ann Arbor, Mich.) containing 0.5% yeast extract (TSBYE) and either lactose (for S. aureus) or glucose (for L. monocytogenes). Antibiotics were added as indicated below.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli strains | ||
| ES1301 | mutS | Promega |
| JM109 | recA | Promega |
| E509 | λDE3, pLYSE | UN-La |
| S. aureus S797A | ptsH | 19 |
| L. monocytogenes Scott A | Wild type | UN-L |
| Plasmids | ||
| pGEM-T | Promega | |
| pALTER | Promega | |
| pRSET | Promega |
UN-L, University of Nebraska-Lincoln.
DNA procedures.
L. monocytogenes Scott A chromosomal DNA was isolated as described by Byun et al. (4), with some modifications. Cells were grown in TSBYE to the late log phase and harvested by centrifugation. Lysozyme (20 mg/ml) and sodium chloride (final concentration, 300 mM) were added prior to phenol-chloroform extraction of the DNA. E. coli was transformed by electroporation. Plasmid DNA was harvested from 1.5 ml of an E. coli culture grown in LB containing either ampicillin (100 μg/ml) or tetracycline (10 μg/ml). The cell pellets were resuspended in 50 μl of H2O prior to addition of 150 μl of phenol and 150 μl of chloroform. The mixture was placed in a microcentrifuge tube containing 0.3 g of high-vacuum grease (Dow Corning, Midland, Mich.), and the mixture was centrifuged for 20 s at 12,000 × g (to quickly separate the aqueous and organic phases). Then 150 μl of chloroform-isoamyl alcohol (24:1) was added, and the mixture was centrifuged for 20 s. The upper aqueous phase was removed, and the nucleic acids were precipitated with 0.1 volume of 3 M sodium acetate and 2 volumes of ice-cold ethanol.
Synthesis of oligonucleotides.
All oligonucleotides were synthesized at the University of Nebraska DNA Synthesis Core Facility. The following deoxyoligonucleotides were used as primers: HPr 1 (5′-GCA TGC CAG AAA CAG GAA TTC ATG CAC-3′), HPr 2 (5′-GCA TGC TTC AGC CAA TCC TTC TTT-3′), HPr 3 (5′-GGA TCC AAT ACC AAG AGA CAT AAC GCC-3′), 5′ HPr (5′-CCG GAT CCA AAT AGT TGT AAC AAT AG-3′), 3′ HPr (5′-CCG GAT CCA GAT AAG CTT TCG CAA TG-3′), Enz1 (5′-GCA TGC GGG TTC ATT TCT TTA GGA AG-3′), Enz1(B) (5′-GGA ACT CCG AAT GAT TTA GAA GG-3′), Enz1(C) (5′-CCT TAA CCA ACC ATA CAA TCC ATC C-3′), Rev HPr (5′-CTA CAA AAC TTG CTT GTT CC-3′), and pALT/Nde (5′-CTT GTT CCA TAT GCC CGC GGC-3′).
PCR conditions.
All PCR were performed in a Cyclogene Dri-Block thermocycler (Techne, Inc.). Each PCR mixture contained 10 ng of chromosomal DNA from L. monocytogenes Scott A as a template, each deoxynucleoside triphosphate at a concentration of 0.1 mM, 0.8 to 2.5 mM MgCl2, 1.5 pM forward primer, 1.5 pM reverse primer, Taq buffer, and 0.2 U of Taq polymerase (Fisher Biotech) in a total volume of 30 μl. The denaturation, annealing, and extension conditions were 94°C for 1 min, 50°C for 2 min, and 72°C for 1.5 min per kb of expected product size, respectively; a total of 30 cycles were used. For inverse PCR, the template DNA was digested with the appropriate restriction enzyme, and the fragments were self-ligated by using T4 DNA ligase (New England Biolabs), which yielded DNA concentrations of 5 to 10 μg/ml (7). Self-ligated monomeric circular DNA was initially denatured by heating it at 99°C for 5 min and then rapidly cooling it in ice water prior to addition of Taq polymerase. After the initial denaturation, inverse PCR were performed as described above.
DNA sequencing and analysis.
Double-stranded DNA sequencing of PCR products was performed by the dideoxy chain-terminating method of Sanger et al. (21). Sequencing reactions were performed with 18- to 26-mer synthetic oligonucleotides and a Sequenase 2.0 kit by using the PCR product sequencing protocol described by the manufacturer (United States Biochemicals). Samples were electrophoresed on 5% denaturing gels. Cloned PCR fragments were resequenced from both directions by workers at the University of Nebraska Sequencing Facility.
Northern blot analysis.
Total RNA was isolated from L. monocytogenes Scott A cells during various stages of growth in TSBYE containing either 8 or 80 mM glucose, as previously described (16). These conditions resulted in broth cultures that had different pHs and residual glucose concentrations. Northern blot analysis was performed as described previously (12), with minor modifications. Each lane contained 10 μg of total cellular RNA that had been denatured in glyoxal loading buffer at 55°C for 45 min. After electrophoresis, the RNA was fixed to a ZetaBind nylon membrane (Cuno Labs) by UV cross-linking. Each blot was prehybridized for 3 h at 65°C in hybridization buffer (16). An HPr-specific probe was generated by PCR by using primers HPr 1 and HPr 2. The resulting 0.2-kb fragment was sequenced to confirm specificity. An enzyme I-specific probe was also generated by PCR by using primers HPr 1 and Enz I. In this case, the resulting 1.2-kb product was subsequently cut with HindIII (New England Biolabs) to generate a 0.89-kb probe internal to the ptsI gene, as confirmed by sequence analysis. Each probe was gel purified by using a GeneClean kit (Bio 101, Inc.) and was labeled by using the protocol supplied with a DECAprime DNA labeling kit (Ambion, Inc.). Unincorporated label was removed by using STE midi Select-D Sephadex G-50 microcentrifuge spin columns (5 Prime - 3 Prime, Inc.).
Primer extension.
Oligonucleotide primer Rev HPr was 5′ end labeled with [γ-32P]ATP (Amersham) by using T4 polynucleotide kinase (New England Biolabs). The oligonucleotide was hybridized with 20 μg of total cellular RNA extracted from L. monocytogenes Scott A, and the annealed primer was extended with SuperScript II reverse transcriptase (Gibco BRL) as described by the manufacturer. The resulting cDNA was analyzed on a sequencing gel adjacent to a DNA sequencing ladder generated from the Rev HPr primer by using the 5′ HPr-3′ HPr PCR product as the template.
Overexpression of HPr in E. coli E509.
An NdeI site was generated at the ptsH start codon by site-directed mutagenesis by using pALTER-1 (Promega) and the pALT/Nde primer. The ptsH gene was removed from the resulting plasmid as an NdeI fragment and was ligated into pRSET(B) (Invitrogen) to create an in-frame fusion with the synthetic ribosome binding site of the vector. The pRSET vector containing the Listeria ptsH gene was transformed into E. coli E509. For overexpression, transformed cells were inoculated into LB containing 200 μg of ampicillin per ml and grown at 37°C overnight without shaking. The culture was then diluted 1:50 with fresh medium at the same temperature and incubated until the cells reached an optical density of 0.6. Isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration, 1 mM) and an additional 100 μg of ampicillin per ml were then added, and the culture was incubated for 2.5 h. Samples (200 μl) were removed before and after IPTG was added and were examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) to confirm induction of HPr synthesis. HPr was purified from 0.2 g (dry weight) of cells by sonication (four 1-min bursts at 60% output; Vibra Cell Sonicator; Sonics and Materials, Danbury, Conn.), and the cellular debris was removed by ultracentrifugation at 40,000 × g for 30 min. The supernatant was heated at 65°C for 20 min, and the precipitated protein (including β-galactosidase) was removed by ultracentrifugation, as described above. Ammonium sulfate (0.42 g/ml) was added to the supernatant, the preparation was incubated for 1 h at 4°C, and the precipitated protein was collected by centrifugation. The resulting pellet was resuspended in TGED buffer, dialyzed three times with 1-liter changes of TGED buffer, and applied to a Q-Sepharose ion-exchange column (ISCO, Lincoln, Nebr.). Bound proteins were eluted with a linear 50 mM to 1.0 M NaCl gradient, and the fractions were analyzed by SDS-PAGE. Fractions containing HPr were pooled, reprecipitated, and centrifuged as described above. The pellet was resuspended in 1.5 ml of TGED buffer and loaded onto a 30-ml Sephadex G-50 column. Peak fractions were pooled, dialyzed overnight in protein storage buffer (20 mM Tris [pH 7.5], 0.1 mM EDTA, 0.1 mM dithiothreitol, 50% glycerol), and stored at −70°C.
Complementation assays.
To measure PTS activity, in vitro complementation assays were performed by using S. aureus S797A cell extracts (11). This organism lacks a functional HPr but possesses the remaining PTS proteins. Strain S797A also lacks β-galactosidase activity. Therefore, it cannot hydrolyze the chromogenic PTS substrate ortho-nitrophenol-β-galactosidase (ONPG). If an exogenous source of HPr is added to S. aureus S797A cell extracts, full PTS activity is restored and ONPG 6-phosphate is formed, which is then hydrolyzed by native phospho-β-galactosidase to form ortho-nitrophenol (ONP). The ONP can be measured spectrophotometrically at 420 nm. S. aureus S797A was grown to mid-log phase in 100 ml of TSBYE containing lactose. The cells were harvested by centrifugation (6,000 × g, 10 min) and resuspended in 1 ml of HPr assay buffer (50 mM Tris-acetate, 5 mM MgCl2; pH 7). The resuspended cells were transferred into a microcentrifuge tube containing 0.2 g of 0.1-mm-diameter glass beads, and the preparation was sonicated (four 20-s bursts at 80% output; Vibra Cell sonicator; Sonics and Materials). The sonicated cells were further disrupted with a mini-bead beater (two 40-s bursts; 5,000 rpm; Biospec Products, Bartlesville, Okla.). The mixture was centrifuged at 10,000 × g for 4 min to remove the glass beads and cell debris. Supernatant samples (100 μl) were transferred to fresh tubes to which 0.2 mM ONPG, 5 mM PEP, 5 mM MgCl2, and 2 ng of the purified HPr fraction were added. The preparations were incubated for 1 h at 25°C, and then 1 volume of 1.0 M sodium carbonate was added to each tube to stop the reaction. Each tube was centrifuged at 14,000 × g for 1 min before the absorbance of the supernatant at 420 nm was determined.
Nucleotide sequence accession number.
The nucleotide sequence of the 1.92-kb PCR fragment containing the ptsH gene sequence and much of the ptsI gene sequence of L. monocytogenes Scott A has been deposited in the GenBank database under accession no. AF030824.
RESULTS
Cloning of ptsH.
We demonstrated previously by performing a Southern blot analysis with a ptsH gene probe from B. subtilis that an L. monocytogenes ptsH homolog is present on a 3.2-kb BamHI fragment. Size-fractioned restriction fragments of L. monocytogenes chromosomal DNA were then used to construct size-fractioned libraries; however, we were not able to recover the ptsH homolog either by colony hybridization or through complementation. After screening more than 60,000 colonies from libraries constructed in high- and low-copy-number vectors, we were unable to obtain complementing or hybridizing clones for reasons discussed below.
Because of the difficulties in using standard cloning techniques to isolate the ptsH gene, PCR was used. The primers used for amplifying the ptsH and ptsI genes were designed based on the nucleotide sequences of the ptsH and ptsI coding regions of B. subtilis, Streptococcus salivarius, Streptococcus mutans, and Enterococcus faecalis. When these primers were used in PCR with L. monocytogenes chromosomal DNA, we obtained the expected 1.2-kb product, as determined by agarose gel electrophoresis. This fragment, which we predicted would carry the C-terminal two-thirds of the ptsH gene and the amino-terminal two-thirds of the ptsI gene, was subsequently cloned into pGEM-T and subjected to nucleotide sequence analysis. The analysis indicated that, as expected, the clone was missing some of the N terminus of ptsH and the C terminus of ptsI (data not shown).
In order to amplify the remaining portion of the gene, we designed internal primers and used them to prime inverse PCR performed with L. monocytogenes DNA that had been partially digested and ligated with ClaI or Sau3AI as the template. The first inverse PCR was performed with primers HPr 2 and EnzI(B) by using ClaI-recircularized fragments as the template. This reaction yielded a single product that was approximately 0.8 kb long. Nucleotide sequence analysis revealed that this product contained overlapping ptsH and ptsI homologs (specifically, bases 146 to 361 and 1238 to 1855) (Fig. 1). A second inverse PCR was carried out to determine the sequence of the promoter region of the ptsH and ptsI genes. In this reaction, primers HPr 3 and EnzI(C) were used in combination with DNA that had been partially digested with Sau3AI and recircularized. This PCR yielded a 1.0-kb product that, when cloned and sequenced to the first Sau3AI site in either direction, provided bases 1 to 264 and 1826 to 1914 (Fig. 1).
FIG. 1.
Nucleotide and deduced amino acid sequences of the ptsH and ptsI genes of L. monocytogenes Scott A. The putative −35 and −10 promoter sequences and ribosome binding sites (RBS) are underlined. The transcriptional start site is indicated by +1. The ptsH stop codon is indicated by an asterisk.
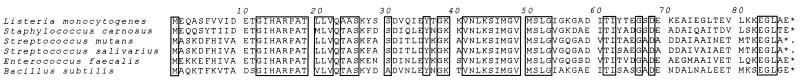
When the overlapping sequences were assembled, a 1,914-base contiguous sequence was constructed that contained two open reading frames whose sizes were consistent with the sizes of previously sequenced ptsH and ptsI genes of other gram-positive bacteria. The first open reading frame (bases 121 to 385) encoded an amino acid sequence which exhibited levels of similarity of >75% with other gram-positive HPr proteins (Fig. 2). This region included highly conserved histidine and serine residues at positions 15 and 46, respectively, that have been shown to be involved in regulating carbohydrate uptake and catabolite repression in B. subtilis. The sequence encoded by the second open reading frame (bases 387 to >1914), which included the ptsI/homolog, exhibited >80% amino acid similarity with other gram-positive PTS enzyme I proteins. Our nucleotide sequence, however, did not encode the C terminus of this protein.
FIG. 2.
Amino acid alignment comparing the HPr protein of L. monocytogenes to the HPr proteins of other gram-positive bacteria. Areas of 100% conservation are enclosed in boxes. The GenBank accession numbers for the nucleotide sequences encoding the proteins are as follows: S. carnosus, X60766; S. mutans, L15191; S. salivarius, Z17217; E. faecalis, Z19137; and B. subtilis, X12832.
ptsH and ptsI are cotranscribed.
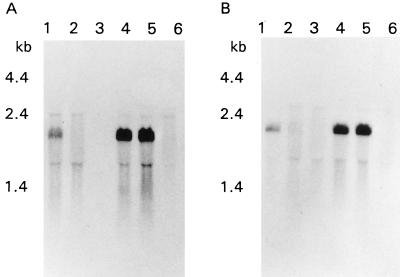
In order to evaluate the transcriptional organization of the ptsH and ptsI genes, a Northern blot analysis was performed by using a variety of growth conditions. RNA was isolated from cells grown in media containing glucose at concentrations at harvest ranging from 0.4 to 73 mM. When either ptsH- or ptsI-specific probes were used, a 2.1-kb transcript was observed, suggesting that the genes are part of a single transcription unit (Fig. 3). Moreover, with each probe, the same transcript was observed at glucose concentrations ranging from 1.2 to 73 mM. The transcript was quite strong at glucose concentrations ranging from 7.4 to 73 mM; however, at concentrations below 1.2 mM the transcript was barely detectable, and it was not observed at a glucose concentration of 0.4 mM.
FIG. 3.
Northern blot analysis of L. monocytogenes Scott A RNA performed with probes specific for ptsH (A) and ptsI (B). (A) The lanes contained total RNA from cells harvested under the following conditions: lane 1, pH 5.4, 1.2 mM glucose; lane 2, pH 5.3, 0.4 mM glucose; lane 4, pH 6.2, 70.6 mM glucose; lane 5, pH 5.34, 64.2 mM glucose; lane 6, pH 4.4, 54.9 mM glucose. Lane 3 contained no RNA. (B) The lanes contained total RNA from cells harvested under the following conditions: lane 1, pH 6.6, 7.4 mM glucose; lane 2, pH 5.4, 1.2 mM glucose; lane 3, pH 5.3, 0.4 mM glucose; lane 4, pH 6.6, 72.9 mM glucose; lane 5, pH 6.2, 70.6 mM glucose; lane 6, pH 4.4, 54.9 mM glucose.
Like the glucose experiments described above, accumulation of the 2.1-kb transcript was affected by pH (Fig. 3). While the transcript was strong at pH values of 6.6 to 5.3, the level of the transcript was reduced at pH 4.4, and it was not detectable at pH 4.2, even though the glucose concentration was more than 50 mM. Thus, given the identical sizes of the transcripts observed with both the ptsH and ptsI probes and given the observation that transcripts hybridizing to each probe responded identically to glucose concentration and pH, we hypothesized that the two genes constitute a single transcription unit whose accumulation is sensitive to glucose concentration and pH or metabolites of glucose.
Mapping of the ptsH promoter.
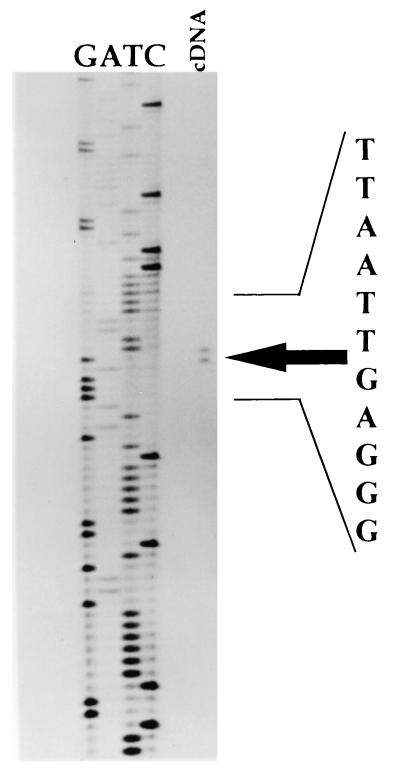
To identify the transcriptional start site of the putative operon, a primer extension analysis was performed with RNA isolated from L. monocytogenes cells grown in TSBYE (Fig. 4). The extension product was electrophoresed next to a sequencing ladder primed with the same primer. The extension product aligned with nucleotides T and G of the sequence shown in Fig. 4 and lies 77 to 78 bases upstream of ptsH. Interestingly, the position of the transcriptional start site is consistent with the position observed in other gram-positive bacteria (GenBank accession no. X12832) (9). Putative −10 (TTT AAT) and −35 (TTG TAA) promoter boxes similar to other L. monocytogenes promoter boxes were also identified. The −35 box is similar to the boxes observed for L. monocytogenes superoxide dismutase (TTG AAA) (3) and the TnpR gene (TTG ACT) (14). The −10 box is also consistent with other L. monocytogenes promoter boxes (5). The 23-bp space between the two putative promoter boxes is uncharacteristic compared with the normal 12- to 18-bp space observed in most promoter regions. However, extended promoter box spacing has been observed previously in L. monocytogenes, including 23 bp between the −10 and −35 promoter boxes of the actA promoter region (GenBank accession no. X59723).
FIG. 4.
Primer extension of the ptsHI transcriptional start site. The position of the cDNA primer extension product is indicated by an arrow.
Cloning of the contiguous ptsH (HPr) gene.
In order to perform genetic and biochemical analyses of the putative ptsH gene and its product, PCR primers (5′ HPr and 3′ HPr) were designed to flank the ptsH promoter and coding region. A 0.4-kb PCR product was readily obtained, and its identity was confirmed by DNA sequence analysis. Cloning of the PCR product, however, was not readily achieved. After several unsuccessful attempts to clone the product into both high-copy-number (pUC18 and pTRKH2) and low-copy-number (pACYC184 and pTRKL2) vectors, the product was finally cloned directly into pGEM-T. During the nucleotide sequence analysis of the cloned PCR product, we found that the 5′ end of the clone began at the ptsH initiation codon and therefore lacked its cognate promoter. This finding is consistent with our previous unsuccessful efforts to clone the intact gene and its promoter. It is also consistent with the observation that PCR products lacking the intact ptsH gene were readily clonable. These results suggest that the gene and/or promoter is toxic to the E. coli host. Toxicity of intact heterologous ptsH genes in E. coli has been reported previously by Gagnon et al. (9).
Function of the ptsH (HPr) protein.
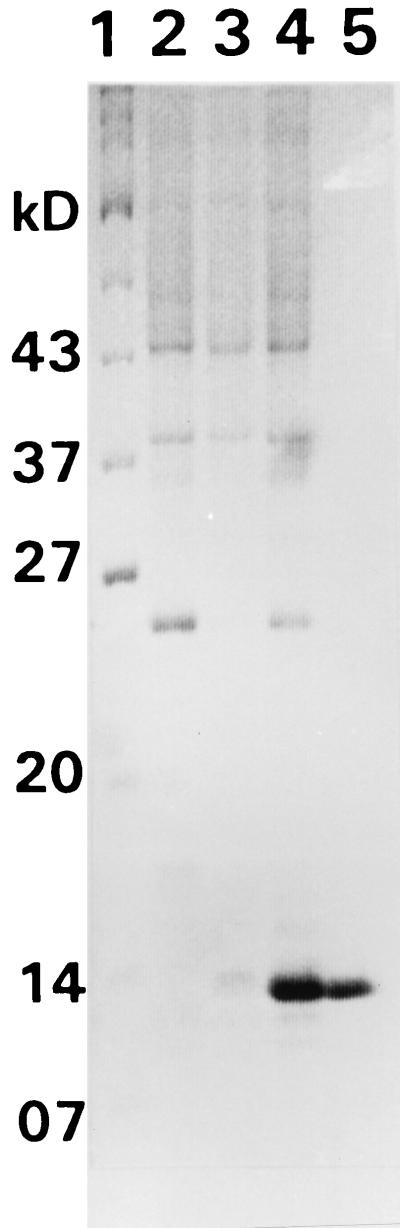
Due to the difficulties in constructing the plasmids necessary for genetic analysis of ptsH, we took a biochemical approach. To facilitate purification of the L. monocytogenes ptsH gene product, the gene was overexpressed in a T7 expression system (Fig. 5). The cloned ptsH PCR product was first moved into the site-directed mutagenesis vector pALTER-1, and an NdeI restriction site was engineered at the putative initiation codon by using the mutagenic oligonucleotide pALT/Nde. Cloning of the 0.3-kb NdeI fragment from the resulting plasmid into the NdeI site of the T7 expression vector pRSET(B) thus created a +1 translational fusion of the ptsH gene to the synthetic ribosome binding site of the vector. The entire ptsH region of the resulting plasmid was subjected to nucleotide sequence analysis to confirm its integrity.
FIG. 5.
SDS-PAGE. Lane 1, protein molecular weight standard; lane 2, E. coli E509 containing pRSET; lane 3, uninduced E. coli E509 containing pRSET-ptsH gene construct; lane 4, IPTG-induced E. coli E509 containing pRSET-ptsH gene construct; lane 5, purified L. monocytogenes HPr. kD, kilodaltons.
To test the function of the L. monocytogenes Scott A HPr protein, in vitro complementation assays were performed. Cell extracts obtained from HPr-deficient S. aureus S797A lacked PTS activity and were unable to convert ONPG to ONPG 6-phosphate, as indicated by the lack of ONP formation by native phospho-β-galactosidase. When Listeria HPr was added, conversion of ONPG to ONPG 6-phosphate and finally to ONP occurred (Table 2), indicating that the L. monocytogenes HPr was active and capable of reconstituting PTS activity in heterologous S. aureus extracts.
TABLE 2.
PTS activity of S. aureus S797A extracts complemented with L. monocytogenes Scott A HPr
| Reaction componentsa | PTS activityb |
|---|---|
| S. aureus extract only | NDc |
| L. monocytogenes HPr only | ND |
| S. aureus extract plus E. coli-expressed L. monocytogenes HPr | 618.5 |
All reaction mixtures contained 5 mM PEP, 5 mM MgCl2, and 0.2 mM ONPG.
Activity is expressed as micromoles of ONP formed per minute per milligram of HPr protein.
ND, not detected.
DISCUSSION
The PTS plays a primary role in carbohydrate uptake and catabolite repression in many gram-negative and gram-positive bacteria (18). In gram-positive bacteria, regulation of PTS activity is associated most directly with the phosphorylation state of the general PTS protein HPr (10, 19). We describe here identification and characterization of the L. monocytogenes ptsH and ptsI genes encoding the HPr and enzyme I proteins, respectively. The predicted amino acid sequence of L. monocytogenes HPr is highly homologous to the amino acid sequences of other gram-positive HPr proteins, including a C-terminal region that is shared by both gram-positive and gram-negative organisms and two regions at positions 22 to 29 and 42 to 56 (Fig. 2) unique to gram-positive organisms. Like all HPr proteins that have been identified thus far, the L. monocytogenes homolog contains a highly conserved histidine at position 15 that has been identified as the site of PEP-dependent phosphorylation by enzyme I in S. aureus (2). A second site of phosphorylation, serine 46, is also found in all gram-positive HPr proteins, including the L. monocytogenes sequence described here. This serine residue is a site of ATP-dependent phosphorylation that potentiates catabolite repression by enhancing the affinity of HPr for the CcpA protein (20). It has been postulated that binding of the HPr-CcpA heterodimer in the proximity of catabolite-repressed promoters is directly responsible for inhibiting the transcription of the promoters in the presence of glucose (20).
The effects of phosphorylation at both histidine 15 and serine 46 of the B. subtilis HPr protein have recently been demonstrated in biochemical experiments in which it was observed that HPr phosphorylated at amino acid position 46 interacted with CcpA but only if histidine 15 was unaltered and in a nonphosphorylated state (20). These analyses were performed with mutant ptsH alleles coding for either unphosphorylatable HPr proteins or HPr proteins which permanently mimic the phosphorylated state. The results suggest that the sensitivity of HPr (serine 46-phosphate)-CcpA complex formation to PEP-dependent phosphorylation of histidine 15 provides a mechanism for coupling catabolite repression to carbohydrate uptake (20). The results of preliminary experiments performed with the H15A, H15D, S46T, and S46D mutants of the L. monocytogenes HPr protein that we constructed also support the hypothesis that these residues play similar roles (6a).
The central region of conserved residues (positions 22 to 29) (Fig. 2) is also observed in the HPr proteins of other gram-positive organisms. One of the most interesting occurrences of this sequence is found in Aspergillus fumigatus (1), a eukaryotic mold lacking a PTS. This region is also present in the HPr protein of a Lactobacillus brevis strain which has been shown to lack a functional PTS (19). Together, these findings suggest that this region may be involved in interactions with non-PTS proteins.
In addition to the conserved amino acid sequences in HPr and enzyme I homologs, we also observed that the L. monocytogenes ptsH and ptsI genes are organized into a single transcription unit. Primer extension analyses revealed a single transcription start site having nucleotide sequences upstream of the start site similar to the nucleotide sequences of other housekeeping promoters of gram-positive bacteria and the consensus promoter of E. coli. However, we observed only a single transcript in the ptsHI region in L. monocytogenes. Other organisms, including E. coli, Salmonella typhimurium, B. subtilis, and S. salivarius, have multiple promoters and/or multiple terminators which give rise to both full-length transcripts and transcripts containing only ptsH (10). In S. salivarius, the ptsH-specific transcript accounts for at least 50% of the ptsH-containing RNA that accumulates. Although ptsHI transcription appears to originate from a single promoter in gram-positive organisms, regulation of ptsHI expression is complex, and it has been proposed that both attenuation and antisense RNAs play regulatory roles (10). We were unable to locate either an open reading frame proceeding in the direction opposite the direction of the L. monocytogenes ptsH gene or terminator sequences in the C-terminal end of the ptsI gene. The fact that only ptsHI transcripts were found and the fact that there are no terminator-like sequences in the ptsI region suggest that regulation of HPr and enzyme I in L. monocytogenes is fundamentally different than regulation of HPr and enzyme I in other gram-positive and gram-negative organisms. Whether the regulatory differences correspond to functional differences in the role of PTS proteins, however, remains to be determined.
When we measured the pattern of ptsHI RNA accumulation in Northern blots, we initially observed decreasing amounts of transcript as the cells approached the stationary phase and depletion of the glucose in the medium (Fig. 3). Whether this pattern of accumulation was the consequence of glucose depletion or a product of the growth state of the cells remains to be determined. The results of experiments in which the glucose concentration was maintained above 40 mM, however, suggest that products of glucose metabolism, not glucose concentration per se, may be the regulatory signal. Although we observed decreasing levels of ptsHI mRNA accumulation as the pH of the medium decreased in these experiments, our results did not distinguish whether pH itself or another signal was responsible for this observation. In addition, the results did not exclude the possibility that multiple signals contributed to ptsHI transcript accumulation. We therefore propose that glucose and pH or a metabolite produced during glucose catabolism serves as a regulatory signal. How this regulation is imposed is unclear at this point. We are currently cloning the L. monocytogenes ptsHI promoter region, including sequences well upstream of the −35 element, in order to construct transcriptional fusions to reporter genes to determine if any regulatory elements of ptsHI transcription exist.
The physical identification of a PTS and information regarding the transcriptional regulation of this PTS in L. monocytogenes contribute to the overall understanding of the physiology of this pathogen. The similarity between the L. monocytogenes ptsHI operon identified here and the ptsHI operons of other gram-positive organisms suggests that these operons have very similar functions. The lack of a terminator sequence in the C-terminal end of ptsI, a characteristic of other gram-positive organisms, suggests that regulation of HPr activity in L. monocytogenes may differ from regulation of HPr activity in other organisms. Further research is required to assess the degree to which the L. monocytogenes PTS is similar to or different from other PTSs.
ACKNOWLEDGMENTS
This research was supported by National Research Initiative Food Safety Program grant 93-37201-9291 from the United States Department of Agriculture. D.P.C. was supported by a graduate research associateship from the University of Nebraska-Lincoln Center for Biotechnology and a graduate fellowship from the Institute of Food Technologists.
Footnotes
Paper no. 12177 in the Journal Series of the Nebraska Agricultural Experiment Station, Lincoln.
REFERENCES
- 1.Barker S, Mathews M L, Bostock A, Burnie J. Identification of a gene encoding an HPr-like protein in Aspergillus fumigatus. J Med Vet Mycol. 1991;29:381–386. doi: 10.1080/02681219180000611. [DOI] [PubMed] [Google Scholar]
- 2.Beyreuther K, Raufuss H, Schrecker O, Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. I. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977;75:275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- 3.Brehm K, Haas A, Goebel W, Kreft J. A gene encoding a superoxide dismutase of the facultative intracellular bacterium Listeria monocytogenes. Gene. 1992;118:121–125. doi: 10.1016/0378-1119(92)90258-q. [DOI] [PubMed] [Google Scholar]
- 4.Byun M O, Kaper J B, Ingram L O. Construction of a new vector for the expression of foreign genes in Zymomonas mobilis. J Ind Microbiol. 1986;1:9–15. [Google Scholar]
- 5.Charpentier E, Gerbaud G, Courvalin P. Characterization of a new class of tetracycline-resistence gene tet(s) in Listeria monocytogenes BM4210. Gene. 1993;131:27–34. doi: 10.1016/0378-1119(93)90665-p. [DOI] [PubMed] [Google Scholar]
- 6.Christensen D P, Hutkins R W. Glucose uptake by Listeria monocytogenes Scott A and inhibition by pediocin JD. Appl Environ Microbiol. 1994;60:3870–3873. doi: 10.1128/aem.60.10.3870-3873.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Christensen, D. P., A. K. Benson, and R. W. Hutkins. Unpublished data.
- 7.Collins F S, Weissman S M. Directional cloning of DNA fragments at a large distance from an initial probe: a circularization method. Proc Natl Acad Sci USA. 1984;81:6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisermann R, Deutscher J, Gonzy-Treboul G, Hengstenberg W. Site-directed mutagenesis with the ptsH gene of Bacillus subtilis. J Biol Chem. 1988;263:17050–17054. [PubMed] [Google Scholar]
- 9.Gagnon G, Vadeboncoeur C, Frenette M. Phosphotransferase system of Streptococcus salivarius: characterization of the ptsH gene and its product. Gene. 1993;136:27–34. doi: 10.1016/0378-1119(93)90443-7. [DOI] [PubMed] [Google Scholar]
- 10.Gagnon G, Vadeboncoeur C, Gauthier L, Frenette M. Regulation of ptsH and ptsI gene expression in Streptococcus salivarius ATCC 25975. Mol Microbiol. 1995;16:1111–1121. doi: 10.1111/j.1365-2958.1995.tb02336.x. [DOI] [PubMed] [Google Scholar]
- 11.Hengstenberg W, Penberthy W K, Hill K L, Morse M L. Phosphotransferase system of Staphylococcus aureus: its requirement for the accumulation and metabolism of galactosides. J Bacteriol. 1969;99:383–388. doi: 10.1128/jb.99.2.383-388.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herold K M, Rothberg P G. Evidence for a labile intermediate in the butyrate induced reduction of the level of c-myc RNA in SW837 rectal carcinoma cells. Oncogene. 1988;3:423–428. [PubMed] [Google Scholar]
- 13.Kvenberg J E. Outbreaks of listeriosis/Listeria-contaminated foods. Microbiol Sci. 1988;5:355–358. [PubMed] [Google Scholar]
- 14.Lebrum M, Audurier A, Cassart P. Plasmid-borne cadmium resistance genes in Listeria monocytogenes are similar to cadA and cadC of Staphylococcus aureus and are induced by cadmium. J Bacteriol. 1994;176:3040–3048. doi: 10.1128/jb.176.10.3040-3048.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell W J, Reizer J, Herring C, Hoischen C, Saier M H., Jr Identification of a phosphoenolpyruvate:fructose phosphotransferase system (fructose-1-phosphate forming) in Listeria monocytogenes. J Bacteriol. 1993;175:2758–2761. doi: 10.1128/jb.175.9.2758-2761.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustapha A, Hutkins R W, Zirnstein G W. Cloning and characterization of the galactokinase gene from Streptococcus thermophilus. J Dairy Sci. 1995;78:989–997. doi: 10.3168/jds.S0022-0302(95)76714-5. [DOI] [PubMed] [Google Scholar]
- 17.Parker C, Hutkins R W. Listeria monocytogenes Scott A transports glucose by high-affinity and low-affinity glucose transport systems. Appl Environ Microbiol. 1997;63:543–546. doi: 10.1128/aem.63.2.543-546.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reizer J, Peterkofsky A, Romano A H. Evidence for the presence of a heat-stable protein (HPr) and ATP-dependent HPr kinase in heterofermentive lactobacilli lacking phosphoenopyruvate:glycose phosphotransferase activity. Proc Natl Acad Sci USA. 1988;85:2041–2045. doi: 10.1073/pnas.85.7.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reizer J, Bergstedt U, Galinier A, Kuster E, Saier M H, Jr, Hillen W, Steinmetz M, Deutscher J. Catabolite repression resistance of gnt operon expression in Bacillus subtilis conferred by mutation of his-15, the site of phosphoenolpyruvate-dependent phosphorylation of the phosphocarrier protein HPr. J Bacteriol. 1996;178:5480–5486. doi: 10.1128/jb.178.18.5480-5486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Rooijen R J, de Vos W M. Molecular cloning, transcriptional analysis, and nucleotide sequence of lacR, a gene encoding the repressor of the lactose phosphotransferase system of Lactococcus lactis. J Biol Chem. 1990;265:18499–18503. [PubMed] [Google Scholar]
- 23.Voskuil M I, Chambliss G H. Significance of HPr in catabolite repression of α-amylase. J Bacteriol. 1996;178:7014–7015. doi: 10.1128/jb.178.23.7014-7015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye J J, Saier M H., Jr Regulation of sugar uptake via the phosphoenolpyruvate-dependent phosphotransferase systems in Bacillus subtilis and Lactococcus lactis is mediated by ATP-dependent phosphorylation of seryl residue 46 in HPr. J Bacteriol. 1996;178:3557–3563. doi: 10.1128/jb.178.12.3557-3563.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye J J, Reizer J, Cui X, Saier M H., Jr Inhibition of the phosphoenolpyruvate:lactose phosphotransferase system and activation of a cytoplasmic sugar-phosphate phosphatase in Lactococcus lactis by ATP-dependent metabolite-activated phosphorylation of serine 46 in the phosphocarrier protein HPr. J Biol Chem. 1994;269:11837–11844. [PubMed] [Google Scholar]