Abstract
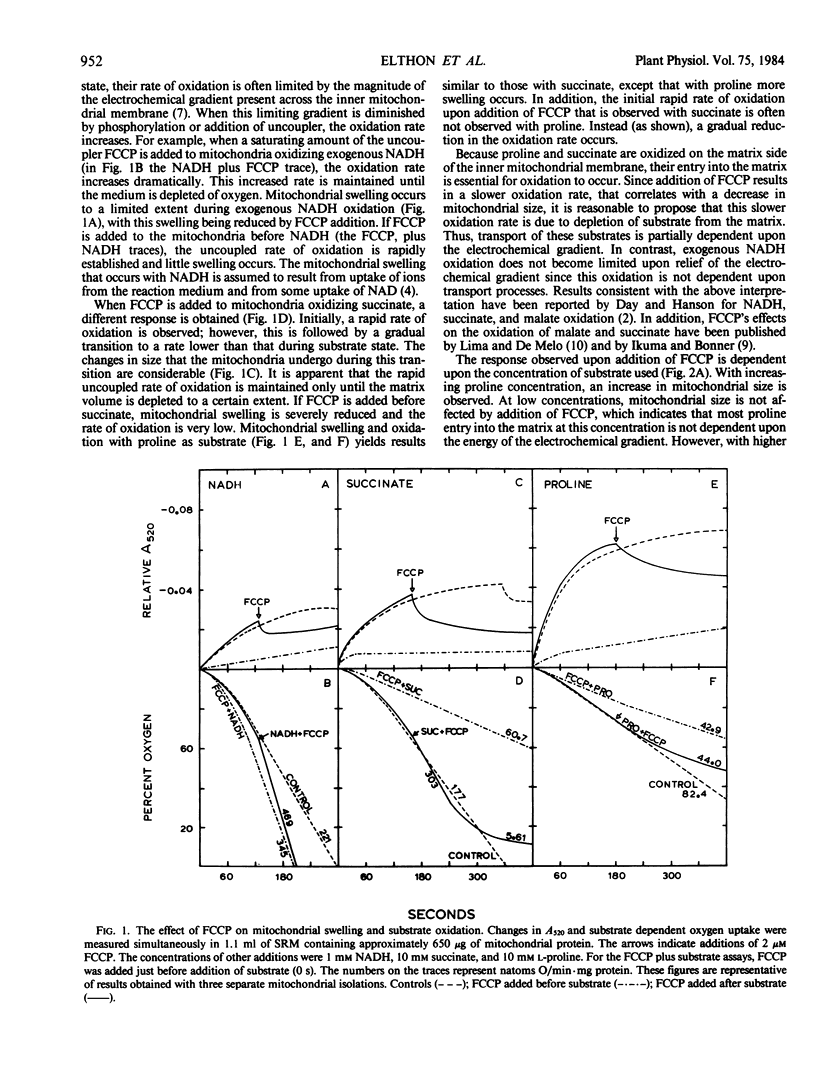
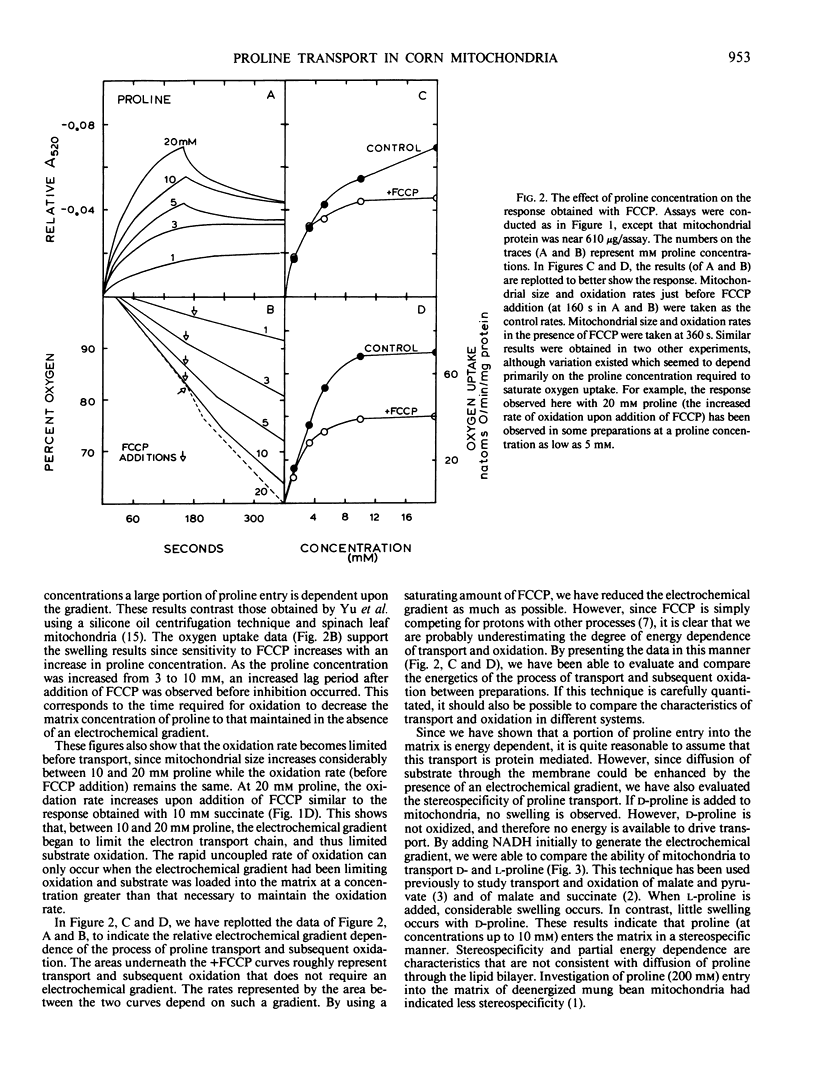
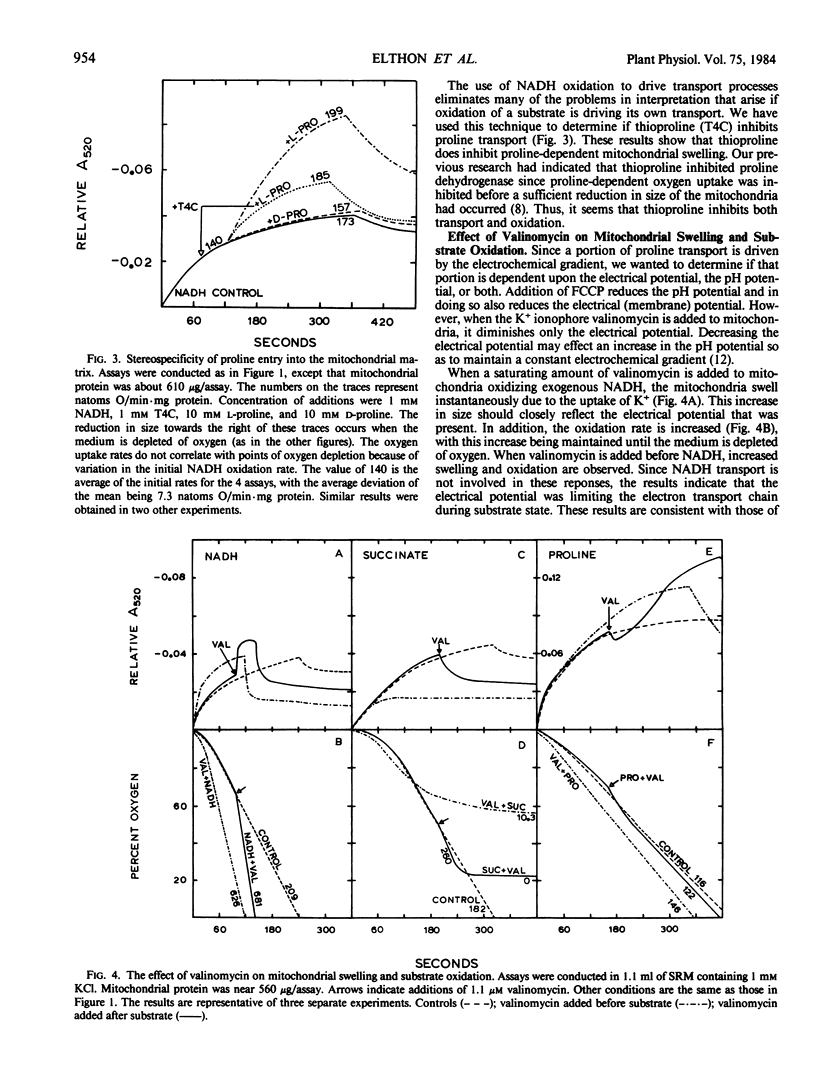
The mechanism of proline entry into the matrix region of isolated corn mitochondria (Zea mays L. Mo17 × B73) was investigated by measuring osmotically induced changes of mitochondrial size (changes in A520) in combination with oxygen uptake measurements. Using NADH oxidation to generate the electrochemical gradient, we have determined that proline transport is stereospecific and that it can be inhibited by the proline analog l-thiazolidine-4-carboxylic acid.
The energetics of proline transport was investigated by measuring the effects of FCCP (p-trifluoromethoxycarbonyl cyanide phenylhydrazone) and valinomycin on mitochondrial swelling and substrate oxidation. Proline transport and resulting oxidation were found to be partially dependent upon the energy of the electrochemical gradient. At low proline concentrations, entry was found to be primarily independent of the gradient (based on insensitivity to FCCP), whereas at higher proline concentrations a gradient-dependent mechanism became involved. Results with valinomycin indicated that proline transport and oxidation are dependent upon the pH potential across the membrane rather than the electrical (membrane) potential.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalieri A. J., Huang A. H. Carrier Protein-mediated Transport of Neutral Amino Acids into Mung Bean Mitochondria. Plant Physiol. 1980 Oct;66(4):588–591. doi: 10.1104/pp.66.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Effect of phosphate and uncouplers on substrate transport and oxidation by isolated corn mitochondria. Plant Physiol. 1977 Feb;59(2):139–144. doi: 10.1104/pp.59.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Hanson J. B. Pyruvate and malate transport and oxidation in corn mitochondria. Plant Physiol. 1977 Apr;59(4):630–635. doi: 10.1104/pp.59.4.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Neuburger M., Douce R., Wiskich J. T. Exogenous NAD Effects on Plant Mitochondria: A Reinvestigation of the Transhydrogenase Hypothesis. Plant Physiol. 1983 Dec;73(4):1024–1027. doi: 10.1104/pp.73.4.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Stewart C. R. Effects of the Proline Analog l-Thiazolidine-4-carboxylic Acid on Proline Metabolism. Plant Physiol. 1984 Feb;74(2):213–218. doi: 10.1104/pp.74.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Stewart C. R. Proline Oxidation in Corn Mitochondria : Involvement of NAD, Relationship to Ornithine Metabolism, and Sidedness on the Inner Membrane. Plant Physiol. 1982 Aug;70(2):567–572. doi: 10.1104/pp.70.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Stewart C. R. Submitochondrial location and electron transport characteristics of enzymes involved in proline oxidation. Plant Physiol. 1981 Apr;67(4):780–784. doi: 10.1104/pp.67.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. II. Effects of DNP, m-Cl-CCP, and Oligomycin on Respiration of Mung Bean Mitochondria. Plant Physiol. 1967 Oct;42(10):1400–1406. doi: 10.1104/pp.42.10.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Stewart C. R. Inhibition of proline oxidation by water stress. Plant Physiol. 1977 May;59(5):930–932. doi: 10.1104/pp.59.5.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskich J. T., Bonner W. D. Preparation and Properties of Sweet Potato Mitochondria. Plant Physiol. 1963 Sep;38(5):594–604. doi: 10.1104/pp.38.5.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Claybrook D. L., Huang A. H. Transport of glycine, serine, and proline into spinach leaf mitochondria. Arch Biochem Biophys. 1983 Nov;227(1):180–187. doi: 10.1016/0003-9861(83)90361-2. [DOI] [PubMed] [Google Scholar]


