Abstract
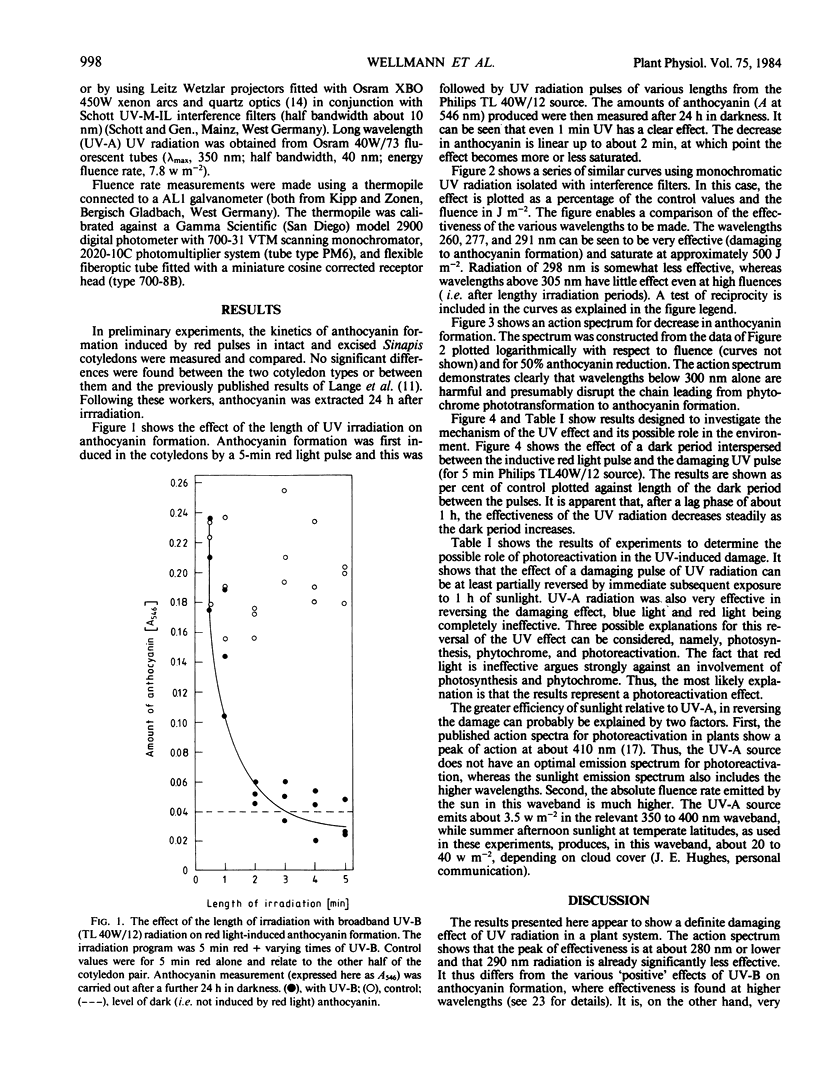
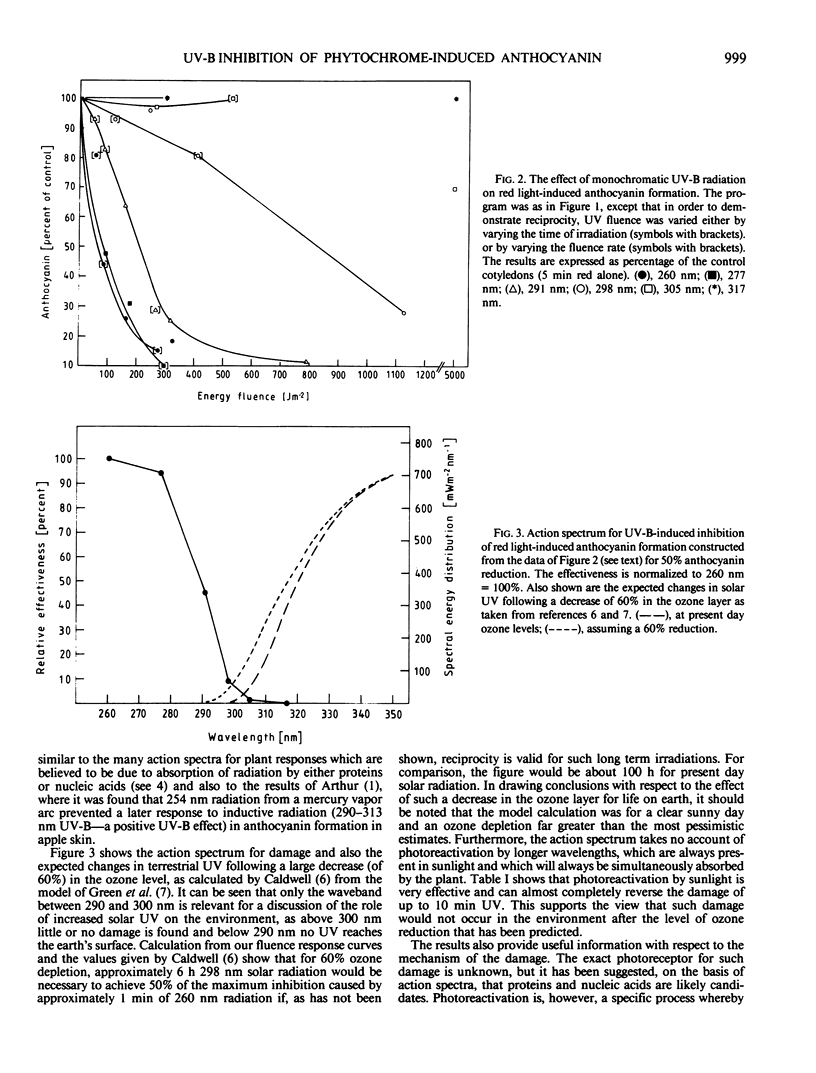
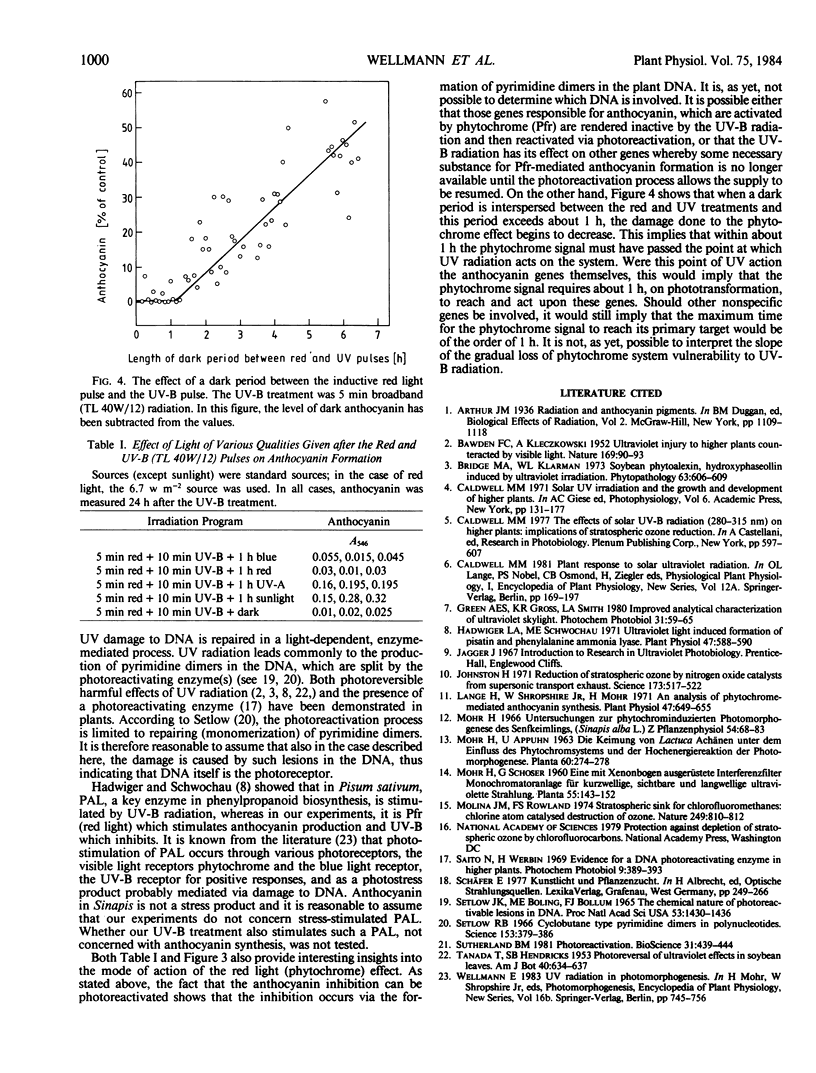
An action spectrum was measured for ultraviolet (UV) radiation-induced damage to (inhibition of) phytochrome-induced anthocyanin formation in cotyledons of 40-hour-old Sinapis alba L. seedlings. The action spectrum showed maximum effectiveness in the 260 to 280 nanometer waveband with little effect above 295 nanometers. The damaging effect of UV could be photorepaired by subsequent exposure to sunlight or to long wavelength (360 nanometers) UV radiation. Because this form of damage is subject to photorepair (photoreactivation), it is probably due to the formation of pyrimidine dimers, and the results suggest that it would not be ecologically relevant even if there was an increase in solar UV due to a decrease in stratospheric ozone levels of about 30%. If a dark period of more than 1 hour is interspersed between the phytochrome induction and the UV irradiation, the inhibition of the phytochrome induction gradually decreases with increasing dark period.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAWDEN F. C., KLECZKOWSKI A. Ultraviolet injury to higher plants counteracted by visible light. Nature. 1952 Jan 19;169(4290):90–91. doi: 10.1038/169090a0. [DOI] [PubMed] [Google Scholar]
- Hadwiger L. A., Schwochau M. E. Ultraviolet Light-induced Formation of Pisatin and Phenylalanine Ammonia Lyase. Plant Physiol. 1971 Apr;47(4):588–590. doi: 10.1104/pp.47.4.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H. Reduction of stratospheric ozone by nitrogen oxide catalysts from supersonic transport exhaust. Science. 1971 Aug 6;173(3996):517–522. doi: 10.1126/science.173.3996.517. [DOI] [PubMed] [Google Scholar]
- Lange H., Shropshire W., Mohr H. An Analysis of Phytochrome-mediated Anthocyanin Synthesis. Plant Physiol. 1971 May;47(5):649–655. doi: 10.1104/pp.47.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N., Werbin H. Evidence for a DNA-photoreactivating enzyme in higher plants. Photochem Photobiol. 1969 Apr;9(4):389–393. doi: 10.1111/j.1751-1097.1969.tb07304.x. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Bollum F. J. The chemical nature of photoreactivable lesions in DNA. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1430–1436. doi: 10.1073/pnas.53.6.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow R. B. Cyclobutane-type pyrimidine dimers in polynucleotides. Science. 1966 Jul 22;153(3734):379–386. doi: 10.1126/science.153.3734.379. [DOI] [PubMed] [Google Scholar]


