Abstract
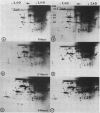
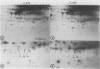
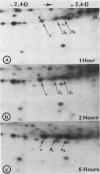
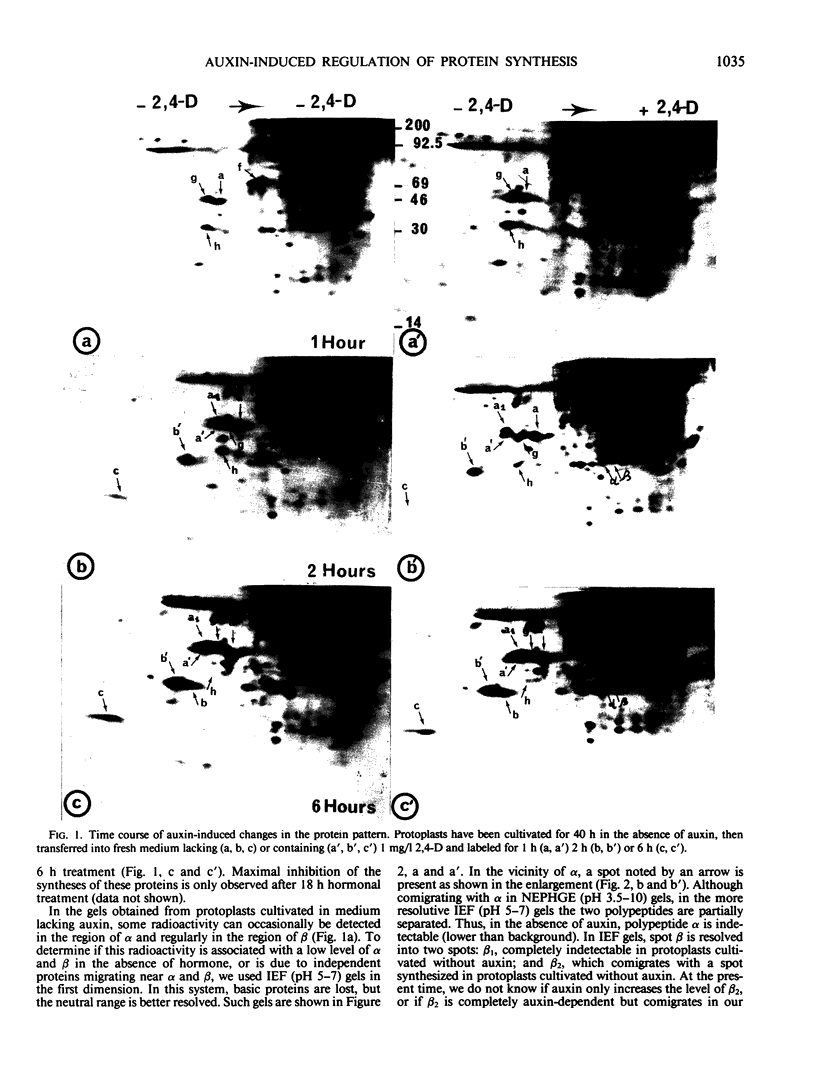
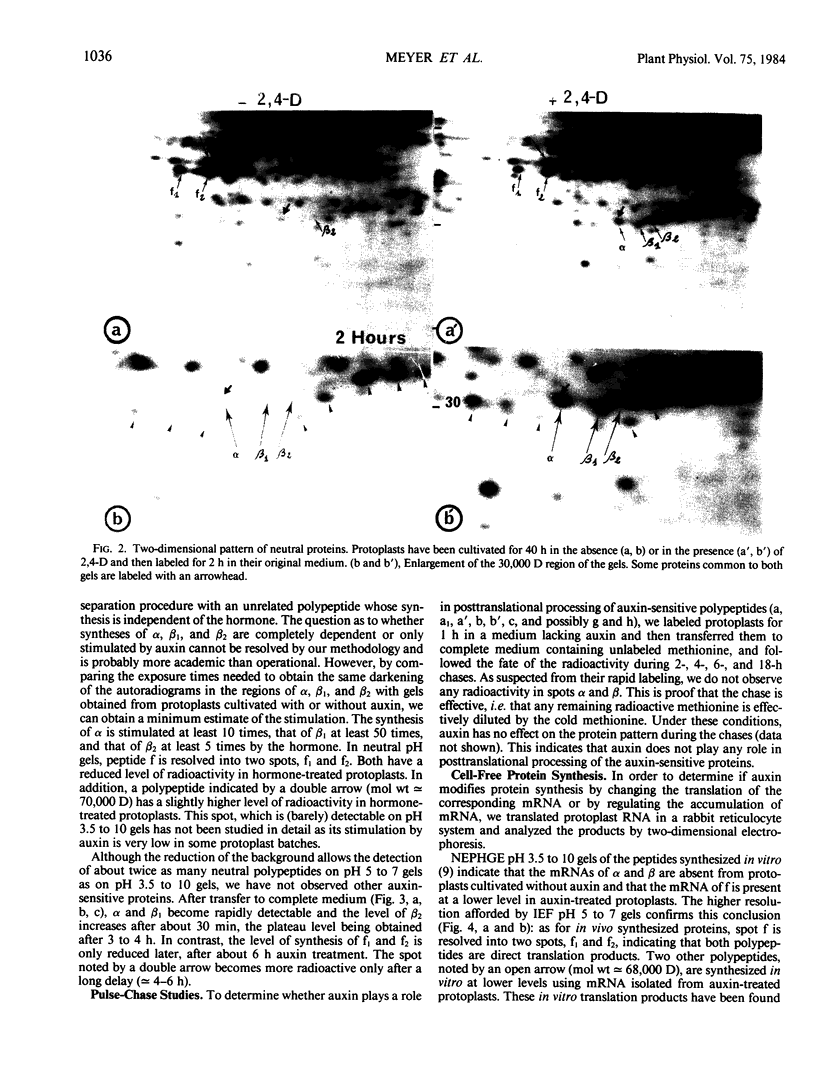
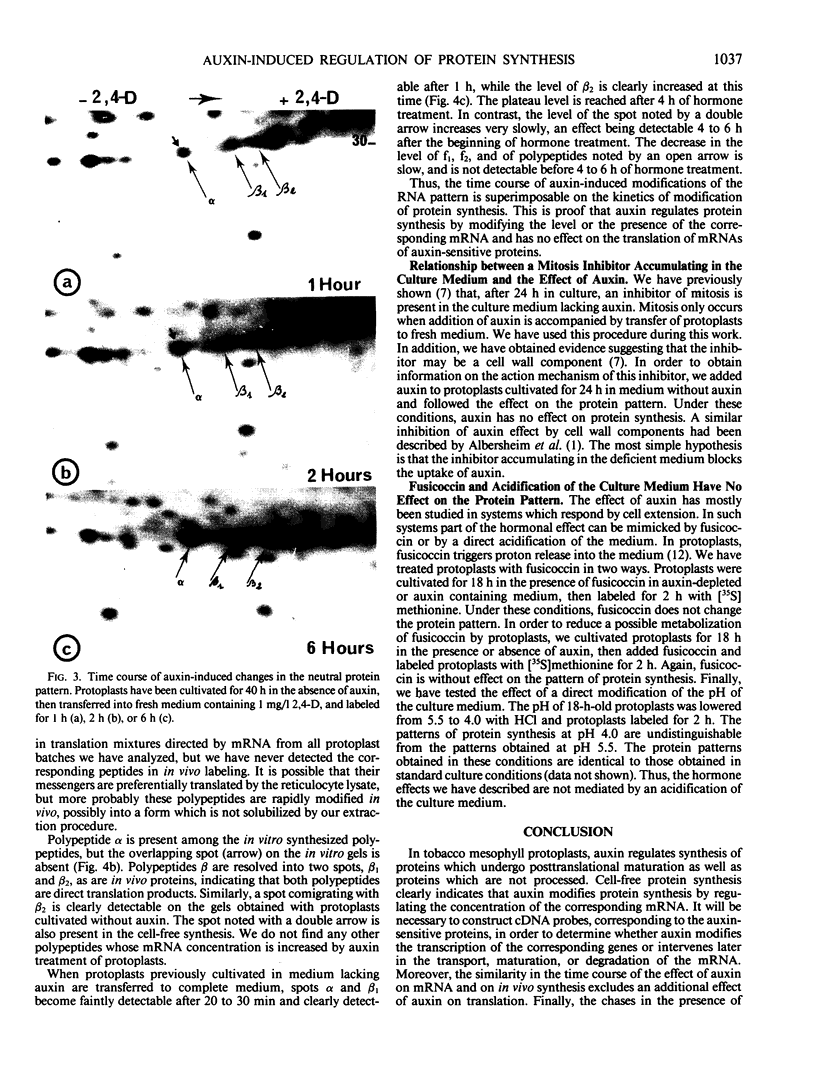
When protoplasts, previously cultivated in a medium lacking auxin, are transferred to complete medium, proteins whose synthesis is stimulated by the hormone become detectable after about 30 minutes and reach a constant level 2 to 4 hours after the beginning of hormonal treatment. In contrast, proteins whose level of synthesis is reduced by auxin, are only affected after 6 hours of treatment.
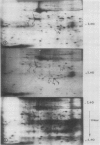
Short radioactive labelings in deficient medium followed by chases in complete medium show that auxin does not interfere with posttranslational processes.
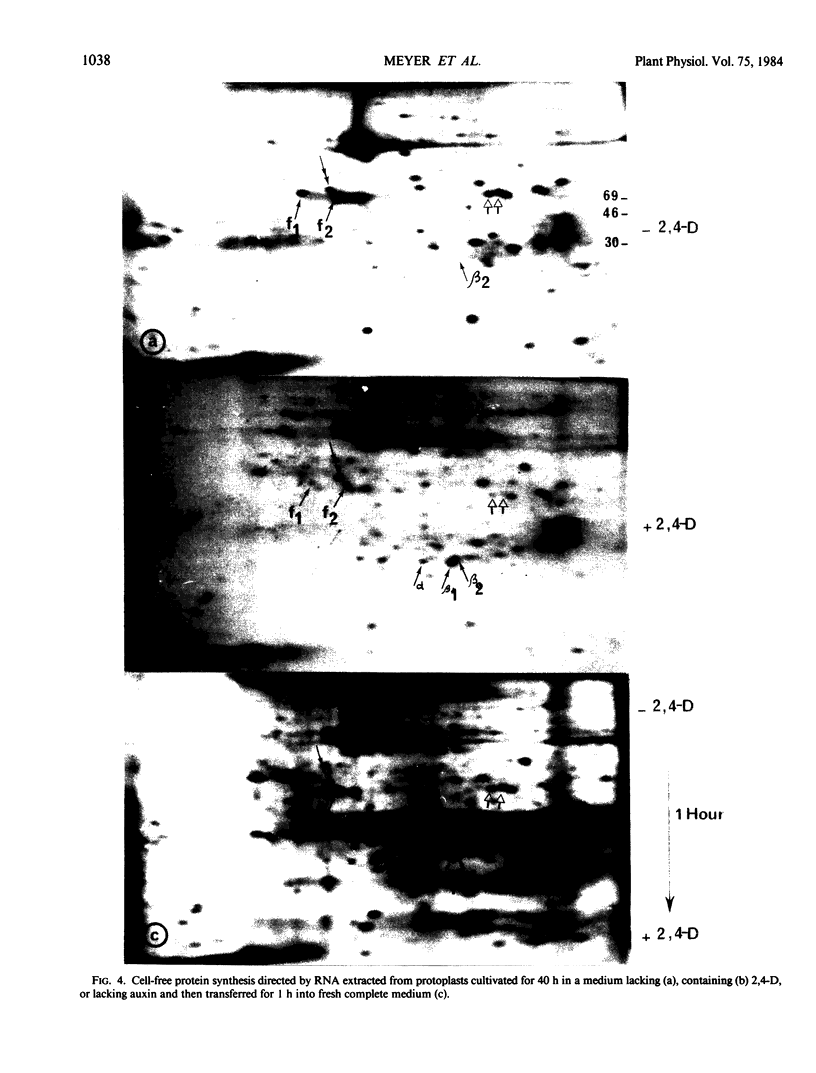
Analysis of in vitro translation products of protoplast RNA shows that the time courses of auxin effects on protein synthesis and mRNA accumulation are perfectly superimposable. This allows us to exclude the possibility that auxin affects the translation process, but indicates that this hormone acts by regulating the concentration of the auxin-sensitive protein mRNAs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpi A., Beevers H. Proteinases and enzyme stability in crude extracts of castor bean endosperm. Plant Physiol. 1981 Mar;67(3):499–502. doi: 10.1104/pp.67.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer Y., Chartier Y. Hormonal Control of Mitotic Development in Tobacco Protoplasts: TWO-DIMENSIONAL DISTRIBUTION OF NEWLY-SYNTHESIZED PROTEINS. Plant Physiol. 1981 Dec;68(6):1273–1278. doi: 10.1104/pp.68.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Theologis A., Ray P. M. Early auxin-regulated polyadenylylated mRNA sequences in pea stem tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):418–421. doi: 10.1073/pnas.79.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhoef L. N., Dute R. R. Auxin-regulated Wall Loosening and Sustained Growth in Elongation. Plant Physiol. 1981 Jan;67(1):146–149. doi: 10.1104/pp.67.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]