Abstract
Simple Summary
We reviewed the available studies assessing salvage surgery after recurrent prostate cancer with primary non-surgical treatment. While the studies used had the potential for bias, due to their retrospective type, we looked at treatment outcomes and toxicity for men treated with a number of salvage radical prostatectomies for recurrent prostate cancer. We demonstrated that SRP can be considered a suitable treatment option for selected patients.
Abstract
The aim of this study was to systematically review the current evidence regarding the oncological and functional outcomes of salvage radical prostatectomy (sRP) for recurrent prostate cancer. A systematic review was conducted throughout September 2022 using the PubMed, Science Direct, Scopus, and Embase databases. Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines were followed to identify eligible studies. A total of 55 studies (3836 patients) met our eligibility criteria. The vast majority of men included had radiation therapy (including brachytherapy) as their first-line treatment (n = 3240, 84%). Other first-line treatments included HIFU (n = 338, 9%), electroporation (n = 59, 2%), proton beam therapy (n = 54, 1.5%), cryotherapy (n = 34, 1%), focal vascular targeted photodynamic therapy (n = 22, 0.6%), and transurethral ultrasound ablation (n = 19, 0.5%). Median preoperative PSA, at the time of recurrence, ranged from 1.5 to 14.4 ng/mL. The surgical approach was open in 2300 (60%) cases, robotic in 1465 (38%) cases, and laparoscopic in 71 (2%) cases. Since 2019, there has been a clear increase in robotic versus conventional surgery (1245 versus 525 cases, respectively). The median operative time and blood loss ranged from 80 to 297 min and 75 to 914 mL, respectively. Concomitant lymph node dissection was performed in 2587 cases (79%). The overall complication rate was 34%, with a majority of Clavien grade I or II complications. Clavien ≥ 3 complications ranged from 0 to 64%. Positive surgical margins were noted in 792 cases (32%). The median follow-up ranged from 4.6 to 94 months. Biochemical recurrence after sRP ranged from 8% to 51.5% at 12 months, from 0% to 66% at 22 months, and from 48% to 59% at 60 months. The specific and overall survival rates ranged from 13.4 to 98% and 62 to 100% at 5 years, respectively. Urinary continence was maintained in 52.1% of cases. sRP demonstrated acceptable oncological outcomes. These results, after sRP, are influenced by several factors, and above all by pre-treatment assessment, including imaging, with the development of mpMRI and metabolic imaging. Our results demonstrated that SRP can be considered a suitable treatment option for selected patients, but the level of evidence remains low.
Keywords: salvage radical prostatectomy, recurrence, prostate cancer, systematic review
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed cancer in men, with an estimated 1.4 million diagnoses recorded worldwide in 2020 [1]. Although active surveillance is increasingly used, most PCa patients undergo definitive local treatment, followed by prostate-specific antigen (PSA) monitoring [2]. However, it is estimated that 27% to 53% of all patients undergoing radical prostatectomy (RP) or radiation therapy (RT) develop biochemical recurrence (BCR) [3]. While there is a standard treatment pathway for post-RP BCR, there is no widely adopted treatment paradigm for BCR after primary nonsurgical treatment. In addition, there have been no randomized trials comparing the oncological outcomes of available salvage therapies, and thus, there is no clear consensus regarding the best treatment option. As such, many patients with BCR after primary nonsurgical treatment receive androgen deprivation therapy (ADT), which denies them any chance of curative therapy [4].
Salvage radical prostatectomy (sRP) is a challenging procedure that is rarely performed, although it represents a guidelines-validated option for BCR after primary nonsurgical treatment. The historical series of sRP with frequent major complications, such as rectal injury and poor functional outcomes [5], have played a major role in the low use of this option in a salvage situation. However, minimally invasive approaches may provide significant improvements, which could lead to improved functional outcomes and reduced complications [6,7]. With the renewed interest in sRP, identifying patients who would benefit most from sRP is crucial to avoid overtreatment and limit treatment-related toxicities.
In this study, we aimed to systematically review the current evidence regarding the oncological and functional outcomes of sRP for recurrent PCa after primary nonsurgical treatment.
2. Methods
2.1. Search Strategy
We conducted a systematic review in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [8].
This protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (Registration Number: CRD42022378227). We conducted a literature search in PubMed/Medline, Embase, and Science Direct databases, to identify reports published through September 2022, which addressed the oncological and functional outcomes of sRP. The search strategy included the following MeSH terms: prostatectomy, Prostate Cancer, Neoplasm Recurrences, treatment, Local, Radiation Therapy, Cryotherapies, Salvage Treatment, Robot-Assisted Surgery, and Surgical Procedure. Initial screening was independently performed by two investigators (A.S. and G.P.) based on the titles and abstracts of the articles to identify ineligible reports. Reasons for exclusions were noted. Potentially relevant reports were subjected to a full-text review, and the relevance of the reports was confirmed after the data extraction process. Disagreements were resolved by consultation with a third co-author (M.B.).
2.2. Study Selection
Studies were deemed eligible if they included men with recurrent PCa after primary nonsurgical treatment (patients), managed with sRP (intervention), and if they assessed oncological and/or functional outcomes (outcome) in randomized controlled trials, nonrandomized prospective studies, and retrospective studies (study design). In case of duplicate publications, either the higher-quality or the most recent publication was selected. Reviews, meta-analyses, editorials, commentaries, authors’ replies, meeting abstracts of unpublished studies, and case reports were excluded, but the reference section was checked for relevant articles. No restriction on the publication language was applied. We searched reports published between January 2008 to September 2022 (Supplementary Material Table S1).
2.3. Data Extraction
Data on studies, patients, treatment, and follow-up were independently extracted by two authors (A.S. and G.P.). We extracted the following variables from the included studies: first author’s name, publication year, sample size, age, pre-sRP PSA, pre- and post-sRP TNM stage, International Society of Urological Pathology (ISUP) score at pre-sRP biopsy, surgical approach, operative time, estimated blood loss, rate and severity of postoperative complications according to the Clavien-Dindo classification, rate of urinary continence, follow-up data, BCR rates, cancer-specific survival, and overall survival.
2.4. Assessment of Methodological Quality
Two authors (A.S. and G.P.) independently assessed the quality of the studies and the risk of bias. The risk of bias was assessed according to EAU recommendations for performing systematic reviews and meta-analysis [9]. The Quality Appraisal tool for case series using a Modified Delphi technique was used for retrospective studies [10].
3. Results
3.1. Study Selection and Characteristics
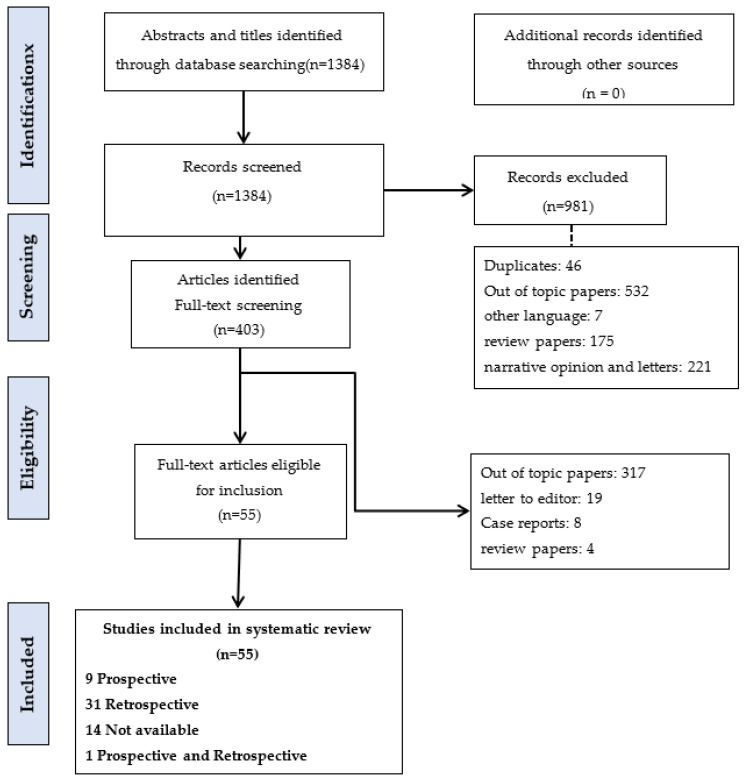
The study selection process is outlined in the PRISMA flow diagram (Figure 1). A total of 403 full-text articles were assessed for eligibility and 55 met our inclusion criteria [7,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64].
Figure 1.
Systematic review PRISMA flow diagram.
The baseline characteristics of the included studies are summarized in Table 1. A total of 3836 patients were included, ranging from 4 to 428 patients per study. The median age of the patients ranged from 59.5 to 71 years, and the median preoperative PSA ranged from 1.5 to 14.4 ng/mL. The vast majority of men included had RT as first-line treatment [Brachytherapy (BT) 632 (16.5%), external-beam radiation therapy (EBRT) 1878 (49%) and BT/EBRT 121 (3%)]. High-Intensity Focused Ultrasound (HIFU) in three hundred and thirty-eight (9%) cases, electroporation in fifty-nine (2%) cases, Proton Beam Therapy (PBT) in fifty-four (1.5%) cases, cryotherapy in thirty-four (1%) cases, focal Vascular Targeted Photodynamic therapy (VTP) in twenty-two (0.6%) cases, transurethral ultrasound ablation (TULSA) in nineteen (0.5%) cases, cryoablation in three (0.07%) cases, cyberknife in two (0.05%) cases, laser ablation in thirteen (0.3%) cases, Cobalt therapy in two (0.05%) cases, and tomography in (0.02%) case. ISUP ≥ 4 was present in 0 to 70% of cases on the initial diagnostic biopsies. Neo-adjuvant androgen deprivation therapy (ADT) at the time of recurrence was used in 266 cases (17%).
Table 1.
Pre-operative characteristics of the included studies.
| Authors (Years) | Number of Patients | Study Type | Inclusion Criteria | Age Median, Years |
Initial Local Therapy Type n (%) |
Pre-sRP ADT n (%) |
Clinical Staging n (%) | Pre-sRP Biopsy n (%) ≥ISUP 4 |
Pre-sRP PSA Median (ng/mL) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| ≤T2 | T3≥ | |||||||||
| Kaouk et al. (2008) [13] | 4 | Retrospective | -a life-expectancy of >10 years -biopsy confirmed recurrence of PCa |
NA | -BT 2 (50) -BT/EBRT 2 (50) |
2 (50) | 3 (75) | NA | 1 (25) | Mean 3.84 |
| Liatsikos et al. (2008) [14] | 12 | NA | -proven biochemical failure of other alternative therapeutic approaches | Mean 63.3 | -HIFU 4 (33) -EBRT 6 (50) -BT 2 (17) |
NA | NA | NA | Mean 12.7 | |
| Kim et al. (2008) [15] | 7 | NA | Biopsy proven, local recurrences | Mean 65.5 | -EBRT 6 (86) -IMRT 1 (14) |
NA | NA | NA | NA | Mean 14.3 |
| Boris et al. (2009) [16] | 11 | Retrospective | -TRUS-guided prostate biopsies that showed persistent cancer after RT -negative preoperative CT and bone scans |
Mean 65 | -BT6 (55) -EBRT 3 (27) -BT/EBRT 1 (9) -IMRT 1 (9) |
0 (0) | 11 (100) | 0 (0) | 3 (27) | Mean 5.2 |
| Seabra et al. (2009) [17] | 42 | Prospective | -biopsy confirmed Recurrence of PCa | 61 | -EBRT | NA | 42 (100) | 0 (0) | 3 (7) | Mean 1.5 |
| Leonardo et al. (2009) [18] | 32 | NA | a life expectancy of more than 10 years, absence of systemic disease and persistent PCa detected by biopsy | 63 | -EBRT | 5 (16) | 25 (78) | 7 (22) | 12 (37.5) | 13 |
| Nunez-Mora et al. (2009) [19] | 9 | NA | All recurrence was histologically confirmed | 59.3 | -BT 5 (55.5) -EBRT 4 (44.5) |
1 (11) | NA | 6 (67) | 9.1 | |
| Paparel et al. (2009) [20] | 146 | Retrospective | -A life expectancy >10 yr -clinically localized prostate cancer determined by biopsy -absence of significant voiding symptoms or urinary incontinence -a negative evaluation for systemic disease |
65 | radiation therapy | NA | 58 (42) | 79 (58) | 16 (11) | 5.1 |
| Heidenreich et al. (2010) [21] | 55 | NA | -A life expectancy >10 yr -clinically organ-confined disease -Absence of locoregional and systemic metastases, -PSA 20 ng/mL |
(3D) EBRT: 19 (34.5) EBRT + BT: 15 (27.5) Seed implantation: 21 (38) |
NA | 44 (80) | 11 (20) | 10 (18.) | <10: 45 (82) 10.1–20: 10 (18) |
|
| Strope et al. (2010) [22] | 6 | Prospective | -biopsy documented locally recurrent or radiation-resistant prostate cancer -Only patients with no evidence of metastatic disease on bone scan and CT scan |
NA | -BT 2 (34) -EBRT 4 (66) |
NA | NA | 2 (34) | Mean 9.3 |
|
| Eandi et al. (2010) [23] | 18 | Retrospective | -Biochemical failure after irradiation |
67 | -BT 8 (44) -EBRT 8 (44) -PBT 2 (12) |
4 (22) | NA | 6 (33) | 6.8 | |
| Chauhan et al. (2011) [24] | 15 | Retrospective | -had biopsy-proven recurrent PCa | 62 | -EBRT 5 (33) -BT 5 (33) -PBT 2 (14) -XRT + BT 3 (20) |
NA | 15 (100) | 0 (0) | 3 (20) | 3.6 |
| Chade et al. (2011) [25] | 404 | Retrospective | -biopsy confirmed Recurrence of PCa |
65 | -BT, EBRT 11 (3) -BT, EBRT, IMRT 2 (0) -BT alone 76 (19) -EBRT, 3-DCRT 5 (1) -EBRT, IMRT 5 (1) -EBRT alone 253 (63) -Unknown 52 (13) |
0 (0) | 262 (55) | 72 (18) | 80 (20) | 4.5 |
| Gorin et al. (2011) [26] | 24 | Retrospective | life expectancy of at least 10 years and a negative metastatic workup | Mean 64.5 | -EBRT 13 (54) -BT 11 (46) |
14 (58) | NA | 9 (37.5) | Mean 8.7 |
|
| Ahallal et al. (2011) [27] | 15 | Retrospective | biopsy-proven local recurrence after cryotherapy or radiation therapy for localized prostate cancer | 62.3 | -EBRT 8 (53) -BT 6 (40) -cryotherapy 1 (7) |
NA | 12 (80) | 1 (7) | 4 (27) | 3.5 |
| Lawrentschuk et al. (2011) [28] | 15 | Prospective | men presenting with an increasing PSA and biopsy-proven PC after primary therapy with HIFU |
64 | HIFU | 1 (7) | 15 (100) | 0 (0) | 0 (0) | 7 |
| Leonardo et al. (2012) [29] | 13 | Retrospective | biopsy-proven local recurrence after HIFU |
61.3 | HIFU | NA | 13 (100) | 0 (0) | 0 (0) | 3.31 |
| Kaffenberger et al. (2013) [30] | 34 | Retrospective | failure of prior definitive therapy | 66.5 | -BT 13 (38) -EBRT 11 (32) -combined BT/EBRT 6 (18) -HIFU 4 (12) |
4 (12) | 32 (94) | 2 (6) | 12 (35) | 3.86 |
| Peters et al. (2013) [31] | 44 | Retrospective | All men showed PSA failure afterward, and recurrences were confirmed by biopsies |
65 | -EBRT 31 (70) -BT 2 (5) -I-125 11 (25) -IMRT 0 (0) |
5 (11) | 30 (69) | 14 (31) | 7 (16) | 0–10 24 (55) >10–20 18 (41) >20 2 (5) |
| Yuh et al. (2014) [32] | 51 | Prospective | -BCR -biopsy confirmed recurrence of PCa -negative CT results and bone scans |
68 | -BT 22 (43.1) -BT+EBRT 1 (2) -Cryoablation 3 (5.9) -EBRT 18 (35.3) -HIFU 1 (2.0) -PBT 6 (11.8) |
18 (19.6) | NA | NA | 5.27 | |
| Zugor et al. (2014) [33] | 13 | Retrospective | -radiation-resistant PCa | 63 | -EBRT 7 (54) -BT 6 (46) |
NA | 13 (100) | 0 (0) | 5 (38.6) | 14.4 |
| Saeedi et al. (2014) [34] | 6 | NA | biopsy confirmed recurrence of PCa |
Mean 59.5 | BT | 2 (33) | 6 (100) | 0 (0) | 0 (0) | 4.2 |
| Bates et al. (2015) [11] | 53 | Retrospective | -PSA concentration >0.2 ng/mL -biopsy confirmed recurrence of PCa |
67 | -EBRT 28 (52.8) -BT 14 (26.4) -IMRT 5 (9.4) -cryotherapy 3 (5.6) -HIFU 3 (5.6) |
NA | 44 (83) | 9 (17) | 16 (30.2) | 3.7 |
| Pokala et al. (2015) [35] | 364 | NA | men 40 to 75 years of age with radio-recurrent prostate cancer |
Mean 64 |
-BT -EBRT -or a combination with the both |
NA | NA | NA | NA | |
| Pearce et al. (2015) [36] | 408 | NA | men with adenocarcinoma of the prostate and those who presented with nonmetastatic disease and no nodal involvement |
Mean 62.5 |
-EBRT 348 (89) -BT 43 (11) |
NA | 167 (63.5) | 96 (36.5) | NA | Mean 12.6 |
| Lebdai et al. (2015) [37] | 19 | Retrospective | biopsy-proven locally persistent or recurrent prostate cancer | 64 | -Focal VTP | NA | NA | 0 (0) | 6.3 | |
| Mandel et al. (2016) [38] | 55 | Retrospective | Low comorbidity, life expectancy of at least 10 years, organ-confined PCa <T2b, Gleason score ≤ 7 and preoperative PSA <10 ng/mL |
Mean 65.4 |
-EBRT 27 (49) -HDR 7 (12.7) -LDR 17 (31) -HIFU 4 (7.3) |
25 (45) | NA | 13 (23.6) | 9.5 | |
| Vora et al. (2016) [39] | 6 | Retrospective | -PSA < 10 ng/mL at recurrence -life expectancy > 10 years at recurrence -negative metastatic workup. |
64.7 | RT | NA | NA | NA | 6.08 | |
| Kenney et al. (2016) [7] | 39 | Retrospective | -BCR after radiation therapy -biopsy confirmed Recurrence of PCa |
66 | -EBRT or PBT 24 (61.5) -BT or BT/EBRT 15 (38.5) |
8 (40) | 31 (79.5) | 6 (15) | 14 (36) | Mean 3.5 |
| Orré et al. (2016) [40] | 7 | NA | Biochemical relapse | 66 | permanent brachytherapy implants | NA | 7 (100) | 0 (0) | 2 (28.5) | 7.13 |
| Vidmar et al. (2017) [41] | 24 | Retrospective | -recurrent or radiation-resistant prostate cancer |
62 | -BT 7 (29) -HIFU 7 (29) -EBRT 10 (42) |
0 (0) | 21 (87) | 2 (8) | 0 (0) | 5.5 |
| Metcalfe et al. (2017) [42] | 70 | Retrospective | biochemical or biopsy-proven failure | 61.06 | -EBRT 42 (60) -BT 14 (20) -Proton 6 (8.6) -EBRT + BT 8 (11.4) |
18 (26) | 60 (88) | 8 (11) | 10 (14) | 5.95 |
| Ogaya-Pinies et al. (2019) [43] | 96 | Prospective | all patients with a localized, biopsy-proven PCa recurrence after radiotherapy or any ablative technique, with a life expectancy of >10 years |
65.75 | -EBRT 37 (38.5) -BT 14 (14.5) -EBRT+BT 13 (13.5) -Cyberknife 3 (3) -Proton beam 1 (1) -Cryotherapy 18 (19) -HIFU 7 (8) -Focal VTP 1 (1) -Electroporation 1 (1) -TULSA 1 (1) |
NA | NA | NA | 4 | |
| Onol et al. (2019) [44] | 94 | Retrospective | -biopsy-proven local recurrence without evidence of metastatic PCa | 65 | -EBRT 39 (31) -IMRT 15 (12) -PBT 3 (2) -BT23 (18) -combined EBRT + BT 14 (11) |
24 (25.5) | 91 (97) | 3 (3) | 36 (38) | Mean 4.53 |
| Devos et al. (2019) [45] | 25 | Retrospective | -BCR -a positive biopsy following EBRT or BT |
65 | -EBRT17 (68) -BT 8 (32) |
NA | NA | 12 (48) | 4.6 | |
| Mohler et al. (2019) [46] | 41 | prospective | -biopsy-proven persistent or recurrent CaP diagnosed ≥ 18 months after radiation therapy with PSA ≤ 20 ng/mL -no radiologic evidence of metastatic disease |
64 | -EBRT 24 (58) -BT 11 (27) -Combined 6 (15) |
0 (0) | 24 (58.5) | 2 (5) | 3 (7) | 4.1 |
| Clery et al. (2019) [47] | 55 | NA | -All patients received radiation therapy -BCR was biopsy-proven in all cases |
64 | -EBRT 30 (55) -BT 10 (18) -HIFU 15 (27) |
8 (14.5) | 45 (81.5) | 2 (4) | 3 (5.5) | 4.96 |
| Herrera-Caceres et al. (2019) [48] | 34 | Retrospective | PCa recurrence after focal therapy | 61 | -Laser ablation 13 (38) -HIFU 19 (56) -Cryotherapy 1 (3) -BT 1 (3) |
NA | NA | 4 (12) | 5.38 | |
| Gontero et al. (2019) [49] | 395 | Retrospective | recurrent PCa | 66.3 | NA | NA | NA | 147 (39) | 6.36 | |
| De Groote et al. (2020) [50] | 106 | Retrospective | -All patients received radiation therapy -All patients had biopsy |
67 | -HIFU 59 (56) -RT27 (25) -BT 10 (9) -ADT 8 (8) -cryotherapy 1 (1) -electroporation /Nanoknife 1 (1) |
8 (8) | 58 (55) | 48 (45) | 27 (25) | 5.6 |
| Nair et al. (2020) [51] | 4 | NA | Recurrent CaP | 69 | transurethral ultrasound ablation (TULSA) | NA | NA | 0 (0) | 4.3 | |
| Thompson et al. (2020) [52] | 53 | Retrospective | Unsuitable for redo FA (e.g., bilateral/ high-risk cancer) or preference towards radical treatment; Age < 75 yo and fit for major surgery; T1-3aN0M0, surgically resectable on MRI and DRE; Accepting of risks and side effects of surgery. |
63 | -HIFU | NA | 40 (89) | 5 (11) | 5 (11) | 6 |
| Nathan et al. (2021) [53] | 135 | Retrospective | Primary treatment failure | 70 | Whole gland therapies: -RT -BT -HIFU Focal gland therapies: -focal HIFU, cryotherapy, and electroporation |
NA | 80 (55) | 61 (45) | 35 (26) | 5.8 |
| Madi et al. (2021) [12] | 26 | Retrospective | -All patients had biopsy-proven prostate cancer recurrence. |
68.5 | -EBRT 18 (69) -BT 4 (15) -Cyberknife 2 (8) -Cryotherapy 2 (8) |
NA | NA | 13 (50) | 5.1 | |
| Rajwa et al. (2021) [54] | 214 | Retrospective | -patients treated with primary radiation therapy-all patients underwent confirmatory biopsy |
69 | -EBRT 167 (78) -BT39 (18) -EBRT + BT 8 (3.7) |
0 (0) | 183 (85.5) |
30 (14) | 86 (40) | 3.8 |
| Bozkurt et al. (2021) [55] | 10 | NA | -clinically organ-confined PCA disease after failure of PBT |
66.8 | PBT | NA | 10 (100) | 0 (0) | 7 (70) | Mean 5.5 |
| Marra et al. (2021) [56] | 414 | Retrospective | Recurrent CaP | 66 | -EBRT 262 (64.5) -BT 106 (25.7) -other primary treatments 56 (13.6) |
NA | NA | 48 (11.5) | 140 (35) | 4.2 |
| Spitznagel et al. (2021) [57] | 13 | Prospective | patients with any detected PCa in the extended follow-up biopsy | 61 | HIFU | NA | 13 (100) | 0 (0) | 0 (0) | 5.4 |
| Wenzel et al. (2021) [58] | 428 | NA | adult patients (≥18 years old) with histologically confirmed adenocarcinoma of the prostate, diagnosed at biopsy |
66 | -EBRT: 316 (74) -BT: 67 (16) -EBRT + BT: 45 (10.5) |
NA | 356 (83) | 43 (11) | 62 (14.5) | 8.8 |
| von Hardenberg et al. (2021) [59] | 44 | Prospective | biopsy-proven (PCa) after FT | 65 | -HIFU 42 (95.5) -VTP 2 (4.5) |
6 (14) | NA | 0 (0) | 5.7 | |
| Nathan et al. (2022) [60] | 100 | Prospective Retrospective |
locally recurrent prostate cancer after ablative therapy failure |
69 | -HIFU 92 (92) -Cryotherapy 5 (5) -Electroporation 3 (3) |
100 (100) | 81 (81) | 19 (19) | 10 (10) | 5.8 |
| Mortensen et al. (2022) [61] | 5 | Retrospective | -BCR following primary external beam radiation -an expected life expectancy of 10 years or more |
71 | EBRT | 5 (100) | 0 (0) | 5 (100) | 4 (80) | Mean 3.34 |
| Van Riel et al. (2022) [62] | 39 | Prospective | Recurrent localised PCa | 64 | irreversible electroporation |
NA | 39 (100) | 0 (0) | 0 (0) | 6 |
| Blazevski et al. (2022) [63] | 15 | Retrospective | patients with histopathologically confirmed residual or recurrent clinically signifcant PCa |
68 | irreversible electroporation |
NA | NA | 0 (0) | 6.6 | |
| Catarino et al. (2022) [64] | 29 | NA | histologically confirmed recurrent PC | 65 | -BT 9 (31) -EBRT 16 (55) -Cobalt therapy 2 (7) -Tomotherapy 1 (3) -BT+ EBRT 1 (3) |
8 (28) | NA | NA | NA | |
PSA: prostate specific antigen, ADT: androgen deprivation therapy, RT: radiation therapy, sRP: salvage radical prostatectomy, ISUP: International Society of Urological Pathology, NA: not available.
3.2. Perioperative Results
The perioperative data are presented in Table 2. Regarding the surgical approach, an open approach was used in 2300 cases (60%), a robotic approach in 1465 cases (38%), and a laparoscopic approach in 71 cases (2%); but since 2019, there has been more frequent use of robotic versus conventional surgery (1245 versus 525 of cases, respectively).
Table 2.
Intra-operative parameters and pathological features of the overall cohort.
| Authors | Surgical Approach n (%) |
Operative Time (min) |
Blood Loss (mL) |
Lymph Node Dissection n (%) |
≥pT3, n (%) |
sRP ISUP ≥4 n (%) |
pN+ n (%) |
PSM n (%) |
Complications * n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kaouk et al. (2008) [13] | RARP: 4 (100) | 125 | 117 | 4 (100) | NA | 2 (67) | 0 (0) | 2 (50) | 0 (0) |
| Liatsikos et al. (2008) [14] | LRP: 12 (100) | 153 | 238 | 8 (66) | 4 (33) | 5 (42) | 0 (0) | 3 (25) | 1 (8) |
| Kim et al. (2008) [15] | Open: 5 (71) RARP: 2 (29) | 292 | 914 | 7 (100) | 2 (28.5) | NA | NA | 1 (14) | 2 (28.5) |
| Boris et al. (2009) [16] | RARP: 11 (100) | 183 | 113 | Standard template 7 (64) Extended template 4 (36) |
8 (73) | 3 (27) | 2 (18) | 3 (27) | 3 (27) |
| Seabra et al. (2009) [17] | NA | 80 | 300 | NA | 11 (26) | 6 (14) | NA | NA | Grade 3a: 21 (50) Grade 3b: 2 (4.8) |
| Leonardo et al. (2009) [18] | Open: 32 (100) | 122 | 550 | 32 (100) | 15 (5) | 20 (6) | 0 (0) | 11 (3) | 4 (12.5) |
| Nunez-Mora et al. (2009) [19] | LRP: 9 (100) | 170 | 250 | 9 (100) | 5 (55.5) | 6 (66) | 2 (22) | 2 (22) | 2 (22) |
| Paparel et al. (2009) [20] | NA | NA | NA | NA | NA | 29 (20) | 18 (13) | 24 (16) | NA |
| Heidenreich et al. (2010) [21] | Open: 55 (100) | 120 | 360 | 55 (100) | 13 (24) | 9 (20) | 9 (20) | 5 (11) | Grade 1: 13 (23.6) Grade 2: 2 (3.6) Grade 3: 2 (3.6) |
| Strope et al. (2010) [22] | RARP: 6 (100) | 356 | 280 | 6 (100) | 1 (16) | NA | 0 (0) | 1 (16) | 2 (34) |
| Eandi et al. (2010) [23] | RARP: 18 (100) | 156 | 150 | 18 (100) | 9 (50) | 4 (22) | NA | 5 (28) | 7 (39) |
| Chauhan et al. (2011) [24] | RARP: 15 (100) | 138 | 75 | 12 (80) | 10 (77) | 7 (47) | 1 (6.6) | 2 (13) | Grade 1: 1 (7) Grade 2: 1 (7) Grade 3: 1 (7) |
| Chade et al. (2011) [25] | Open: 404 (100) | NA | NA | 58 (14) | NA | 96 (24) | 65 (16) | 99 (25) | NA |
| Gorin et al. (2011) [26] | Open: 24 (100) | NA | 415 | 15 (63) | 13 (54) | NA | 2 (13.3) | 11 (46) | NA |
| Ahallal et al. (2011) [27] | Open: 11 (73) RARP: 4 (27) |
235 | 200 | 15 (100) | 9 (60) | 7 (47) | 2 (13) | 2 (13) | Grade 1: 3 (20) Grade 2: 2 (13) Grade 3: 0 (0) |
| Lawrentschuk et al. (2011) [28] | Open: 15 (100) | 135 | NA | 13 (87) | 9 (64) | 4 (27) | NA | 4 (27) | 1 (7) |
| Leonardo et al. (2012) [29] | LRP: 12 (100) | 220 | 150 | 13 (100) | 8 (61.5) | 2 (15) | 0 (0) | 2 (15) | Grade 1: 3 (23) Grade 2: 1 (8) Grade 3: 2 (15) |
| Kaffenberger et al. (2013) [30] | RARP: 34 (100) | 176 | NA | 29 (85) | 16 (47) | 9 (26) | N+: 0 (0) Nx: 5 (15) |
9 (26) | Grade 1: 11 (32) Grade 2: 1 (3) Grade 3: 1 (3) |
| Peters et al. (2013) [31] | Open: 44 (100) | NA | NA | NA | NA | NA | NA | NA | NA |
| Yuh et al. (2014) [32] | RARP: 51 (100) | 179 | 175 | 43 (84) | 26 (51) | 11 (21.6) | 3 (6) | 16 (31.4) | Grade 1 2: 13 (25.5) Grade 3 4: 22 (43) |
| Zugor et al. (2014) [33] | RARP: 13 (100) | 154 | 130 | 13 (100) | 6 (46) | 7 (54) | 0 (0) | 0 (0) | Minor complications 4 (30.7) Grade 1: 2 (15.3) Grade 3a: 2 (15.3) Major complications 0 (0) |
| Saeedi et al. (2014) [34] | Open: 6 (100) | NA | NA | 6 (100) | 1 (17) | 0 (0) | 1 (17) | 2 (33) | 2 (33) |
| Bates et al. (2015) [11] | RARP: 53 (100) | 128 Console time: 80 |
100 | NA | 26 (49) | 19 (36) | NA | 10 (19) | Grade 1 2: 1 (2) Grade 3 4: 0 (0) |
| Pokala et al. (2015) [35] | NA | NA | NA | 286 (79) | 186 (51) | NA | 40 (11) | NA | NA |
| Pearce et al. (2015) [36] | NA | NA | NA | 273 (75) | 169 (49) | 19 (6.2) | 122 (30) | 124 (34) | NA |
| Lebdai et al. (2015) [37] | Open: 12 (63) RARP: 5 (26) LRP: 2 (11) |
150 | 400 | 19 (100) | 7 (37) | 1 (5) | 1 (5) | 9 (47) | Grade 1: 1 (5) Grade 2: 1 (5) Grade 3: 1 (5) |
| Mandel et al. (2016) [38] | Open: 55 (100) | NA | 725 | 55 (100) | 22 (40.5) | 13 (23.6) | 12 (22) | 15 (27.5) | Grade 3: 7 (12.7) |
| Vora et al. (2016) [39] | RARP: 6 (100) | NA | NA | NA | NA | NA | NA | NA | 1 (16.7) |
| Kenney et al. (2016) [7] | Open: 19 (49) RARP: 20 (51) |
297 | 623 | 39 (100) | 24 (61.5) | 18 (46) | 5 (13) | 6 (15) | Grade 1 2: 43 (77) Grade 3 4: 13 (23) |
| Orré et al. (2016) [40] | RARP: 7 (100) | 142 | NA | 2 (28.5) | 5 (71) | NA | 4 (57) | 1 (14) | 2 (28.5) |
| Vidmar et al. (2017) [41] | RARP: 12 (50) Open: 12 (50) |
180 | 300 | 4 (33) | 7 (63) | 4 (44) | 1 (8) | 6 (50) | 0 (0) |
| Metcalfe et al. (2017) [42] | NA | NA | NA | 70 (100) | 42 (60) | 14 (20) | 38 (54) | 14 (20) | NA |
| Ogaya-Pinies et al. (2019) [43] | RARP: 96 (100) | 125 | 100 | 85 (89) | 22 (23) | 8 (8) | 29 (30) | 16 (17) | Grade 1: 20 (21) Grade 2: 1 (1) Grade 3: 3 (3) Grade 4: 1 (1) |
| Onol et al. (2019) [44] | RARP: 126 (100) | 129 Console time: 84 |
107 | 94 (100) | 47 (50) | 40 (42.6) | 10 (10.6) | 16 (17) | Clavien 1: 9 (9.7) Clavien 2: 11 (11.8) Clavien 3a: 2 (2.2) Clavien 3b: 1 (1.1) Clavien 4a: 1 (1.1) |
| Devos et al. (2019) [45] | Open: 23 (92) RARP: 2 (8) |
166 | 808 | 23 (92) | 14 (56) | 12 (48) | 7 (28) | 11 (44) | 22 (100) Grade 1: 1 (4) Grade 2: 5 (20) Grade 3: 16 (64) |
| Mohler et al. (2019) [46] | Open: 41 (100) | 213 | NA | 41 (100) | 23 (57) | 24 (58.5) | 5 (12) | 7 (17) | 44 (100) |
| Clery et al. (2019) [47] | RARP: 44 (80) Open: 11 (20) |
150 | 300 | 55 (100) | 31 (56) | 15 (27) | 6 (11) | 4 (7) | Grade 1: 44 (80) Grade 2: 1 (1.8) Grade 3: 2 (2.3) |
| Herrera-Caceres et al. (2019) [48] | Open: 28 (82) LRP: 1 (3) RARP: 5 (15) |
NA | 512 | 34 (100) | 20 (59) | 2 (6) | NA | 13 (38) | Intraoperative complications: Cystotomy 2 (6) |
| Gontero et al. (2019) [49] | Open: 186 (47) RARP: 209 (53) |
221 | 468.5 | 337 (85) | 217 (55) | 170 (43) | 63 (16) | NA | 146 (37) |
| De Groote et al. (2020) [50] | RARP: 106 (100) | 142 | 200 | NA | 70 (66) | 23 (22) | NA | RT: 14 (52) | 8 (8) Grade 3a: 1 (1) |
| Nair et al. (2020) [51] | Open: 4 (100) | 210 | 866 | 4 (100) | 3 (75) | 1 (25) | NA | 2 (50) | 1 (25) |
| Thompson et al. (2020) [52] | RARP: 53 (100) | Console time: 140 | 200 | NA | 34 (64.5) | 5 (11) | NA | 23 (44) | Grade 1: 4 (9) Grade 2: 3 (7) Grade 3: 1 (2) |
| Nathan et al. (2021) [53] | RARP: 135 (100) | 165 | 200 | 25 (18.5) | 77 (57) | 26 (29) | NA | 51 (38) | Grade 1: 9 (7) Grade 2: 7 (5) Grade 3–5: 2 (1.5) |
| Madi et al. (2021) [12] | RARP: 26 (100) | 170.5 | 75 | 26 (100) | 11 (42) | 15 (58) | 1 (4) | 8 (31) | 4 (15) Grade 1: 1 (4) Grade 2: 0 (0) Grade 3: 3 (11) |
| Rajwa et al. (2021) [54] | NA | 198 | 600 | 214 (100) | 159 (74) | 86 (40.1) | 40 (19) | 43 (20) | Grade 1: 21 (9.8) Grade 2: 167 (78) Grade 3: 26 (12) |
| Bozkurt et al. (2021) [55] | RARP: 10 (100) | 230.7 | 745 | 10 (100) | 8 (80) | 6 (60) | 2 (20) | 2 (20) | Grade 1–2: 19 (90) Grade 3–4: 5 (30) |
| Marra et al. (2021) [56] | Open: 216 (52) RARP: 198 (48) |
186.5 | 300 | 349 (84.3) | 218 (53) | 151 (40) | 65 (16.0) | 122 (29.7) | Grade 1–2: 144 (41.5) Grade 3–4: 65 (19) |
| Spitznagel et al. (2021) [57] | RARP: 13 (100) | 260 | 230 | 13 (100) | 3 (23) | 1 (8) | 1 (8) | 1 (8) | Grade 1: 2 (15) Grade 2: 0 (0) Grade 3: 4 (31) |
| Wenzel et al. (2021) [58] | NA | NA | NA | 428 (100) | 47 (11) | 17 (4) | 24 (6) | NA | NA |
| von Hardenberg et al. (2021) [59] | Open: 16 (36) LRP: 3 (7) RARP: 25 (57) |
NA | NA | NA | 14 (32) | 16 (36) | 3 (7) | 10 (23) | NA |
| Nathan et al. (2022) [60] | RARP: 100 (100) | 170 | 200 | NA | NA | NA | NA | 38 (38) | Grade 1: 6 (6) Grade 2: 2 (2) Grade 3: 1 (1) |
| Mortensen et al. (2022) [61] | RARP: 5 (100) | 205 | 120 | 0 (0) | 3 (60) | NA | 3 (60) | 3 (60) | Grade 1: 3 (60) Grade 2: 1 (20) |
| Van Riel et al. (2022) [62] | LRP: 3 (8) RARP: 36 (92) |
NA | 182 | 9 (23) | 18 (46) | 8 (21) | 0 (0) | 10 (26) | NA |
| Blazevski et al. (2022) [63] | RARP: 15 (100) | NA | 200 | 4 (27) | 6 (40) | 6 (40) | 1 (7) | 1 (7) | 0 (0) |
| Catarino et al. (2022) [64] | LRP: 29 (100) | 90 | 200 | 25 (86) | 19 (65.5) | 13 (45) | 5 (17) | 8 (28) | Grade 2: 4 (14) Grade 3: 3 (10) |
NA: not available, sRP: salvage radical prostatectomy, PSM: positive surgical margins, RT: radiation therapy. * According to classification of Clavien-Dindo.
A total of 45 studies reported data on concomitant lymph node dissection. The median number of nodes yielded was reported in 14 studies and ranged from 6 to 17, including 593 (20.5%) patients which were staged pN+ at final pathology.
Regarding pathological features, stage ≥pT3, positive surgical margins, and pN+ status ranged from 5 to 75%, 25 to 82%, and 3 to 60%, respectively. The pathological Gleason score was ≥8 in 6 to 67% of cases. These data were missing in nine studies.
3.3. Complications and Functional Results
The reported postoperative complications are summarized in Table 2. The overall complication rate was 34%; with a majority of Clavien grade I or II complications. Clavien grade ≥3 complications ranged from 0 to 64%. The complete urinary continence rate (no pad use) was 52.1% (Table 3). The rates of urinary continence were 56% and 47%, respectively, in minimally invasive (i.e., laparoscopic and robotic) and open approaches.
Table 3.
Oncological and functional outcomes of the overall cohort.
| Authors | Median Follow-Up (Months) |
BCR n (%) | Recurrence Free Survival (RFS) (%) |
Cancer-Specific Survival (%) | Overall Survival (%) | Urinary Continence n (%) |
|---|---|---|---|---|---|---|
| Kaouk et al. (2008) [13] | 5 | NA | NA | NA | NA | At 1 month: 3 (75) |
| Liatsikos et al. (2008) [14] | Mean 20 | At 12 months: 1 (8) | NA | NA | NA | 10 (83) |
| Kim et al. (2008) [15] | NA | NA | NA | NA | NA | NA |
| Boris et al. (2009) [16] | 20.5 | At 43 months: 3 (30) | NA | NA | NA | 6 (54.5) |
| Seabra et al. (2009) [17] | 18 | NA | NA | NA | NA | 12 (28) |
| Leonardo et al. (2009) [18] | 35 | 8 (25) | NA | NA | NA | At 1 year: 0 pads per day: 7 (22) 1–2 pads per day: 20 (62.5)>2 pads per day: 5 (15.5) |
| Nunez-Mora et al. (2009) [19] | 26.8 | At 16 months: 2 (22) | NA | NA | NA | complete continence:3 (33) 1–2 pads per day:4 (44) |
| Paparel et al. (2009) [20] | 4.6 y | 65 (44.5) | 5 year: 54 | NA | NA | NA |
| Heidenreich et al. (2010) [21] | 23 | NA | NA | NA | NA | At 1 year: complete continence: 44 (80) |
| Strope et al. (2010) [22] | 15 | At 6 weeks: 2 (34) | NA | NA | NA | At 1 year: 2.3 pads per day: 4 (66) |
| Eandi et al. (2010) [23] | 18 | At 18 months: 2 (33) | NA | NA | NA | 6 (33) |
| Chauhan et al. (2011) [24] | 4.6 | At 5 months: 4 (28.6) | NA | NA | NA | 11 (71.4) |
| Chade et al. (2011) [25] | 4.4 y | At 5y: 48 | NA | At 10y: 83 | NA | NA |
| Gorin et al. (2011) [26] | 63 | At 2y: 14 (58) | 5 year: 40 | NA | 5 year: 90 | 23 (96) |
| Ahallal et al. (2011) [27] | 8 | NA | NA | NA | NA | 0 pads per day: 7 (47) 1–2 pads per day: 7 (47) |
| Lawrentschuk et al. (2011) [28] | NA | NA | NA | NA | NA | At 1 year:0 pads per day: 6 (60) |
| Leonardo et al. (2012) [29] | 14 | at 10 months: 1 (8) | NA | NA | NA | 0 pads per day: 9 (69)2 pads per day:4 (31) |
| Kaffenberger et al. (2013) [30] | 16.1 | At 16 months: 6 (18) | NA | NA | NA | 20 (65) |
| Peters et al. (2013) [31] | 60 | At 22 months: 29 (66 ) | NA | NA | NA | NA |
| Yuh et al. (2014) [32] | 36 | At 3 years: 57 | NA | NA | 5 year: 100 | At 6 month: 23 (45) |
| Zugor et al. (2014) [33] | 23 | 3 (23) | NA | NA | NA | At 12 month: 7 (54) |
| Saeedi et al. (2014) [34] | NA | NA | NA | NA | NA | At 12 month: 0 pads per day: 5 (83) 1 pads per day:1 (17) |
| Bates et al. (2015) [11] | 26 | At 13 months: 8 (15) | NA | NA | NA | At 36 month: 41 (77) |
| Pokala et al. (2015) [35] | NA | NA | NA | 10 years: 88.6 | 10 years: 77.5 | NA |
| Pearce et al. (2015) [36] | NA | NA | NA | NA | NA | NA |
| Lebdai et al. (2015) [37] | NA | NA | NA | NA | NA | At 10 month: Completely continent: 13 (68) ≤1 pad/day: 5 (27) 3 pads/day: 1 (5) |
| Mandel et al. (2016) [38] | 36 | 23 (42) | 5-year: 48.7 | NA | 5-year: 88.7 | 41 (74) |
| Vora et al. (2016) [39] | NA | NA | NA | NA | NA | 1 (16.7) |
| Kenney et al. (2016) [7] | 16.8 | NA | 9.5 months robotic | NA | NA | 4 (10) |
| Orré et al. (2016) [40] | NA | NA | NA | NA | NA | At 12 month: 4 (57) |
| Vidmar et al. (2017) [41] | 25 | NA | NA | NA | NA | 7 (30) |
| Metcalfe et al. (2017) [42] | 2.79 y | At 5 months: 35 (51.5) | Median: 2.78 | NA | NA | NA |
| Ogaya-Pinies et al. (2019) [43] | 14 | At 1 year: 15 (16) | NA | NA | NA | At 12 month: 0 pads per day: 55 (57) 1–2 pads per day: 25 (26) |
| Onol et al. (2019) [44] | 32 | 16 (17) Radiation group 6 (19) Focal ablation group |
5-year: 56 | NA | NA | At 1 year Overall full (no pads): 37 (39.2) social (0–1 pad/day): 48 (51.3) |
| Devos et al. (2019) [45] | 43 | NA | NA | 5-year: 74 | 5-year: 62 | 4 (16) |
| Mohler et al. (2019) [46] | 91 | NA | At 10y: 33 | NA | At 10y: 52 | At 12 year: 6 (15) |
| Clery et al. (2019) [47] | 24 | At 13 months: 17 (31) | NA | 5 years: 80 | NA | 27 (49.1) |
| Herrera-Caceres et al. (2019) [48] | 52 | At 42 months: 7 (21) | NA | NA | NA | ≤1 pad: 31 (91) ≥2 pads: 2 (6) |
| Gontero et al. (2019) [49] | 3 years | NA | NA | NA | NA | At 12 month: fully continent: 221 (56) |
| De Groote et al. (2020) [50] | 2.1 years | At 25 months: 26 (24) | 5-year: 60 | NA | NA | At 2 years or more fully continent: 53 (50) socially continent: 35 (33) |
| Nair et al. (2020) [51] | 43 | 2 (50) | NA | NA | NA | Continent:1 (25) 0–1 pads per day: 2 (50) |
| Thompson et al. (2020) [52] | 18 | At 3 months: 8 (16) | NA | NA | NA | Pad-free at 12-months: 35 (65.5) Socially continent at 12-mo (0–1 pad): 46 (86) |
| Nathan et al. (2021) [53] | 17 | At 26 months: 17 (33) | 5 years: 60 | NA | 129 (96) | At 12 month: fully continent: 90 (67) |
| Madi et al. (2021) [12] | 18 | NA | NA | NA | NA | 14 (100) |
| Rajwa et al. (2021) [54] | 25.3 | NA | NA | NA | NA | NA |
| Bozkurt et al. (2021) [55] | 32 | NA | NA | NA | NA | 0–1 pads per day: 2 (20) 2 pads per day: 6 (60) |
| Marra et al. (2021) [56] | 36 | At 12 months: 115 (31) | NA | 5 years: 98 | 5 years: 92 | 85 (28.2) |
| Spitznagel et al. (2021) [57] | 12 | At 12 months: 1 (8) | NA | NA | NA | No incontinence: 3 (20) Mild incontinence: 3 (20) Moderate incontinence: 6 (50) |
| Wenzel et al. (2021) [58] | 74 | NA | NA | 5 years: 13.4 | NA | NA |
| von Hardenberg et al. (2021) [59] | 28 | NA | 3 years: 80 | NA | NA | NA |
| Nathan et al. (2022) [60] | 16.5 | At 16.5 months: 31 (23) | 5 years: 75 | NA | NA | At 12 month: 77 (77) |
| Mortensen et al. (2022) [61] | 13 | NA | NA | NA | NA | 5 (100) |
| Van Riel et al. (2022) [62] | 17.7 | At 6 months: 1 (2.5) | NA | 100 | 100 | 34 (94.4) |
| Blazevski et al. (2022) [63] | 22 | At 22 months: 0 (0) | NA | NA | NA | Pad free at 3 months: 14 (93) Pad free at 6 months: 1 (7) |
| Catarino et al. (2022) [64] | 94 | At 61 months: 17 (59) | 5 years: 50 | NA | NA | At 12 month: Pad-free continence: 6 (21) Mild incontinence: 12 (41) |
NA: not available, RFS: recurrence-free survival, BCR: biochemical recurrence.
The urinary continence rate in the primary non-radiation-treatment group (HIFU, electroporation, proton beam therapy, cryotherapy, focal vascular targeted photodynamic therapy, and transurethral ultrasound ablation) was 67% versus 55% in patients formerly treated by RT.
3.4. Oncological Results
The median follow-up ranged from 4.6 to 94 months (36, 22, and 39.5 months, respectively, in laparoscopic, robotic, and open approaches). Biochemical recurrence ranged from 8% to 51.5% at 12 months, from 0% to 66% at 22 months, and from 48% to 59% at 60 months. Specific and overall survival rates ranged from 13.4 to 98% and 62 to 100% at 5 years, respectively (Table 3).
The rates of BCR were 20%, 27%, and 47%, respectively, in laparoscopic, robotic, and open approaches. Overall survival was 100% and 98% in the laparoscopic and robotic groups, respectively, and 74% in the open surgery group. The rates of BCR were 36% and 21% in the group of patients treated by non-radiation therapy and RT, respectively. Overall survival was 98% in the group of other primary treatments and 85% for patients treated by RT.
4. Discussion
sRP for recurrent PCa after primary non-surgical treatment failure is challenging for urologists due to its aggressive features and technical demands. The majority of PCa patients who present recurrent disease after RT are therefore treated with palliative ADT while a salvage treatment initiated early may change the disease course. As a result, only 1% of the patients recurring after RT indeed undergo salvage surgery [65].
In the present systematic review, we found that sRP may represent a good alternative that can be provided to carefully selected patients. It may lead to a durable response if initiated early and may delay progression and use for systemic therapies.
The introduction of minimally invasive approaches regarding sRP could be associated with many advantages, such as decreasing the rates of overall and high-grade complications (i.e., Clavien > 2). The robotic approach has been also associated with lower rates of blood loss, rectal injury, anastomotic stricture, and postoperative incontinence [66]. Recently, it has been suggested that the Retzius-sparing approach could also be interesting as it allows a meticulous dissection near the often fibrotic rectal plane. Using this approach, Madi et al. only noted one intraoperative urine leak in their salvage Retzius-sparing (SRS) group [12]. Taken together, the implementation of a minimally invasive approach in sRP has led to a renewed interest in this option for managing recurrence after the primary nonsurgical management of PCa.
One of the major limitations attributed to sRP is the poor functional outcomes regarding the urinary continence associated with this option. Thus, we found an overall complication rate of 34%, including rectal wounds, ureteral complications, rectourethral fistula, lymphoceles, anastomotic leakage, and urinary tract infections, which is in line with a previous report from Matei et al., who reported a Clavien > 2 complication rate of 0–33% [66].
However, the functional results widely differed between the studies included in this systematic review. Continence rates reported after sRP ranged from 10 to 100%. This heterogeneity could be explained, once again, by the surgical approach used. Robotic-assisted sRP appeared to improve the early return to continence, compared to open surgery series. This is thought to be due to the support of the surrounding ligaments to the anterior urethra, which helps to maintain sphincteric integrity after SRS [67,68]. Mason et al. suggested that continence outcomes were significantly improved in the SRS group for the treatment of radioresistant prostate cancer [69].
Oncological outcomes after sRP are influenced by several factors and may vary depending on the patient/tumor characteristics, type of initial treatment, surgical approach used, length of follow-up, and, above all, pre-treatment assessment (including imaging, with the development of MRI and metabolic imaging). At the mid-term follow-up, we found that the oncological outcomes were acceptable, as a significant proportion of men were disease-free after five years (i.e., the BCR-free survival rate ranged from 48% to 59% at 60 months). In addition, cancer-specific survival and overall survival rates ranged from 13.4 to 98% and 62 to 100% at 5 years, respectively. However, long-term data remain poorly reported in the literature. Two series showed a 10-year BCR-free survival of 31% and 37%, respectively [25,36]. We therefore encourage further studies evaluating long-term oncological outcomes in these patients.
Our study has several strengths, including the important number of studies/patients included, with a variety of nonsurgical primary treatments with a clear distinction between them, the inclusion of most updated data, and their careful review for study inclusion.
Some limitations must be acknowledged. The main limitation is the significant risk of bias, as all included studies were retrospective, which prevented us from reaching a high level of evidence and from providing clear recommendations. Finally, the heterogeneity regarding the surgical approach used, the type of initial local, and the functional erectile results, which are not reported in our review, are important limitations to notice. Of note, although we performed a systematic review, a meta-analysis was not possible given the heterogeneity of the studies in terms of the initial treatment proposed and the surgical approach.
5. Conclusions
sRP appears to be feasible with acceptable morbidity in well-selected PCa patients who recur after primary non-operative surgical treatment. The development of a minimally invasive approach and the improvement of surgical techniques are considered to be two key factors in improving perioperative outcomes. However, the level of evidence remains low as comparative and long-term data are lacking.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15225485/s1, Table S1: Risk of bias in non-randomized trials using the ROBINS-I tool.
Author Contributions
Conceptualization, A.S. and G.P.; methodology, A.S. and G.P.; validation, A.S. and G.P.; formal analysis, A.S. and G.P.; writing—original draft preparation, A.S. and G.P.; writing—review and editing, A.S., A.R., C.D., E.B., G.F., G.F.H., G.C., G.R., J.B.B., L.B., R.R.-P., M.G., M.B., G.P. and M.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Culp M.B., Soerjomataram I., Efstathiou J.A., Bray F., Jemal A. Recent Global Patterns in Prostate Cancer Incidence and Mortality Rates. Eur. Urol. 2020;77:38–52. doi: 10.1016/j.eururo.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Chen J., Oromendia C., Halpern J.A., Ballman K.V. National trends in management of localized prostate cancer: A population based analysis 2004–2013. Prostate. 2018;78:512–520. doi: 10.1002/pros.23496. [DOI] [PubMed] [Google Scholar]
- 3.Cornford P., van den Bergh R.C.N., Briers E., Van den Broeck T., Cumberbatch M.G., De Santis M., Fanti S., Fossati N., Gandaglia G., Gillessen S., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. [DOI] [PubMed] [Google Scholar]
- 4.Jones J.S. Radiorecurrent prostate cancer: An emerging and largely mismanaged epidemic. Eur. Urol. 2011;60:411–412. doi: 10.1016/j.eururo.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Coelho R.F., Patel M.B., Chauhan S., Orvieto M.A., Liss M., Ahlering T., Ferrigni R., Castle E., Joseph J., Sivaraman A., et al. Salvage robotic-assisted radical prostatectomy (SRALP) for treatment of radio-recurrent prostate cancer: Description of technique and multi-institutional outcomes. J. Endourol. 2010;24:A347. doi: 10.1016/S1569-9056(10)61170-0. [DOI] [Google Scholar]
- 6.Chade D.C., Eastham J., Graefen M., Hu J.C., Karnes R.J., Klotz L., Montorsi F., van Poppel H., Scardino P.T., Shariat S.F. Cancer control and functional outcomes of salvage radical prostatectomy for radiation-recurrent prostate cancer: A systematic review of the literature. Eur. Urol. 2012;61:961–971. doi: 10.1016/j.eururo.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Zargar H., Lamb A.D., Rocco B., Porpiglia F., Liatsikos E., Davis J., Coelho R.F., Pow-Sang J.M., Patel V.R., Murphy D.G. Salvage robotic prostatectomy for radio recurrent prostate cancer: Technical challenges and outcome analysis. Minerva Urol. Nefrol. 2017;69:26–37. doi: 10.23736/S0393-2249.16.02797-1. [DOI] [PubMed] [Google Scholar]
- 8.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knoll T., Omar M.I., Maclennan S., Hernández V., Canfield S., Yuan Y., Bruins M., Marconi L., Van Poppel H., N’Dow J., et al. Key steps in conducting systematic reviews for underpinning clinical practice guidelines: Methodology of the European Association of Urology. Eur. Urol. 2018;73:290–300. doi: 10.1016/j.eururo.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Moga C., Guo B., Schopfocher D.H.C. Development of a Quality Appraisal Tool for Case Series Studies Using a Modified Delphi Technique. Institute of Health Economics (IHE); Edmonton, AB, USA: 2012. [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. Salvage robot assisted radical prostatectomy: A propensity matched study of perioperative, oncological and functional outcomes. Eur. J. Surg. Oncol. 2015;41:1540–1546. doi: 10.1016/j.ejso.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Madi R., Sayyid R.K., Hiffa A., Thomas E., Terris M.K., Klaassen Z. Early Experience with Salvage Retzius-sparing Robotic-assisted Radical Prostatectomy: Oncologic and Functional Outcomes. Urology. 2021;149:117–121. doi: 10.1016/j.urology.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 13.Kaouk J.H., Hafron J., Goel R., Haber G.P., Jones J.S. Robotic salvage retropubic prostatectomy after radiation/brachytherapy: Initial results. BJU Int. 2008;102:93–96. doi: 10.1111/j.1464-410X.2008.07570.x. [DOI] [PubMed] [Google Scholar]
- 14.Liatsikos E., Bynens B., Rabenalt R., Kallidonis P., Do M., Stolzenburg J.U. Treatment of patients after failed high intensity focused ultrasound and radiotherapy for localized prostate cancer: Salvage laparoscopic extraperitoneal radical prostatectomy. J. Endourol. 2008;22:2295–2298. doi: 10.1089/end.2008.9713. [DOI] [PubMed] [Google Scholar]
- 15.Kim S.P., Hollenbeck B.K., Parker W.R., Labo J., Wood D.P., Jr. Feasibility and safety of robot-assisted salvage prostatectomy for recurrent prostate cancer following radiation therapy. J. Robot. Surg. 2008;2:81–83. doi: 10.1007/s11701-008-0082-x. [DOI] [PubMed] [Google Scholar]
- 16.Boris R.S., Bhandari A., Krane L.S., Eun D., Kaul S., Peabody J.O. Salvage robotic-assisted radical prostatectomy: Initial results and early report of outcomes. BJU Int. 2009;103:952–956. doi: 10.1111/j.1464-410X.2008.08245.x. [DOI] [PubMed] [Google Scholar]
- 17.Seabra D., Faria E., Dauster B., Rodrigues G., Fava G. Critical analysis of salvage radical prostatectomy in the management of radioresistant prostate cancer. Int. Braz. J. Urol. 2009;35:43–48. doi: 10.1590/S1677-55382009000100007. [DOI] [PubMed] [Google Scholar]
- 18.Leonardo C., Simone G., Papalia R., Franco G., Guaglianone S., Gallucci M. Salvage radical prostatectomy for recurrent prostate cancer after radiation therapy. Int. J. Urol. 2009;16:584–586. doi: 10.1111/j.1442-2042.2008.02209.x. [DOI] [PubMed] [Google Scholar]
- 19.Nuñez-Mora C., García-Mediero J.M., Cabrera-Castillo P.M. Radical laparoscopic salvage prostatectomy: Medium-term functional and oncological results. J. Endourol. 2009;23:1301–1305. doi: 10.1089/end.2009.0019. [DOI] [PubMed] [Google Scholar]
- 20.Paparel P., Cronin A.M., Savage C., Scardino P.T., Eastham J.A. Oncologic outcome and patterns of recurrence after salvage radical prostatectomy. Eur. Urol. 2009;55:404–410. doi: 10.1016/j.eururo.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Heidenreich A., Richter S., Thüer D., Pfister D. Prognostic parameters, complications, and oncologic and functional outcome of salvage radical prostatectomy for locally recurrent prostate cancer after 21st-century radiotherapy. Eur. Urol. 2010;57:437–443. doi: 10.1016/j.eururo.2009.02.041. [DOI] [PubMed] [Google Scholar]
- 22.Strope S.A., Coelho M., Wood D.P., Hollenbeck B.K. Robot-assisted salvage prostatectomy: Evaluation of initial patient-reported outcomes. J. Endourol. 2010;24:425–427. doi: 10.1089/end.2009.0143. [DOI] [PubMed] [Google Scholar]
- 23.Eandi J.A., Link B.A., Nelson R.A., Josephson D.Y., Lau C., Kawachi M.H., Wilson T.G. Robotic assisted laparoscopic salvage prostatectomy for radiation resistant prostate cancer. J. Urol. 2010;183:133–137. doi: 10.1016/j.juro.2009.08.134. [DOI] [PubMed] [Google Scholar]
- 24.Chauhan S., Patel M.B., Coelho R., Liss M., Rocco B., Sivaraman A.K., Palmer K.J., Coughlin G.D., Ferrigni R.G., Castle E.P., et al. Preliminary analysis of the feasibility and safety of salvage robot-assisted radical prostatectomy after radiation failure: Multi-institutional perioperative and short-term functional outcomes. J. Endourol. 2011;25:1013–1019. doi: 10.1089/end.2010.0564. [DOI] [PubMed] [Google Scholar]
- 25.Chade D.C., Shariat S.F., Cronin A.M., Savage C.J., Karnes R.J., Blute M.L., Briganti A., Montorsi F., van der Poel H.G., Van Poppel H., et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: A multi-institutional collaboration. Eur. Urol. 2011;60:205–210. doi: 10.1016/j.eururo.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorin M.A., Manoharan M., Shah G., Eldefrawy A., Soloway M.S. Salvage open radical prostatectomy after failed radiation therapy: A single center experience. Cent. Eur. J. Urol. 2011;64:144–147. doi: 10.5173/ceju.2011.03.art9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahallal Y., Shariat S.F., Chade D.C., Mazzola C., Reuter V.E., Sandhu J.S., Laudone V.P., Touijer K.A., Guillonneau B.D. Pilot study of salvage laparoscopic prostatectomy for the treatment of recurrent prostate cancer. BJU Int. 2011;108:724–728. doi: 10.1111/j.1464-410X.2010.09924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrentschuk N., Finelli A., Van der Kwast T.H., Ryan P., Bolton D.M., Fleshner N.E., Trachtenberg J., Klotz L., Robinette M., Woo H. Salvage radical prostatectomy following primary high intensity focused ultrasound for treatment of prostate cancer. J. Urol. 2011;185:862–868. doi: 10.1016/j.juro.2010.10.080. [DOI] [PubMed] [Google Scholar]
- 29.Leonardo C., Franco G., De Nunzio C., Tubaro A., Salvitti M., Tartaglia N., Simonelli G., De Dominicis C. Salvage laparoscopic radical prostatectomy following high-intensity focused ultrasound for treatment of prostate cancer. Urology. 2012;80:130–133. doi: 10.1016/j.urology.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Kaffenberger S.D., Keegan K.A., Bansal N.K., Morgan T.M., Tang D.H., Barocas D.A., Penson D.F., Davis R., Clark P.E., Chang S.S., et al. Salvage robotic assisted laparoscopic radical prostatectomy: A single institution, 5-year experience. J. Urol. 2013;189:507–513. doi: 10.1016/j.juro.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters M., Moman M.R., van der Poel H.G., Vergunst H., Jan de Jong I., Vijverberg P.L.M., Battermann J.J., Horenblas S., van Vulpen M. Patterns of outcome and toxicity after salvage prostatectomy salvage cryosurgery and salvage brachytherapy for prostate cancer recurrences after radiation therapy: A multi-center experience and literature review. World J. Urol. 2013;31:403–409. doi: 10.1007/s00345-012-0928-8. [DOI] [PubMed] [Google Scholar]
- 32.Yuh B., Ruel N., Muldrew S., Mejia R., Novara G., Kawachi M., Wilson T. Complications and outcomes of salvage robot-assisted radical prostatectomy: A single-institution experience. BJU Int. 2014;113:769–776. doi: 10.1111/bju.12595. [DOI] [PubMed] [Google Scholar]
- 33.Zugor V., Labanaris A.P., Porres D., Heidenreich A., Witt J.H. Robot-assisted radical prostatectomy for the treatment of radiation-resistant prostate cancer: Surgical, oncological and short-term functional outcomes. Urol. Int. 2014;92:20–26. doi: 10.1159/000351948. [DOI] [PubMed] [Google Scholar]
- 34.Saeedi Y., Pop M., Jacqmin D. Salvage radical prostatectomy for brachytherapy failure: Preliminary results. Prog. Urol. 2014;24:266–270. doi: 10.1016/j.purol.2013.08.314. [DOI] [PubMed] [Google Scholar]
- 35.Pokala N., Huynh D.L., Henderson A.A., Johans C. Survival Outcomes in Men Undergoing Radical Prostatectomy after Primary Radiation Treatment for Adenocarcinoma of the Prostate. Clin. Genitourin. Cancer. 2016;14:218–225. doi: 10.1016/j.clgc.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 36.Pearce S.M., Richards K.A., Patel S.G., Pariser J.J., Eggener S.E. Population-based analysis of salvage radical prostatectomy with examination of factors associated with adverse perioperative outcomes. Urol. Oncol. 2015;33:163.e1–163.e6. doi: 10.1016/j.urolonc.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Lebdai S., Villers A., Barret E., Nedelcu C., Bigot P., Azzouzi A.-R. Feasibility, safety, and efficacy of salvage radical prostatectomy after Tookad® Soluble focal treatment for localized prostate cancer. World J. Urol. 2015;33:965–971. doi: 10.1007/s00345-015-1493-8. [DOI] [PubMed] [Google Scholar]
- 38.Mandel P., Steuber T., Ahyai S., Kriegmair M., Schiffmann J., Boehm K., Heinzer H., Michl U., Schlomm T., Haese A., et al. Salvage radical prostatectomy for recurrent prostate cancer: Verification of European Association of Urology guideline criteria. BJU Int. 2016;117:55–61. doi: 10.1111/bju.13103. [DOI] [PubMed] [Google Scholar]
- 39.Vora A., Agarwal V., Singh P., Patel R., Rivas R., Nething J., Muruve N. Single-institution comparative study on the outcomes of salvage cryotherapy versus salvage robotic prostatectomy for radio-resistant prostate cancer. Prostate Int. 2016;4:7–10. doi: 10.1016/j.prnil.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orré M., Piéchaud T., Sargos P., Richaud P., Roubaud G., Thomas L. Oncological and functional results of robotic salvage radical prostatectomy after permanent brachytherapy implants. Cancer Radiother. 2017;21:119–123. doi: 10.1016/j.canrad.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Vidmar R., Marcq G., Flamand V., Fantoni J.C., Hénon F., Villers A., Ouzzane A. Salvage radical prostatectomy for recurrent prostate cancer. Morbidity, oncological and functional results. Prog. Urol. 2017;27:458–466. doi: 10.1016/j.purol.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Metcalfe M.J., Troncoso P., Guo C.C., Chen H.-C., Bozkurt Y., Ward J.F., Pisters L.L. Salvage prostatectomy for post-radiation adenocarcinoma with treatment effect: Pathological and oncological outcomes. Can. Urol. Assoc. J. 2017;11:E277–E284. doi: 10.5489/cuaj.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogaya-Pinies G., Linares-Espinos E., Hernandez-Cardona E., Jenson C., Cathelineau X., Sanchez-Salas R., Patel V. Salvage robotic-assisted radical prostatectomy: Oncologic and functional outcomes from two high-volume institutions. World J. Urol. 2019;37:1499–1505. doi: 10.1007/s00345-018-2406-4. [DOI] [PubMed] [Google Scholar]
- 44.Onol F.F., Bhat S., Moschovas M., Rogers T., Ganapathi H., Roof S., Rocco B., Patel V. Comparison of outcomes of salvage robot-assisted laparoscopic prostatectomy for post-primary radiation vs focal therapy. BJU Int. 2019;125:103–111. doi: 10.1111/bju.14900. [DOI] [PubMed] [Google Scholar]
- 45.Devos B., Al Hajj Obeid W., Andrianne C., Diamand R., Peltier A., Everaerts W., Van Poppel H., Van Velthoven R., Joniau S. Salvage high-intensity focused ultrasound versus salvage radical prostatectomy for radiation-recurrent prostate cancer: A comparative study of oncological, functional, and toxicity outcomes. World J. Urol. 2019;37:1507–1515. doi: 10.1007/s00345-019-02640-x. [DOI] [PubMed] [Google Scholar]
- 46.Mohler J.L., Halabi S., Ryan S.T., Al-Daghmin A., Sokoloff M.H., Steinberg G.D., Sanford B.L., Eastham J.A., Walther P.J., Morris M.J., et al. Management of recurrent prostate cancer after radiotherapy: Long-term results from CALGB 9687 (Alliance), a prospective multi-institutional salvage prostatectomy series. Prostate Cancer Prostatic Dis. 2019;22:309–316. doi: 10.1038/s41391-018-0106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clery R., Grande P., Seisen T., Gobert A., Duquesne I., Villers A., Olivier J., Bernhard J.C., Robert G., Beauval J.B., et al. Outcomes after salvage radical prostatectomy and first-line radiation therapy or HIFU for recurrent localized prostate cancer: Results from a multicenter study. World J. Urol. 2019;37:1491–1498. doi: 10.1007/s00345-019-02683-0. [DOI] [PubMed] [Google Scholar]
- 48.Herrera-Caceres J.O., Nason G.J., Salgado-Sanmamed N., Goldberg H., Woon D.T.S., Chandrasekar T., Ajib K., Tan G.H., Alhunaidi O., van der Kwast T., et al. Salvage radical prostatectomy following focal therapy: Functional and oncological outcomes. BJU Int. 2019;125:525–530. doi: 10.1111/bju.14976. [DOI] [PubMed] [Google Scholar]
- 49.Gontero P., Marra G., Alessio P., Filippini C., Oderda M., Munoz F., Linares E., Sanchez-Salas R., Challacombe B., Dasgupta P., et al. Salvage Radical Prostatectomy for Recurrent Prostate Cancer: Morbidity and Functional Outcomes from a Large Multicenter Series of Open versus Robotic Approaches. J. Urol. 2019;202:725–731. doi: 10.1097/JU.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 50.De Groote R., Nathan A., De Bleser E., Pavan N., Sridhar A., Kelly J., Sooriakumaran P., Briggs T., Nathan S. Techniques and Outcomes of Salvage Robot-Assisted Radical Prostatectomy (sRARP) Eur. Urol. 2020;78:885–892. doi: 10.1016/j.eururo.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Nair S.M., Stern N., Dewar M., Siddiqui K., Smith E., Gomez J.A., Moussa M., Chin J.L. Salvage open radical prostatectomy for recurrent prostate cancer following MRI-guided transurethral ultrasound ablation (TULSA) of the prostate: Feasibility and efficacy. Scand. J. Urol. 2020;54:215–219. doi: 10.1080/21681805.2020.1752795. [DOI] [PubMed] [Google Scholar]
- 52.Thompson J.E., Sridhar A.N., Shaw G., Rajan P., Mohammed A., Briggs T.P., Nathan S., Kelly J.D., Sooriakumaran P. Peri-operative, functional and early oncologic outcomes of salvage robotic-assisted radical prostatectomy after high-intensity focused ultrasound partial ablation. BMC Urol. 2020;20:81. doi: 10.1186/s12894-020-00656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nathan A., Fricker M., De Groote R., Arora A., Phuah Y., Flora K., Patel S., Kasivisvanathan V., Sridhar A., Shaw G., et al. Salvage Versus Primary Robot-assisted Radical Prostatectomy: A Propensity-matched Comparative Effectiveness Study from a High-volume Tertiary Centre. Eur. Urol. Open Sci. 2021;27:43–52. doi: 10.1016/j.euros.2021.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajwa P., Schuettfort V.M., Quhal F., Mori K., Katayama S., Laukhtina E., Pradere B., Motlagh R.S., Mostafaei H., Grossmann N.C., et al. Role of systemic immune-inflammation index in patients treated with salvage radical prostatectomy. World J. Urol. 2021;39:3771–3779. doi: 10.1007/s00345-021-03715-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bozkurt Y., Atar M., Pisters L.L. Early Experience with Salvage Robotic-Assisted Radical Prostatectomy in Proton Beam Radiotherapy Failures. Balk. Med. J. 2021;38:310–315. doi: 10.5152/balkanmedj.2021.21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marra G., Karnes R.J., Calleris G., Oderda M., Alessio P., Palazzetti A., Battaglia A., Pisano F., Munegato S., Munoz F., et al. Oncological outcomes of salvage radical prostatectomy for recurrent prostate cancer in the contemporary era: A multicenter retrospective study. Urol. Oncol. 2021;39:296.e21–296.e29. doi: 10.1016/j.urolonc.2020.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Spitznagel T., Hardenberg J.V., Schmid F.A., Rupp N.J., Westhoff N., Worst T.S., Weis C.-A., Mortezavi A., Eberli D. Salvage Robotic-assisted Laparoscopic Radical Prostatectomy Following Focal High-Intensity Focused Ultrasound for ISUP 2/3 Cancer. Urology. 2021;156:147–153. doi: 10.1016/j.urology.2021.04.059. [DOI] [PubMed] [Google Scholar]
- 58.Wenzel M., Würnschimmel C., Nocera L., Ruvolo C.C., Tian Z., Shariat S.F., Saad F., Briganti A., Graefen M., Becker A., et al. Salvage Radical Prostatectomy: Baseline Prostate Cancer Characteristics and Survival across SEER Registries. Clin. Genitourin. Cancer. 2021;19:e255–e263. doi: 10.1016/j.clgc.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 59.von Hardenberg J., Cash H., Koch D., Borkowetz A., Bruendl J., Leyh-Bannurah S.-R., Kuru T.H., Kowalewski K.-F., Schindele D., Mala K.S., et al. Triggers and oncologic outcome of salvage radical prostatectomy, salvage radiotherapy and active surveillance after focal therapy of prostate cancer. World J. Urol. 2021;39:3747–3754. doi: 10.1007/s00345-021-03700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nathan A., Ng A., Mitra A., Sooriakumaran P., Davda R., Patel S., Fricker M., Kelly J., Shaw G., Rajan P., et al. Comparative Effectiveness Analyses of Salvage Prostatectomy and Salvage Radiotherapy Outcomes Following Focal or Whole-Gland Ablative Therapy (High-Intensity Focused Ultrasound, Cryotherapy or Electroporation) for Localised Prostate Cancer. Clin. Oncol. R. Coll. Radiol. 2022;34:e69–e78. doi: 10.1016/j.clon.2021.10.012. [DOI] [PubMed] [Google Scholar]
- 61.Mortensen M.A., Poulsen C.A., Ahlgren G., Madsen K., Poulsen M.H. Introduction of salvage prostatectomy in Denmark: The initial experience. BMC Res. Notes. 2022;15:185. doi: 10.1186/s13104-022-06076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Riel L.A.M.J.G., Geboers B., Kabaktepe E., Blazevski A., Reesink D.J., Stijns P., Stricker P.D., Casanova J., Dominguez-Escrig J.L., de Reijke T.M., et al. Outcomes of salvage radical prostatectomy after initial irreversible electroporation treatment for recurrent prostate cancer. BJU Int. 2022;130:611–618. doi: 10.1111/bju.15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blazevski A., Gondoputro W., Scheltema M.J., Amin A., Geboers B., Barreto D., Haynes A.-M., Shnier R., Delprado W., Agrawal S., et al. Salvage robot-assisted radical prostatectomy following focal ablation with irreversible electroporation: Feasibility, oncological and functional outcomes. BMC Urol. 2022;22:28. doi: 10.1186/s12894-022-00978-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Catarino R., Otta-Oshiro R.J., Lista-Mateos F., García-Mediero J.M., Nunez-Mora C. Outcomes of laparoscopic salvage radical prostatectomy after primary treatment of prostate cancer. Cent. Eur. J. Urol. 2022;75:59–64. doi: 10.5173/ceju.2022.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cary K.C., Paciorek A., Fuldeore M.J., Carroll P.R., Cooperberg M.R. Temporal trends and predictors of salvage cancer treatment after failure following radical prostatectomy or radiation therapy: An analysis from the CaPSURE registry. Cancer. 2014;120:507–512. doi: 10.1002/cncr.28446. [DOI] [PubMed] [Google Scholar]
- 66.Matei D.V., Ferro M., Jereczek-Fossa B.A., Renne G., Crisan N., Bottero D., Mazzarella C., Terracciano D., Autorino R., De Cobelli O. Salvage radical prostatectomy after external beam radiation therapy: A systematic review of current approaches. Urol. Int. 2015;94:373–382. doi: 10.1159/000371893. [DOI] [PubMed] [Google Scholar]
- 67.Egan J., Marhamati S., Carvalho F.L., Davis M., O’Neill J., Lee H., Lynch J.H., Hankins R.A., Hu J.C., Kowalczyk K.J. Retzius-sparing Robot-assisted Radical Prostatectomy Leads to Durable Improvement in Urinary Function and Quality of Life Versus Standard Robot-assisted Radical Prostatectomy without Compromise on Oncologic Efficacy: Single-surgeon Series and Step-by-step Guide. Eur. Urol. 2021;79:839–857. doi: 10.1016/j.eururo.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 68.Sayyid R.K., Sherwood D., Simpson W.G., Terris K., Klaassen Z., Madi R. Retzius-sparing robotic-assisted laparoscopic radical prostatectomy: Racial considerations for 250 consecutive cases. J. Robot. Surg. 2020;15:221–228. doi: 10.1007/s11701-020-01096-1. [DOI] [PubMed] [Google Scholar]
- 69.Mason J.B., Hatch L., Dall C., Kowalczyk K.J. Salvage Retzius-Sparing Radical Prostatectomy: A Review of Complications, Functional Outcomes, and Oncologic Outcomes. Curr. Oncol. 2022;29:9733–9743. doi: 10.3390/curroncol29120764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are contained within the article.