Abstract
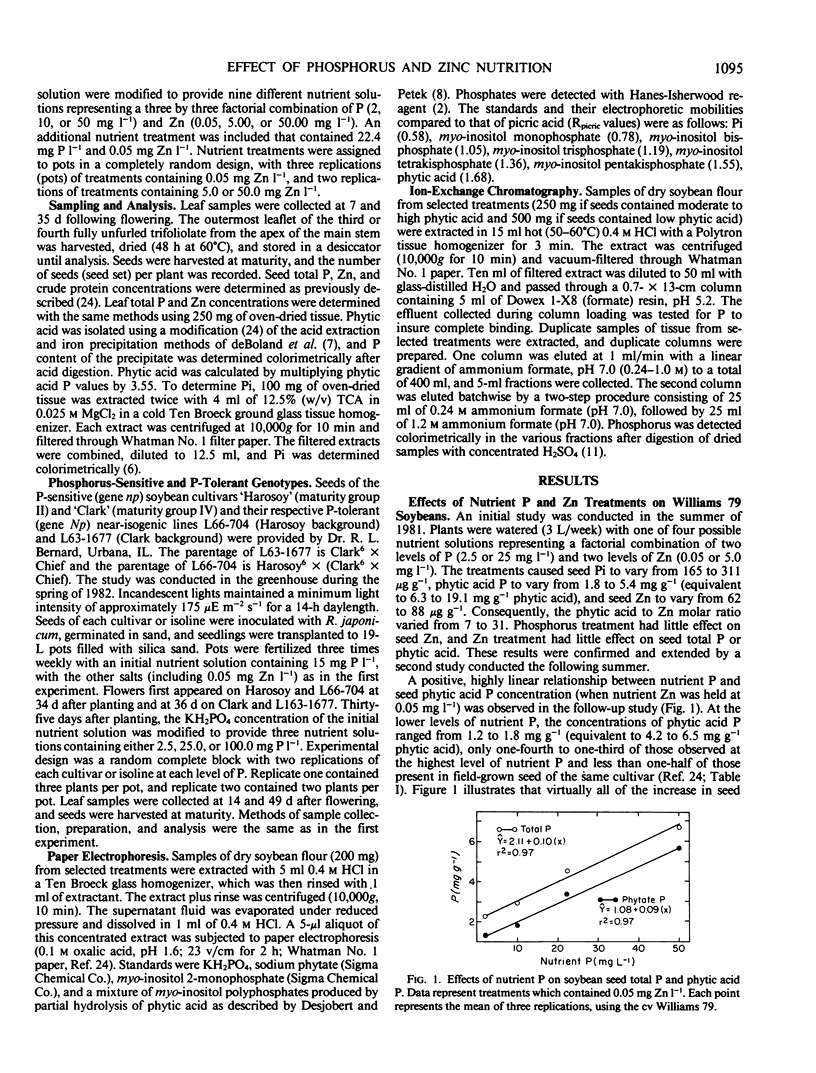
The relationships between nutrient P and Zn levels and the phytic acid, P, and Zn concentrations in soybean (Glycine max L. Merr. cv `Williams 79') seed were studied. Phytic acid increased linearly from 4.2 to 19.2 milligrams per gram as nutrient P treatment was varied from 2.0 to 50 milligrams per liter and Zn was held constant at 0.05 milligrams per liter. Leaf P concentration during seed development was found to be closely related to the concentrations of seed P and phytic acid. Leaf and seed Zn concentrations both responded positively to increasing nutrient Zn treatment. The effects of P treatment on plant and seed P and phytic acid were largely independent of the effects of Zn treatment on leaf and seed Zn. Phytic acid to Zn molar ratios ranging from 3.6 to 33.8 were observed.
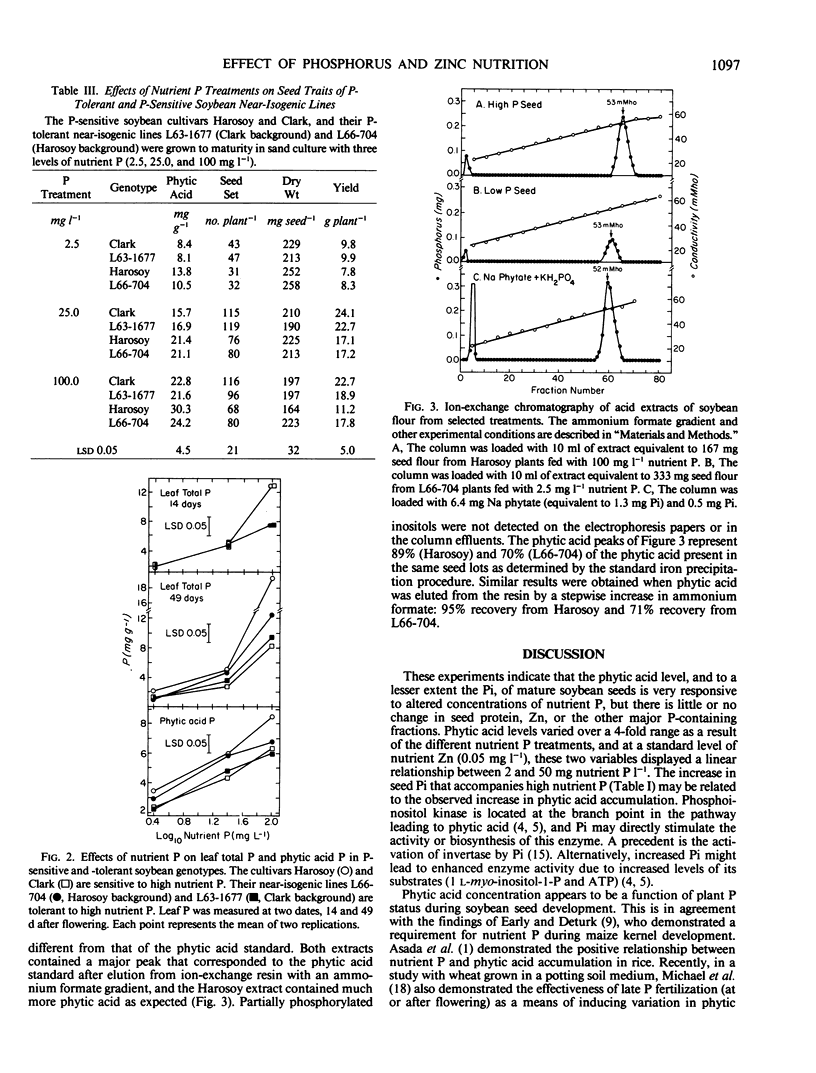
The effects of nutrient P treatments on the concentrations of phytic acid, seed P, and leaf P were also studied in the P-sensitive (gene np) cultivars `Harosoy' and `Clark' and their respective P-tolerant (gene Np) near-isogenic lines L66-704 and L63-1677. In general, the positive relationships observed among nutrient P, leaf P, seed P, and phytic acid concentrations were similar to those observed in the studies with Williams 79. When fertilized with low or moderate nutrient P (2.5 and 25.0 milligrams P per liter, respectively) no significant differences in any parameter were observed between Harosoy or Clark and their respective P-tolerant isolines. When fertilized with high nutrient P (100 milligrams P per liter), Harosoy seed had a significantly higher concentration of phytic acid (30 milligrams per gram) than did seed of its P-tolerant near-isogenic line L66-704 (24.2 milligrams per gram phytic acid), whereas no significant difference was observed between Clark and its P-tolerant near-isogenic line L63-1677 (22.8 and 21.6 milligrams per gram, respectively). Variation in the phytic acid concentrations in the mature seed of the cultivars and isolines more closely paralleled leaf P concentrations observed during seed development (49 days after flowering), than those observed at the onset of seed development (14 days after flowering). Electrophoresis and ion-exchange chromatography revealed that partially phosphorylated intermediates do not appear when phytic acid accumulation is greatly reduced by limiting the nutrient P or when accumulation is greatly accelerated by excess P.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Tanaka K., Kasai Z. Formation of phytic acid in cereal grains. Ann N Y Acad Sci. 1969 Oct 17;165(2):801–814. [PubMed] [Google Scholar]
- BANDURSKI R. S., AXELROD B. The chromatographic identification of some biologically important phosphate esters. J Biol Chem. 1951 Nov;193(1):405–410. [PubMed] [Google Scholar]
- DESJOBERT A., PETEK F. Chromatographie sur papier des esters phosphoriques de l'inositol; application a l'étude de la dégradation hydrolytique de l'inositolhexaphosphate. Bull Soc Chim Biol (Paris) 1956 Sep 4;38(5-6):871–883. [PubMed] [Google Scholar]
- Foote B. D., Howell R. W. Phosphorus Tolerance and Sensitivity of Soybeans as Related to Uptake and Translocation. Plant Physiol. 1964 Jul;39(4):610–613. doi: 10.1104/pp.39.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleas D., Harland B. F. Phytate content of foods: effect on dietary zinc bioavailability. J Am Diet Assoc. 1981 Oct;79(4):433–436. [PubMed] [Google Scholar]
- Seiffert U. B., Agranoff B. W. Isolation and separation of inositol phosphates from hydrolysates of rat tissues. Biochim Biophys Acta. 1965 Jun 1;98(3):574–581. doi: 10.1016/0005-2760(65)90154-2. [DOI] [PubMed] [Google Scholar]
- Solomons N. W. Biological availability of zinc in humans. Am J Clin Nutr. 1982 May;35(5):1048–1075. doi: 10.1093/ajcn/35.5.1048. [DOI] [PubMed] [Google Scholar]
- Welch R. M., House W. A. Availability to rats of zinc from soybean seeds as affected by maturity of seed, source of dietary protein, and soluble phytate. J Nutr. 1982 May;112(5):879–885. doi: 10.1093/jn/112.5.879. [DOI] [PubMed] [Google Scholar]
- de Boland A. R., Garner G. B., O'Dell B. L. Identification and properties of "phytate" in cereal grains and oilseed products. J Agric Food Chem. 1975 Nov-Dec;23(6):1186–1189. doi: 10.1021/jf60202a038. [DOI] [PubMed] [Google Scholar]


