Abstract
Chaetomium thermophilium was isolated from composting municipal solid waste during the thermophilic stage of the process. C. thermophilium, a cellulolytic fungus, exhibited laccase activity when it was grown at 45°C both in solid media and in liquid media. Laccase activity reached a peak after 24 h in liquid shake culture. Laccase was purified by ultrafiltration, anion-exchange chromatography, and affinity chromatography. The purified enzyme was identified as a glycoprotein with a molecular mass of 77 kDa and an isoelectric point of 5.1. The laccase was stable for 1 h at 70°C and had half-lives of 24 and 12 h at 40 and 50°C, respectively. The enzyme was stable at pH 5 to 10, and the optimum pH for enzyme activity was 6. The purified laccase efficiently catalyzed a wide range of phenolic substrates but not tyrosine. The highest levels of affinity were the levels of affinity to syringaldazine and hydroxyquinone. The UV-visible light spectrum of the purified laccase had a peak at 604 nm (i.e., Cu type I), and the activity was strongly inhibited by Cu-chelating agents. When the hydrophobic acid fraction (the humic fraction of the water-soluble organic matter obtained from municipal solid waste compost) was added to a reaction assay mixture containing laccase and guaiacol, polymerization took place and a soluble polymer was formed. C. thermophilium laccase, which is produced during the thermophilic stage of composting, can remain active for a long period of time at high temperatures and alkaline pH values, and we suggest that this enzyme is involved in the humification process during composting.
Humic substances (HS) are defined as relatively high-molecular-weight, dark-colored organic materials that are produced during chemical and biological transformation of plant, animal, and human wastes (28, 29, 31). HS are major organic components of soils, natural waters, marine and lake sediments, and coals. The end product of composting (HS) resembles soil organic matter. It has been suggested that the rapid humification process that takes place during composting can serve as a model for the process in soil (1, 13, 22, 23). Stevenson (31) hypothesized that the formation of polyaromatic HS is associated with phenoloxidase, laccase, and peroxidase activities in the soil. Laccase-like enzymes oxidize the phenolic nucleus by one electron, forming a phenoxy free radical, which causes spontaneous polymerization (7, 33, 36, 40, 41). The synthesis of polyphenols is an essential step in the formation of HS from phenols, quinones, carbohydrates, and N sugars.
The relatively short time required to form HS during composting and the presence of laccase-like activity in compost at the thermophilic stage (10) are consistent with the hypothesis that enzymatic phenol oxidation is a major driving force in humification. Thus, our objectives were to isolate a laccase-producing microorganism from composting municipal solid waste (MSW), to purify and characterize the laccase, and to study its possible role in HS formation.
MATERIALS AND METHODS
Organisms.
Chaetomium thermophilium (the culture collection of the Department of Plant Pathology and Microbiology, The Hebrew University of Jerusalem, Rehovot, Israel) was isolated from composted MSW in March 1996. The organism was grown at 45°C and then maintained at 4°C. The culture medium contained 2% malt extract and 2% agar.
Isolation of fungi.
Composted MSW samples were collected from the Amnir Recycling Industries compost facility in Afula, Israel. Samples (10 kg) of compost at the thermophilic stage were taken from 2- to 12-week-old compost at four different times. Compost subsamples (10 g, fresh weight) were each placed in 100 ml of sterile water and shaken at 150 rpm for 30 min at room temperature. Then, 100-μl portions of the suspensions were inoculated onto plates containing malt extract (1.5%, wt/vol), agar (2%, wt/vol), and tannic acid (0.5%, wt/vol), and the plates were incubated at 45°C for 4 days to identify microorganisms that could oxidize phenol (3). Microorganisms isolated at this stage were examined further to determine whether they had laccase activity (an ability to oxidize syringaldazine) by dripping syringaldazine (1 mM in ethanol) onto the colonies. Active strains exhibited pink haloes within a few minutes.
Pure strains were grown at 45°C on potato dextrose agar (PDA), which contained (per liter) 39 g of PDA (Difco) and 50 ml of a microelement stock solution containing (per liter) 140 mg of MnSO4, 40 mg of ZnNO3 · 4H2O, 1 g of Ca(NO3)2 · 4H2O, and 60 mg of CuSO4 · 5H2O.
Culture conditions.
For studies of laccase production, 20 cylinders (diameter, 10 mm) of C. thermophilium grown on PDA plates were used to inoculate 250-ml Erlenmeyer flasks containing 60 ml of liquid culture medium. The liquid culture medium contained (per liter) 5 g of glucose, 0.6 g of l-aspargine, 1 g of KH2PO4, 1 g of MgSO4 · 7H2O, 0.5 g of KCl, 0.5 g of yeast extract, 10 mg of FeSO4 · 7H2O, and 50 ml of the microelement stock solution. The pH of the medium was adjusted to 6.0 with 2 M NaOH. Cultures were incubated at 45°C on a rotary shaker (125 rpm).
For large-scale laccase production, a 48-h-old liquid culture was homogenized by using an Ultra-turrax (Ystral GmbH, Dottingen, Germany) at one-third the maximal speed. Seventy-five milliliters of the blended culture was used to inoculate a 1.5-liter fermentor (model F-2000; New Brunswick Scientific Corp., Edison, N.J.). The fermentation was carried out by using a stirrer speed of 200 rpm and an aeration rate of 20 liters min−1 at 45°C for 48 h.
Enzyme assay.
Enzyme activity was determined in triplicate by monitoring the absorbance at 530 nm with a model 8452A diode array spectrophotometer (Hewlett-Packard); syringaldazine (ɛmax = 6.4 × 104 M−1 cm−1; Sigma Chemical Co., St. Louis, Mo.) was used as the substrate. The reaction mixture (1 ml) contained 10 μl of the enzyme sample and 0.1 mM syringaldazine in 50 mM sodium phosphate buffer (pH 6.0) and was incubated at 50°C for 5 min. Enzyme activity was expressed in units; 1 U was defined as 1 μmol of product formed per min.
To determine the optimum temperature for the enzyme, activity was measured at 10, 20, 30, 40, 50, 60, 70, and 80°C. To estimate enzyme thermal stability, activity was measured after preincubation at 40, 50, 60, 70, and 80°C.
To estimate the optimum pH for the enzyme, activity was monitored at pH values ranging from 3 to 10 by using the following buffers: 50 mM citrate buffer for pH 3 to 6; 50 mM phosphate buffer for pH 6 to 8; and 50 mM Tris buffer for pH pH 8 to 10. To determine the pH stability of the enzyme, a 100×-concentrated enzyme stock solution was incubated for 1 h at the pH values described above at 4°C. Activity was measured in 50 mM sodium phosphate buffer (pH 6.0) at 50°C for 5 min by using 0.1 mM syringaldazine as the substrate.
The effects of sodium azide, EDTA, and thioglycolic acid (0.01 to 10, 1 to 25, and 0.1 to 1 mM, respectively) on enzyme activity were determined after 15 min of preincubation of the enzyme with the various inhibitors at 4°C.
Purification of extracellular laccase from C. thermophilium.
The supernatant of a 48-h C. thermophilium culture was obtained from the fermentor by filtration through a 0.45-μm-pore-size membrane filter (Supor-450; Gelman Sciences, Ann Arbor, Mich.). The remaining purification steps were carried out at 4°C. The culture liquid was concentrated to a volume of 100 ml with a tangential flow membrane filter (Filton Technology Corp., Northborough, Mass.) by using a 10-kDa filter cassette and a pressure of 0.12 MPa. The concentrated culture was dialyzed overnight against 10 mM Tris buffer (pH 8.2) and then loaded onto a DEAE-Sepharose anion-exchange column (Pharmacia Biotech, Uppsala, Sweden) equilibrated with 10 mM Tris buffer (pH 8.2). Ten column volumes of the same buffer were passed through the column before elution with a linearly increasing NaCl concentration gradient (0 to 0.5 M) in the same buffer. The fractions containing laccase activity were pooled, concentrated with an Amicon cell (10-kDa-molecular-mass-cutoff membrane; Spectrum Medical Industries Inc., Los Angeles, Calif.), and loaded onto a concanavalin A-Sepharose 4B (Sigma) column which was equilibrated with 50 mM phosphate buffer (pH 6.0) containing 0.5 M NaCl, 0.1 mM Ca2+, and 0.1 mM Mn2+. The enzyme was eluted from the column with a linearly increasing 0 to 0.5 M α-d mannopyranoside gradient in the same buffer.
Gel electrophoresis.
To determine the purity of the protein and its molecular weight, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a 10% polyacrylamide gel containing 0.1% SDS. Samples (10 μg of protein) were treated before they were loaded onto the gel with 0.5% SDS and 5% β-mercaptoethanol and were boiled at 100°C for 10 min. The protein was visualized by staining the gel with both Coomassie blue G-250 (Bio-Rad) and silver and was compared with low-range molecular weight markers (Bio-Rad).
Isoelectric point determination.
Analytical isoelectric focusing was performed with a mini isoelectric focusing cell (Bio-Rad) by using a 2-μg sample of purified enzyme and a pH 3 to 9 gradient. The pure protein was visualized with Coomassie blue R-250. pH 4.5 to 9.6 markers (Bio-Rad) were included.
Substrate specificity.
Substrate specificity was determined by oxygen consumption studies. Oxygen concentrations were measured with a model 5775 oxygen electrode (Yellow Springs Instrument Co., Yellow Springs, Ohio). The assay was performed by using 10 μg of purified laccase at 50°C along with 50 mM phosphate buffer (pH 6). The concentration of oxygen at 50°C air saturated water was 190 μmol/ml. To identify the pure enzyme as laccase, the presence of H2O2 as a coproduct was determined as follows. The purified enzyme was assayed by using syringaldazine or guaiacol as a substrate, coupled with horseradish peroxidase (HRP) type 1 (Sigma) and phenol red. HRP was omitted from the control. Absorbance at 610 nm was recorded after 10 min of incubation at 30°C, and the H2O2 level was estimated by determining the difference between the experiment and the control.
N-terminal amino acid sequencing.
The purified laccase was loaded onto an SDS-PAGE gel. After the gel was electrophoresed, the protein was transferred to a polyvinylidene fluoride membrane (Millipore Corp., Bedford, Mass.) by electroblotting and then stained. The polyvinylidene fluoride membrane slice containing the laccase was excised and sequenced by workers at the Protein Research Center at the Technion, Israel Institute of Technology.
Polymerization studies.
To study the effect of laccase isolated from C. thermophilium, we used a soluble organic matter fraction of MSW compost. This fraction was extracted from 77-day-old compost. The fraction selected was the hydrophobic acid (HoA) fraction, which was part of the compost water-soluble organic matter that was adsorbed to XAD-8 resin and displaced by 0.1 M NaOH (11). The HoA fraction and a peat soil fulvic acid (FA) (34) were used as models to demonstrate humification.
To study polymerization by the laccase in the presence of HoA, assay mixtures containing the following compounds were monitored: (i) 1 mg of HoA; (ii) 1 mg of HoA and 10 μg of purified laccase; (iii) 1 mg of HoA and 0.1 mg of guaiacol; (iv) 1 mg of HoA, 0.1 mg of guaiacol, and 10 μg of purified laccase; and (v) 0.1 mg of guaiacol and 10 μg of purified laccase. An enzyme preparation that was boiled for 10 min was used as a control in the assays in which laccase was included. All of the mixtures were dissolved in 50 mM phosphate buffer (pH 6.0), incubated at 45°C for 18 h, and then centrifuged (10 min, 10,000 × g) to obtain clear solutions. A similar study in which FA was used instead of HoA was also performed. One milliliter of each clarified assay solution was analyzed by Sephacryl S-200 (Pharmacia) gel filtration chromatography by using a column (650 by 16 mm) connected to a fast protein liquid chromatograph (ÄKTA Explorer 100; Pharmacia Biotech). The column void volume was 58 ml. The running conditions were as follows: flow rate, 30 ml h−1; and pressure, 0.4 MPa. The absorbance at 254 nm was recorded every 5.12 s. The assay mixture containing guaiacol and laccase could not be loaded onto the gel filtration column because of the formation of an insoluble precipitate.
Other procedures.
Protein concentrations in extracellular extracts were determined by using Coomassie blue R-250 reagent (6) and bovine serum albumin as the standard. Glucose contents were determined by using Glucostat reagent (Glucose HK 50; Sigma) as recommended by the manufacturer. For UV-visible light spectroscopic characterization of the Cu centers of the C. thermophilium laccase, the absorbance spectrum was recorded by using 1 mg of purified enzyme per ml in phosphate buffer (pH 6.0).
RESULTS
Isolation of microorganisms with laccase activity.
Screening of thermophilic microorganisms for laccase activity was performed by using 25 samples taken from composted MSW at the thermophilic stage. In this study we focused on one isolate of C. thermophilium, a thermophilic ascomycete that grows at temperatures ranging from 35 to 60°C and exhibits optimum linear growth (3.2 cm day−1) at 50°C (linear growth rates of 0.55, 1.4, 2.7, 2.8, and 0.5 cm day−1 were observed at 35, 40, 45, 55, and 60°C, respectively).
C. thermophilium growth parameters.
When C. thermophilium was grown in liquid culture, laccase activity was detectable at 12 h and reached a maximum value of 3.5 U after 24 h. Laccase activity could still be detected after 10 days at a level of 0.45 U. The glucose concentration decreased from 5 to 0.1 g/liter, and the amount of biomass accumulated increased to 1 mg/ml; stationary phase was reached after 48 h.
Large-scale production and purification of laccase.
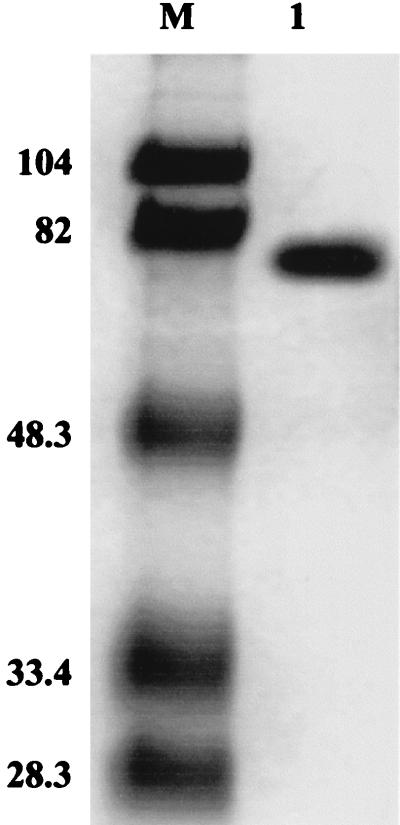
C. thermophilium was grown in a fermentor until the maximum laccase yield was obtained (48 h). The results of the laccase purification procedure are summarized in Table 1. The laccase fraction was obtained by elution with 0.1 to 0.15 M NaCl through an anion-exchange (DEAE) column. Binding of the enzyme to a concanavalin A column indicated that the purified laccase was a glycoprotein. The pure laccase produced one band on an SDS-PAGE gel at a molecular mass of approximately 77 kDa (Fig. 1). A similar result was obtained when 10 μg of the purified enzyme was visualized by silver staining. The isoelectric point of the C. thermophilium laccase was 5.1. The UV-visible light spectrum of the purified C. thermophilium laccase had a very small peak of absorbance at 604 nm, which is typical of type I Cu.
TABLE 1.
Purification of laccase from C. thermophilium
| Step | Vol (ml) | Protein concn (μg/ml) | Total activity (U) | Sp act (U/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|---|
| Culture supernatant | 1,300 | 4 | 22 | 3.6 | 100 | |
| Ultrafiltration | 110 | 16 | 19 | 11 | 84 | 3 |
| DEAE | 26 | 36 | 17 | 19 | 79 | 5 |
| Concanavalin A | 1.2 | 190 | 8.4 | 37 | 40 | 10 |
FIG. 1.
Coomassie blue-stained PAGE gel containing purified C. thermophilium laccase. Lane M contained standard molecular mass protein markers, including phosphorylase b (molecular mass, 104 kDa), bovine serum albumin, (82 kDa), ovalbumin (48.3 kDa), carbonic anhydrase (33.4 kDa), and soybean trypsin inhibitor (28.3 kDa). Lane 1 contained 10 μg of purified C. thermophilium laccase.
Effects of pH and temperature on laccase stability and activity.
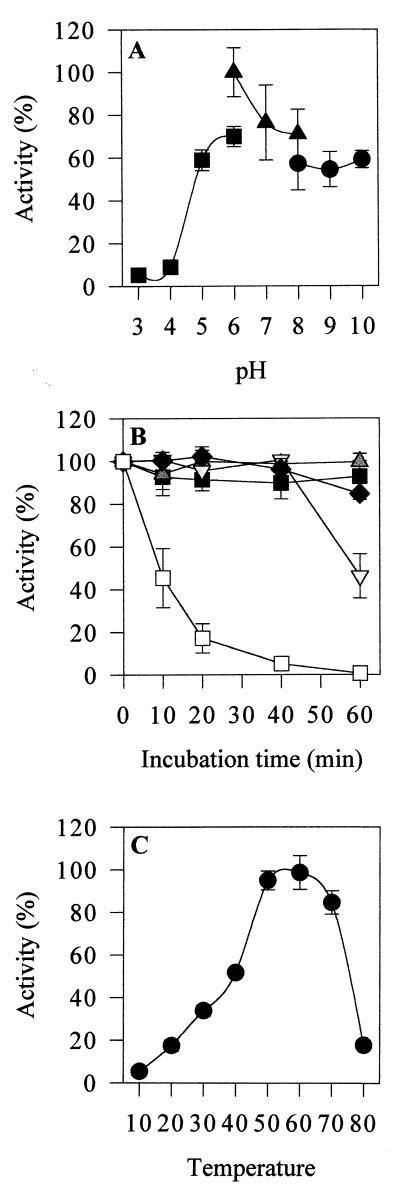
The purified laccase was stable after 1 h of preincubation at pH 5 to 10 (Fig. 2A). Phosphate buffer enhanced laccase activity compared to the activities in both Tris and citrate buffers. The optimum pH for laccase oxidation of syringaldazine was 6. The enzyme retained 20 and 65% of its maximum activity (pH 6 activity) at pH 5 and 8, respectively, but was inactive at pH 4 and 9. Thermal stability was measured after up to 48 h of preincubation. The laccase had a half-lives of 24 h at 40°C and 12 h at 50°C. It was stable for 1 h during preincubation at 70°C but for only 8 min during preincubation at 80°C (Fig. 2B). Preincubation at 40 to 60°C increased enzyme activity compared to the control activity (the activity of the enzyme without preincubation). Thus, we calibrated enzyme activity by using an enzyme preparation preincubated for 2 min at 50°C (100% activity). The optimal temperature range for activity of the purified laccase was 50 to 60°C (Fig. 2C). Enzyme activity was ≤20% at temperatures below 20°C and above 70°C, as determined during 10-min reactions.
FIG. 2.
Effect of pH and temperature on purified C. thermophilium laccase. (A) Enzyme stability after preincubation at various pH values in citrate buffer (■), phosphate buffer (▴), and Tris buffer (•). (B) Enzyme stability after preincubation at 40°C (■), 50°C (▴), 60°C (▾), 70°C (⧫), and 80°C (□). (C) Enzyme activity at various temperatures.
Substrate specificity and inhibition studies.
Laccase is a phenoloxidase which catalyzes the reduction of molecular oxygen to water as a coproduct during the oxidation of a phenolic substrate. Assay mixtures containing HRP were monitored, and no H2O2 was detected. In addition, no oxygen uptake occurred when tyrosine was used as a substrate. Several substituted phenols and aromatic amines were studied as possible substrates (Table 2). The relationship between oxygen uptake (laccase activity) and substrate concentrations produced typical Michaelis-Menten curves. C. thermophilium laccase exhibited high catalytic activity towards a wide range of substrates (o- and p-phenols, syringic acid, and 3,4-dihydroxyphenylalanine). Syringaldazine, which is considered a specific substrate for laccase (36), and hydroxyquinone had the lowest Km values, whereas guaiacol had the highest Km. The highest Vmax was obtained with gallic acid.
TABLE 2.
Kinetic parameters of C. thermophilium laccase for oxidation of various substrates (obtained by measuring the oxygen consumption rates with 10 μg of purified enzymea
| Substrateb | Km (mM) | Vmax (μM O2 min−1) |
|---|---|---|
| ABTS | 0.19 | 2.6 |
| l-DOPA | 0.10 | 1.9 |
| 2,6-DMP | 0.096 | 3.4 |
| Ferulic acid | 0.27 | 3.8 |
| Gallic acid | 0.13 | 5.0 |
| Guaiacol | 0.40 | 2.5 |
| Hydroxyquinone | 0.036 | 1.7 |
| Syringaldazine | 0.034 | 4.1 |
| Vanillic acid | 0.15 | 2.1 |
All values are the means of duplicate measurements, and the coefficients of variation were less than 5%.
ABTS, 2,2′-azinobis(3-ethylbenz-thiazoline-6-sulfonic acid); l-DOPA, l-3,4-dihydroxyphenylalanine; 2,6-DMP, 2,6-dimethoxyphenol.
The purified laccase was strongly inhibited by Cu-chelating agents. The order of inhibition was thioglycolic acid > sodium azide > EDTA (Table 3).
TABLE 3.
Inhibition of laccase purified from C. thermophilium
| Inhibitor | Concn (mM) | % Inhibitiona |
|---|---|---|
| Sodium azide | 0.01 | 12 |
| 0.05 | 84 | |
| 0.1 | 96 | |
| 10 | 96 | |
| EDTA | 1 | 34 |
| 2 | 39 | |
| 5 | 67 | |
| 10 | 95 | |
| 25 | 100 | |
| Thioglycolic acid | 0.1 | 91 |
| 0.2 | 94 | |
| 1.0 | 100 |
All values are the means of triplicate measurements, and the coefficients of variation were less than 5%; 100% activity was the activity obtained when 17-U/ml assay mixture containing 0.1 mM syringaldazine as a substrate was used.
N-terminal amino acid sequencing.
The N-terminal amino acid sequence of the C. thermophilium laccase was unique did not exhibit homology to the sequences of other fungal laccases (Table 4).
TABLE 4.
Comparison of the N-terminal amino acid sequences of C. thermophilium laccase and other ascomycete laccases
| Microorganism | N-terminal amino acid sequence | Reference |
|---|---|---|
| Chaetomium thermophilium | F N P P L L P S L E P | |
| Aspergillus nidulans | M Y L S T V L F P L L | 2 |
| Botrytis cinerea | G T T M H W H G I R Q | 8 |
| Cryphonectria parasitica | M P S F F R A L F S L | 12 |
| Cryptococcus neoformans | X K T D E S P E A V S | 40 |
| Neurospora crassa | G G G G G C N S P T N | 19 |
| Podospora anserina | A P S L P G V P R E V | 17 |
Polymerization study.
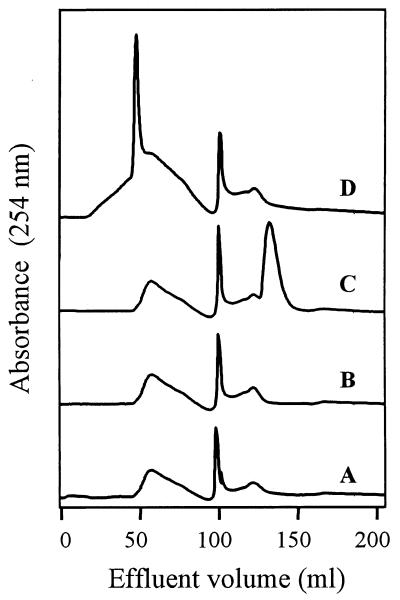
When guaiacol was incubated with laccase in the presence of HoA or FA (extracted from compost and soil, respectively), the color of the mixture changed from light yellow-brown to dark purple after 18 h of incubation. The purple product (oxidized guaiacol bound to HoA or FA) was soluble, and no precipitation was observed. This newly formed compound had a higher molecular weight than either HoA or FA (Fig. 3). As a control, guaiacol alone was incubated with the enzyme. The color of the assay mixture changed rapidly to purple-brown, and a purple precipitate was observed. When HoA or FA was incubated with laccase, no change in color was observed, although both HoA and FA served as laccase substrates (the rates of oxygen consumption were 14.6 and 7.1 μmol of O2 mg−1 min−1, respectively). No color change or oxygen consumption was observed in the control mixtures that contained boiled enzyme.
FIG. 3.
Fast protein liquid gel filtration chromatography profiles of polymerization assay mixtures containing the HoA fraction (line A), HoA and laccase (line B), HoA and guaiacol (line C), and HoA, guaiacol, and laccase (line D).
DISCUSSION
Chaetomium spp. are cellulolytic fungi that are often isolated from decomposed organic matter and mushroom compost (9, 14, 16, 32). In this work we focused on the laccase of a thermophilic strain of C. thermophilium isolated from composted MSW. The optimal temperature range for fungal laccase activity is 30 to 60°C (25, 27, 30, 39, 41). However, none of the laccases studied previously was produced by thermophilic fungi. The laccase examined in this study had a temperature optimum between 50 and 60°C and was stable at higher temperatures than the laccases purified from mesophilic fungi, such as Pyncoporus cinnabarinus (15).
Fungal laccases are generally active at low pH values (pH 3 to 5) (4, 15, 18, 25, 27, 30). In contrast, the C. thermophilium laccase exhibited maximum activity at pH 6 to 8 and resembled the laccases isolated directly from composted MSW (10).
C. thermophilium laccase, like other fungal laccases (7), nonspecifically oxidized a wide range of substrates, but not tyrosine. The nature and substitution of the phenolic ring affected the oxidation rate and the affinity of the laccase. Monohydroxy-substituted phenols (hydroxyquinone and l-3,4-dihydroxyphenylalanine) had lower oxidation rates than methoxylated compounds (guaiacol and ferulic acid). Addition of a carboxylic acid to the aromatic ring increased the oxidation rate (cf. vanillic acid and guaiacol). Increasing the number of methoxy substituents also increased the oxidation rate (cf. 2,6 dimethoxyphenol and guaiacol or 2,6 dimethoxyphenol and syringaldazine). In general, increasing the number of substituted methoxyl groups increased the oxidation rate.
C. thermophilium laccase was strongly inhibited by Cu-chelating agents. The purified laccase was sensitive to 0.1 mM sodium azide and thioglycolic acid, like other fungal laccases (4, 15). The C. thermophilium laccase was much less sensitive to metal chelation by EDTA than the laccases of Coriolus hirsutus and P. cinnabarinus (15). In general, the inhibition profile of C. thermophilium laccase was similar to that of laccase A extracted directly from composted MSW (10).
The N-terminal sequence of the C. thermophilium laccase does not exhibit homology to other fungal laccase N-terminal sequences, although similar oxidative activities were observed. It is worth noting that the other known ascomycete laccase N-terminal amino acid sequences do not show sequence similarity, unlike the amino acid sequences of some laccases of lignin-degrading basidiomycetes (2, 8, 12, 15, 17, 19, 25, 38).
Fungal laccases purified from cultures or soils often polymerize phenolic substrates in vitro (5, 24, 35). Laccases can transform guaiacol, 1-naphthol, and vanillic acid into oligomeric products (dimers to pentamers) (33). In vitro experiments have revealed enzymatic covalent binding of aromatic xenobiotic compounds (2,4-dichlorophenol and pentachlorophenol) to humic acid or FA (21, 26). This binding demonstrates the importance of the oxidative coupling reaction (detoxification) and repolymerization in the environment (7, 20).
Aromatic and phenolic compounds are precursors in the formation of HS, and several authors (31, 37) have suggested that laccase plays a role in humification in soils. One hypothesis for humic acid formation is that quinones polymerize to form humic macromolecules. According to this hypothesis, polyphenols are formed from lignin degradation products or are synthesized by microorganisms from nonaromatic sources. Previously, we (11) suggested that one mechanism for humification in compost involves the formation of water-soluble apolar aromatic compounds (HoA) during the last stage of composting. This fraction resembles low-molecular-weight FA. We tested this hypothesis by incubating mixtures of guaiacol and HoA or FA with laccase. The reaction of C. thermophilium laccase with HoA and guaiacol resulted in the formation of a new high-molecular-weight soluble product by a coupling reaction. This mechanism may explain the increasing aromaticity of the HoA fraction during composting (11).
We conclude that laccase produced by thermophilic fungi such as C. thermophilium could be involved in polymerization that yields humic macromolecules. This reaction represents one way in which humification may occur during composting. In this process, natural and xenobiotic phenols are oxidized by laccase present in the compost environment, resulting in the formation of free radicals which can be spontaneously bound to soluble high-molecular-weight compounds, which results in humic macromolecules.
ACKNOWLEDGMENTS
This research was sponsored in part by GIF.
We thank R. A. Samson (Centaalbureau voor Schimmelcultures) for assistance with C. thermophilium identification.
REFERENCES
- 1.Adani F, Genevini P L, Tambone F. A new index of organic matter stability. Compost Sci Utiliz. 1995;3:25–37. [Google Scholar]
- 2.Aramayo R, Timberlake W E. Sequence and molecular structure of the Aspergillus nidulans yA (laccase I) gene. Nucleic Acids Res. 1990;18:3415–3415. doi: 10.1093/nar/18.11.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavendamm W. Uber das Vorkommen und den Nachweis von Oxydasen bei holzzerstoerenden Pilzen. Z Pflanzenkr Pflanzenschutz. 1928;38:257–276. [Google Scholar]
- 4.Bollag J M, Leonowicz A. Comparative studies of extracellular fungal laccase. Appl Environ Microbiol. 1984;48:849–853. doi: 10.1128/aem.48.4.849-854.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bollag J M, Liu S Y, Minard R D. Enzymatic oligomerization of vanillic acid. Soil Biol Biochem. 1982;14:157–163. [Google Scholar]
- 6.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Call H P, Mücke I. History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (lignozym®-process) J Biotechnol. 1997;53:163–202. [Google Scholar]
- 8.Cantone F A, Staples R C. A laccase-cDNA from Botrytis cinerea. Phytopathology. 1993;83:1383–1383. [Google Scholar]
- 9.Chang Y, Hudson H J. The fungi of wheat straw compost. Trans Br Mycol Soc. 1967;50:649–666. [Google Scholar]
- 10.Chefetz B, Kerem Z, Chen Y, Hadar Y. Laccase from composted municipal solid waste: purification and characterization. Soil Biol Biochem. 1998;30:1091–1098. [Google Scholar]
- 11.Chefetz B, Hatcher P G, Hadar Y, Chen Y. Characterization of dissolved organic matter extracted from composted municipal solid waste. Soil Sci Soc Am J. 1998;62:326–332. [Google Scholar]
- 12.Choi G H, Larson T G, Nuss D L. Molecular analysis of the laccase gene from chestnut blight fungus and selective suppression of its expression in an isogenic hypovirulent strain. Mol Plant Microbe Interact. 1992;5:119–128. doi: 10.1094/mpmi-5-119. [DOI] [PubMed] [Google Scholar]
- 13.Ciavatta C, Govi M, Pasotti L, Sequi P. Changes in organic matter during stabilization of compost from municipal solid waste. Bioresource Technol. 1993;43:141–145. [Google Scholar]
- 14.Deacon J W. Decomposition of filter paper by thermophilic fungi acting singly, in combination and in sequence. Trans Br Mycol Soc. 1985;85:633–670. [Google Scholar]
- 15.Eggert C, Temp U, Eriksson K L. The ligninolytic system of the white rot fungus Pyncoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksen J, Goksoyr J. Cellulases from Chaetomium thermophilium var. dissitum. Eur J Biochem. 1977;77:445–450. doi: 10.1111/j.1432-1033.1977.tb11685.x. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Larrea J, Stahl U. Isolation and characterization of a laccase gene from Podospora anserina. Mol Gen Genet. 1996;252:539–551. doi: 10.1007/BF02172400. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima Y, Kirk T K. Laccase component of the Ceriporiopsis subvermispora lignin-degrading system. Appl Environ Microbiol. 1995;61:872–876. doi: 10.1128/aem.61.3.872-876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germann U A, Müller G, Hunziker P E, Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. J Biol Chem. 1988;263:885–896. [PubMed] [Google Scholar]
- 20.Haars A, Hüttermann A. Function of laccase in the white-rot fungus Fomes annosus. Arch Microbiol. 1980;125:233–237. [Google Scholar]
- 21.Hatcher P G, Bortiatynski J M, Minard R D, Dec J, Bollag J M. Use of high-resolution 13C NMR to examine the enzymatic covalent binding of 13C-labeled 2,4-dichlorophenol to humic substances. Environ Sci Technol. 1993;27:2098–2103. [Google Scholar]
- 22.Inbar Y, Chen Y, Hadar Y. Humic substances formed during the composting of organic matter. Soil Sci Soc Am J. 1990;54:1316–1323. [Google Scholar]
- 23.Jimenez E, Garcia P. Determination of maturity indices for city refuse composts. Agric Ecosyst Environ. 1992;38:331–343. [Google Scholar]
- 24.Katase T, Bollag J M. Transformation of trans-4-hydroxycinnamic acid by a laccase of the fungus Trametes versicolor: its significance in humification. Soil Sci. 1991;151:291–296. [Google Scholar]
- 25.Munoz C, Guillen F, Martinez A T, Martinez M J. Laccase isoenzymes of Pleurotus eryngii: characterization, catalytic properties, and participation in activation of oxygen and Mn2+ oxidation. Appl Environ Microbiol. 1997;63:2166–2174. doi: 10.1128/aem.63.6.2166-2174.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nanny M A, Bortiatynski J M, Tien M, Hatcher P G. Investigation of enzymatic alterations of 2,4-dichlorophenol using 13C-nuclear magnetic resonance in combination with site-specific 13C-labeling: understanding the environmental fate of this pollutant. Environ Toxicol Chem. 1996;15:1857–1864. [Google Scholar]
- 27.Nishizawa Y, Nakabayashi K, Shinagawa E. Purification and characterization of laccase from white rot fungus Trametes sanguinea M85-2. J Ferment Bioeng. 1995;80:91–93. [Google Scholar]
- 28.Rüttimann-Johnson C, Lamar R T. Polymerization of pentachlorophenol and ferulic acid by fungal extracellular lignin-degrading enzymes. Appl Environ Microbiol. 1996;62:3890–3893. doi: 10.1128/aem.62.10.3890-3893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shevchenko S M, Bailey G W. Life after death: lignin-humic relationships reexamined. Crit Rev Environ Sci Technol. 1996;26:95–154. [Google Scholar]
- 30.Slomczynski D, Nakas J P, Tanenbaun S W. Production and characterization of laccase from Botrytis cinerea 61-34. Appl Environ Microbiol. 1995;61:907–912. doi: 10.1128/aem.61.3.907-912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson F J. Humus chemistry. New York, N.Y: John Wiley & Sons Inc.; 1994. [Google Scholar]
- 32.Straatsma G, Samson R A, Olijnsma T W, Op-Den-Camp H J M, Gerrits J P G, Griensven L J L D, Van-Griensven L J L D. Ecology of thermophilic fungi in mushroom compost, with emphasis on Scytalidium thermophilum and growth stimulation of Agaricus bisporus mycelium. Appl Environ Microbiol. 1994;60:454–458. doi: 10.1128/aem.60.2.454-458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suflita J M, Bollag J M. Polymerization of phenolic compounds by soil-enzyme complex. Soil Sci Soc Am J. 1981;45:297–302. [Google Scholar]
- 34.Tarchitzky J, Chen Y, Banin A. Humic substances and pH effects on sodium- and calcium-montmorillonite flocculation and dispersion. Soil Sci Soc Am J. 1993;57:367–372. [Google Scholar]
- 35.Tatsumi K, Freyer A, Minard R D, Bollag J M. Enzymatic coupling of chloroanilines with syringic acid, vanillic acid and protocatechuic acid. Soil Biol Biochem. 1994;26:735–742. [Google Scholar]
- 36.Thurston C F. The structure and function of fungal laccase. Microbiology. 1994;140:19–26. [Google Scholar]
- 37.Varadachari C, Ghosh K. On humus formation. Plant Soil. 1984;77:305–313. [Google Scholar]
- 38.Williamson P R. Biochemical and molecular characterization of the diphenol oxidase of Cryptococus neoformans: identification as a laccase. J Bacteriol. 1994;176:656–664. doi: 10.1128/jb.176.3.656-664.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood D A. Inactivation of extracellular laccase during fruiting of Agaricus bisporus. J Gen Microbiol. 1980;117:339–345. [Google Scholar]
- 40.Yaropolov A I, Skorobogatko O V, Vartanov S S, Varfolomeyev S D. Laccase properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol. 1994;49:257–280. [Google Scholar]
- 41.Youn H D, Hah Y C, Kang S. Role of laccase in lignin degradation by white rot fungi. FEMS Microbiol Lett. 1995;132:183–188. [Google Scholar]