Abstract
Tritiated 5-azidoindole-3-acetic acid (5-N3-[7-3H]IAA), a photoaffinity labeling agent, was used to photolabel proteins of a crude microsomal preparation from maize (Zea mays L., Bear Hybrid, WF9 × BR38) coleoptile. Approximately 50% of the bound radioactivity was solubilized in 5 molar urea containing Triton X-100, and the extract was fractionated using a variety of techniques. High performance liquid chromatography demonstrated that, although many membrane proteins incorporated tritiated label, only a few showed reduced incorporation in the presence of excess indole-3-acetic acid. By contrast, no detectable reduction in incorporation was observed in the presence of excess naphthalene-1-acetic acid. Results from isoelectric focusing gel electrophoresis indicate that the proteins that showed reduced incorporation of photolyzed 5-N3-[7-3H]IAA in the presence of IAA fell into two main groups: one which focuses between pH 5.2 and 5.7 (pI 4.8-5.3) and another around pH 6.2 (pI 5.8). In sodium dodecylsulfate polyacrylamide gel electrophoresis, the proteins migrated as four bands with apparent molecular weights of 60, 49, 45, and 37 kilodaltons. The auxin-transport inhibitor, 2,3,5-triiodobenzoic acid, competes for the labeling by 5-N3-[7-3H]IAA, suggesting that some of these proteins may be involved in auxin transport.
Full text
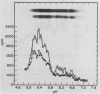
PDF
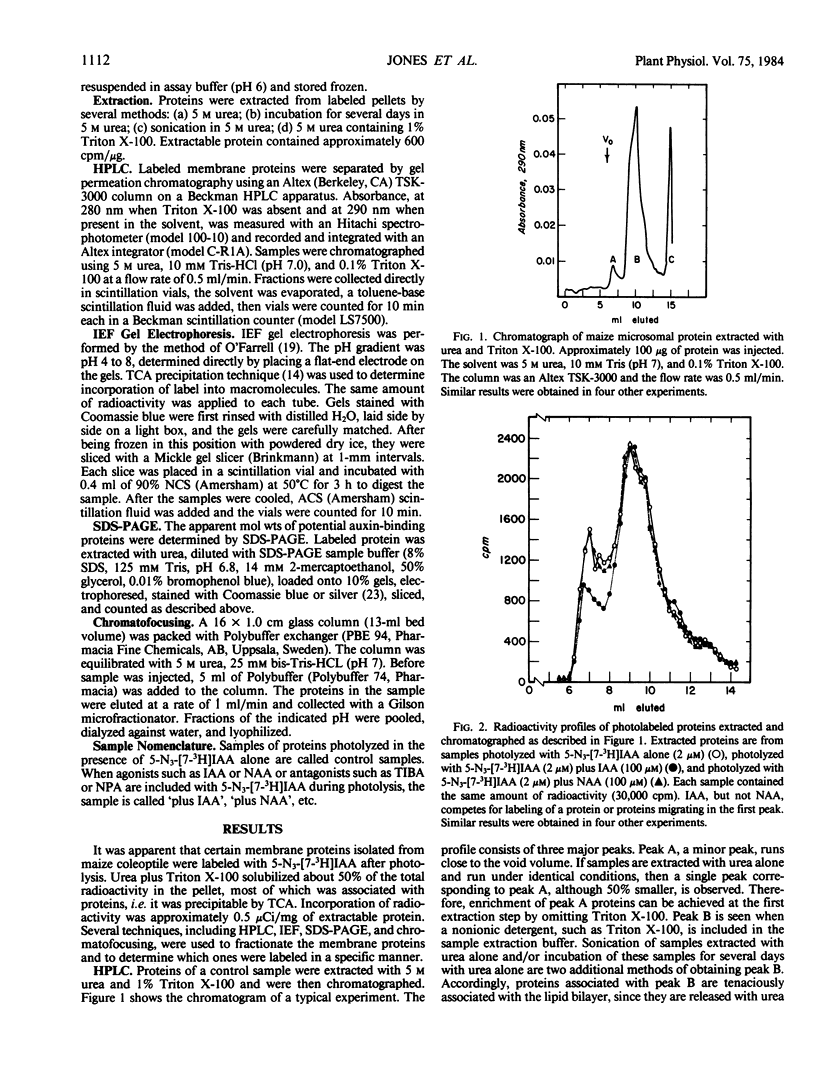
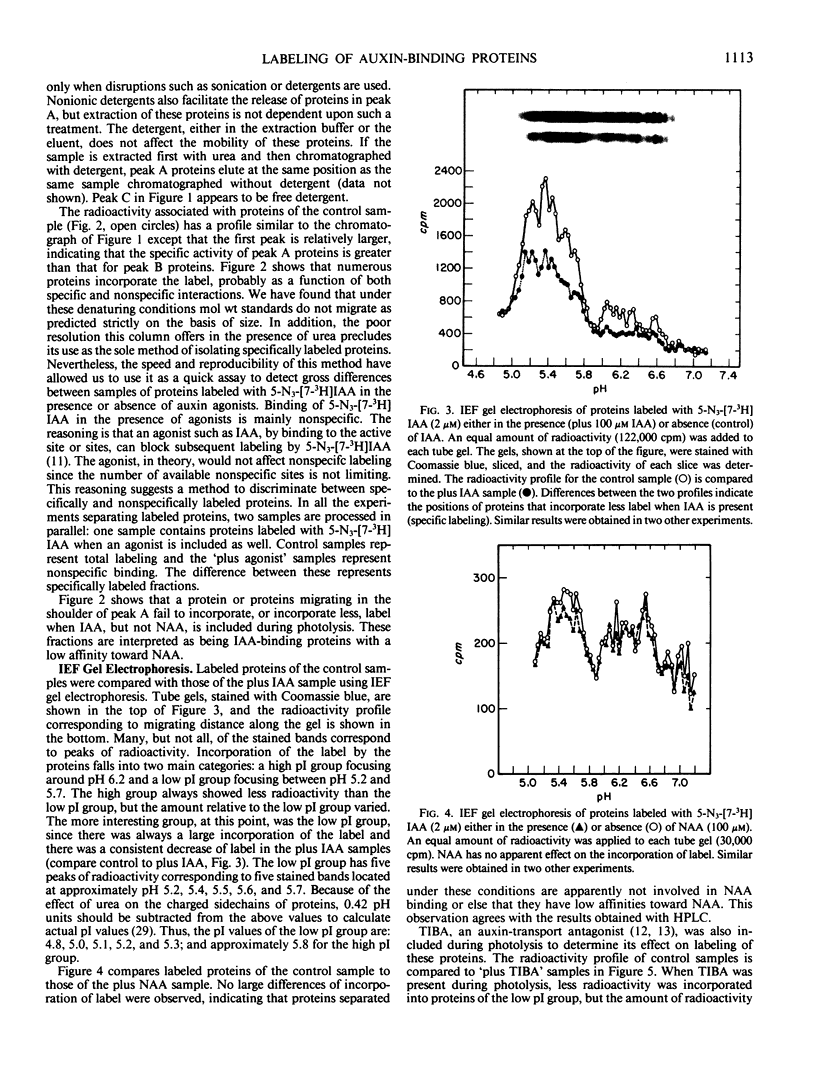
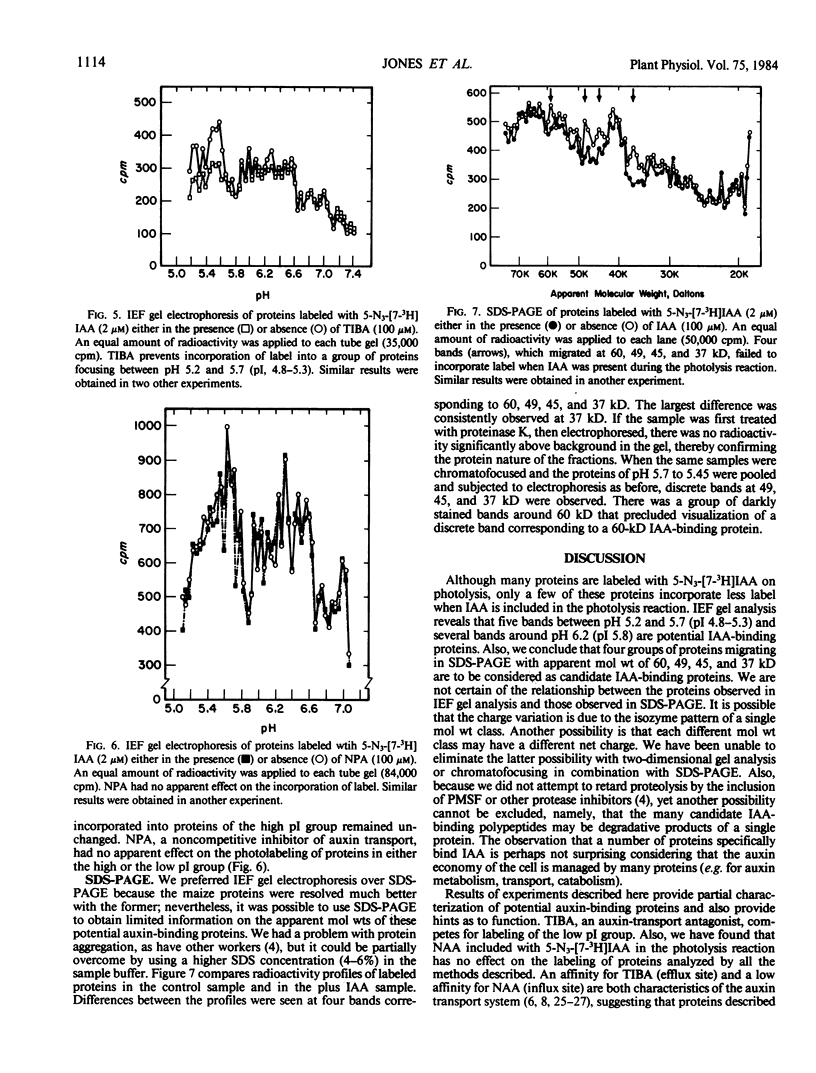


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chowdhry V., Westheimer F. H. Photoaffinity labeling of biological systems. Annu Rev Biochem. 1979;48:293–325. doi: 10.1146/annurev.bi.48.070179.001453. [DOI] [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Properties of a Solubilized Microsomal Auxin-binding Protein from Coleoptiles and Primary Leaves of Zea mays. Plant Physiol. 1978 Jul;62(1):152–157. doi: 10.1104/pp.62.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Jones A. M., Melhado L. L., Ho T. H., Leonard N. J. Azido auxins: quantitative binding data in maize. Plant Physiol. 1984 Feb;74(2):295–301. doi: 10.1104/pp.74.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katekar G. F., Geissler A. E. Auxin Transport Inhibitors: III. Chemical Requirements of a Class of Auxin Transport Inhibitors. Plant Physiol. 1977 Dec;60(6):826–829. doi: 10.1104/pp.60.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katekar G. F., Geissler A. E. Auxin Transport Inhibitors: IV. EVIDENCE OF A COMMON MODE OF ACTION FOR A PROPOSED CLASS OF AUXIN TRANSPORT INHIBITORS: THE PHYTOTROPINS. Plant Physiol. 1980 Dec;66(6):1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANS R. J., NOVELLI G. D. A convenient, rapid and sensitive method for measuring the incorporation of radioactive amino acids into protein. Biochem Biophys Res Commun. 1960 Nov;3:540–543. doi: 10.1016/0006-291x(60)90171-6. [DOI] [PubMed] [Google Scholar]
- Melhado L. L., Jones A. M., Ho T. H., Leonard N. J. Azido auxins : photolysis in solution and covalent binding to soybean. Plant Physiol. 1984 Feb;74(2):289–294. doi: 10.1104/pp.74.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhado L. L., Jones A. M., Leonard N. J., Vanderhoef L. N. Azido auxins: synthesis and biological activity of fluorescent photoaffinity labeling agents. Plant Physiol. 1981 Aug;68(2):469–475. doi: 10.1104/pp.68.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Solubilization and purification of a prolactin receptor from the rabbit mammary gland. J Biol Chem. 1974 Dec 25;249(24):7902–7911. [PubMed] [Google Scholar]
- Sussman M. R., Gardner G. Solubilization of the receptor for N-1-naphthylphthalamic Acid. Plant Physiol. 1980 Dec;66(6):1074–1078. doi: 10.1104/pp.66.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M., Krull U. J., Venis M. A. A chemoreceptive bilayer lipid membrane based on an auxin-receptor ATPase electrogenic pump. Biochem Biophys Res Commun. 1983 Jan 14;110(1):300–304. doi: 10.1016/0006-291x(83)91295-0. [DOI] [PubMed] [Google Scholar]
- Ui N. Isoelectric points and conformation of proteins. I. Effect of urea on the behavior of some proteins in isoelectric focusing. Biochim Biophys Acta. 1971 Mar 23;229(3):567–581. [PubMed] [Google Scholar]
- Walton J. D., Ray P. M. Evidence for Receptor Function of Auxin Binding Sites in Maize : RED LIGHT INHIBITION OF MESOCOTYL ELONGATION AND AUXIN BINDING. Plant Physiol. 1981 Dec;68(6):1334–1338. doi: 10.1104/pp.68.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]