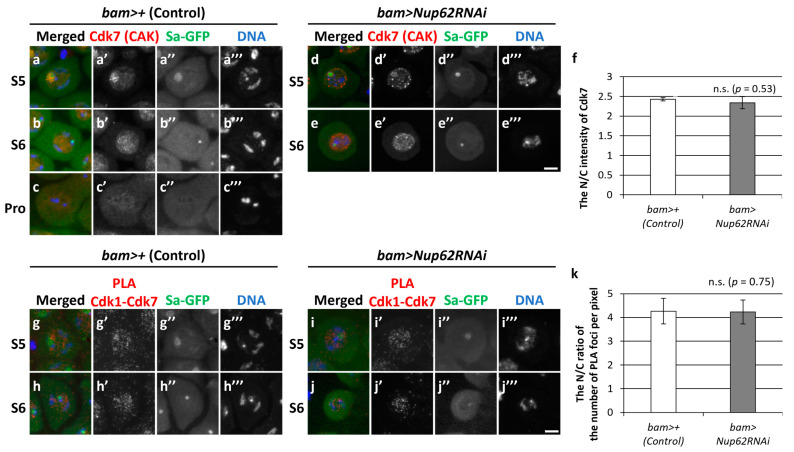
Figure 5.
Intracellular localization of Cdk7 and in situ PLA results to monitor a close association between Cdk1 and Cdk7 in spermatocytes at S5, S6, and Prophase I (Pro). (a–e) Anti-Cdk7 immunostaining of normal (a–c) and Nup62-silenced (d,e) spermatocytes in the growth phase ((a,d) at S5; (b,e) at S6) and prophase (Pro) (c). Images show anti-Cdk7 immunofluorescence (red in (a–e), white in (a’–e’)), Sa-GFP fluorescence for visualizing the nucleolus (green in (a–e), white in (a’’–e’’)), and DNA staining with DAPI (blue in (a–e), white in (a’’’–e’’’)). Scale bar: 10 μm. (f) Ratio of the intensity of anti-Cdk7 immunofluorescence in the nucleus to that in the cytoplasm. The fluorescence intensity of the spermatocytes at S5 to Pro was measured. The mean ratio of the intensity in the cytoplasm to that in the nucleus (N/C intensity) was displayed as a white bar (control cells) or a gray bar (Nup62RNAi cells). Data are presented as means ± 95% CIs (n >16 cells for each genotype). Significance was tested using the Mann–Whitney test; n.s.: not significant. (g–j) In situ PLA to detect the close interaction between Cdk1 and Cdk7 in normal (g,h) and Nup62-silenced (i,j) spermatocytes at S5 (g,i) and S6 (h,j). Scale bar: 10 μm. (k) Ratio of the number of PLA signals in the nucleus to that in the cytoplasm. The number of PLA-positive foci in the nucleus or cytoplasm of each spermatocyte at the S5 stage was counted. The number per pixel in each compartment was calculated. The ratio of the number in the cytoplasm to that in the nucleus (N/C intensity) was displayed on the y-axis. Data are presented as means ± 95% CIs (n > 300 cells). Significance was tested using the Mann–Whitney test.