Abstract
The iron(III) reductase activity of Geobacter sulfurreducens was determined with the electron donor NADH and the artificial electron donor horse heart cytochrome c. The highest reduction rates were obtained with Fe(III) complexed by nitrilotriacetic acid as an electron acceptor. Fractionation experiments indicated that no iron(III) reductase activity was present in the cytoplasm, that approximately one-third was found in the periplasmic fraction, and that two-thirds were associated with the membrane fraction. Sucrose gradient separation of the outer and cytoplasmic membranes showed that about 80% of the iron(III) reductase was present in the outer membrane. The iron(III) reductase could be solubilized from the membrane fraction with 0.5 M KCl showing that the iron(III) reductase was weakly bound to the membranes. In addition, solubilization of the iron(III) reductase from whole cells with 0.5 M KCl, without disruption of cells, indicated that the iron(III) reductase is a peripheral protein on the outside of the outer membrane. Redox difference spectra of KCl extracts showed the presence of c-type cytochromes which could be oxidized by ferrihydrite. Only one activity band was observed in native polyacrylamide gels stained for the iron(III) reductase activity. Excision of the active band from a preparative gel followed by extraction of the proteins and sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed the presence of high-molecular-mass, cytochrome-containing proteins in this iron(III) reductase activity band. From these experimental data it can be hypothesized that the iron(III) reductase of G. sulfurreducens is a peripheral outer membrane protein that might contain a c-type cytochrome.
Dissimilatory microbial iron(III) reduction has been proposed as the most important chemical change that takes place in the development of certain anaerobic soils and sediments (26). Iron(III) reduction may have been the first globally significant mechanism for the oxidation of organic matter to carbon dioxide (17). Several studies have demonstrated that iron(III) reduction accounts for the oxidation of up to 65% of organic matter in anaerobic sediments (7, 17). Microbial oxidation coupled with iron(III) reduction is an important natural mechanism for contaminant removal from groundwater or shallow aquifers polluted with landfill leachates (1). Furthermore, iron(III) reduction can influence the biogeochemical cycles of important nutrients, such as carbon, sulfur, and phosphorus (17, 28). Up to now, numerous iron(III)-reducing bacteria have been described in the literature (8, 16). These bacteria are versatile towards electron donors and acceptors which can be used for growth.
Although iron(III)-reducing bacteria have been studied for many years, little is known about the biochemistry and the mechanism of iron(III) reduction. Iron(III)-reducing bacteria are able to utilize Fe(III) either chelated or in insoluble inorganic minerals such as ferrihydrite, goethite, and others. The question arises whether Fe(III) is taken up and reduced in the periplasm or cytoplasm or whether electrons are transferred to Fe(III) minerals outside of the cells. Evidence has been presented that a direct contact between the cells and the metal oxide is necessary for the conservation of energy (3). Iron(III) reductase activity was found to be predominantly in the membranes of the iron(III)-reducing bacteria (23). In the case of Shewanella putrefaciens, 54 to 56% of this activity was localized in the outer membrane (23). The involvement of b- and c-type cytochromes as electron carriers in the iron(III) respiration chain has been reported previously (23). In addition, it has been shown that the outer membranes of anaerobically grown cells of S. putrefaciens have a high cytochrome content (22). This suggests that those cytochromes localized in the outer membrane may play a key role in iron(III) reduction. In this study, we report investigations on the localization of the iron(III) reductase activity of Geobacter sulfurreducens with a new enzyme assay. Results obtained by isolating the outer membrane and by treating whole cells with a high KCl concentration to solubilize the iron(III) reductase indicated that the iron(III) reductase of G. sulfurreducens is a peripheral protein of the outer membrane. Evidence is presented that a c-type cytochrome is involved in iron(III) reduction.
MATERIALS AND METHODS
Growth conditions.
G. sulfurreducens was cultivated anaerobically in 1-liter bottles filled with 0.7 liter of defined medium (5). One liter of basal medium contained the following: 2.5 g of NaHCO3, 1.5 g of NH4Cl, 0.68 g of NaH2PO4, 0.1 g of KCl, 6.8 g of CH3COONa, 13.7 g of Fe(III)-citrate, 10 ml of vitamin solution, and 10 ml of trace element solution, whose composition was as described previously (19). The pH was adjusted to 6.8. The bottles were sealed with butyl rubber stoppers, and the headspace was replaced with N2-CO2 (80%-20% [vol/vol]). Cultures were incubated in the dark for 4 days without shaking at 30°C.
Cell harvest and preparation of subcellular fractions.
All steps were carried out in an anaerobic glove box (Coy Laboratory, Ann Arbor, Mich.). If not mentioned otherwise, N2-saturated Tris buffer (100 mM, pH 8.1), containing 0.1 mM dithiothreitol, was used. G. sulfurreducens cultures in the late-growth phase were acidified to pH 5 to dissolve iron(II) precipitates, harvested by centrifugation (10,000 × g, 20 min, 4°C), and washed three times with Tris buffer. To prepare cellular fractions, a protocol adapted from a method described by Myers and Myers (22) was used. For the preparation of spheroplasts, cells (110 mg of protein) were resuspended in 30 ml of the Tris buffer containing 25% (wt/vol) sucrose. To accomplish cell wall lysis, a 1/10 volume of a lysozyme solution (6.4 mg · ml−1) and a 1/10 volume of a Na2-EDTA solution (20 mg · ml−1) were added with constant stirring at 15-min intervals. Finally, MgCl2 (0.1 M) was added to a final concentration of 12.8 mM. Separation of spheroplasts from the periplasmic fraction was obtained by centrifugation at 20,000 × g for 30 min. Spheroplasts (pellet) were resuspended in Tris buffer to a final protein concentration of 9 mg/ml.
To obtain the membrane and soluble fraction, a few crystals of DNase were added to an EDTA-lysozyme-treated cell suspension, and the cells were broken by ultrasonic treatment (10 times, 100 W, 30 s) with a sonifier (model 250; Branson Ultrasonics, Danbury, Conn.) in an ice-water bath. Cell debris was removed by centrifugation (4,000 × g, 10 min, 4°C). The crude extract (supernatant) was centrifuged at 200,000 × g (1 h, 4°C), yielding the soluble fraction containing the cytoplasmic and periplasmic fractions and the membrane fraction (pellet). The latter was resuspended in Tris buffer to a final concentration of 15 mg/ml. The protein concentration was determined by using bicinchoninic acid as described previously (30), with bovine serum albumin as a standard.
Separation of outer and cytoplasmic membranes.
Separation of outer and cytoplasmic membranes was achieved according to a procedure adapted from Myers and Myers (22). An aliquot of 1 ml of the membrane fraction containing 1,585 mg of protein was layered onto 10.5 ml of a 50 to 80% (wt/wt) sucrose gradient and centrifuged for 17 h at 82,500 × g. Two colored discrete bands were observed, one between 5 and 8 cm from the bottom of the tube (low-density band) and one on the bottom of the tube (high-density band). The bands were retrieved from the tube by removing fractions of 0.5 to 1 ml with a syringe. The fractions were resuspended in and subsequently dialyzed for 5 h at 4°C against a 10 mM HEPES buffer containing 0.1 mM dithioerythreitol (DTE). To identify the fraction containing the outer membrane, the content of KDO (2-keto-3-deoxyoctonate), a specific constituent of the lipopolysaccharide in the outer membrane, was determined (13).
Enzyme assay.
Iron(III) reductase activity was assayed anaerobically in 1-cm cuvettes by photometric monitoring of the appearance of Fe(II) over time as adapted from the method of Lascelles and Burke (15). The reaction mixture contained either 1 mM NADH or 4 μM dithionite-reduced horse heart cytochrome c as the electron donor, 0.5 mM ferrozine as the Fe(II) chelating agent, and either 0.15 mM Fe(III)-nitriloacetic acid (NTA), Fe(III)-citrate, or Fe(III)-EDTA as electron acceptor in N2-saturated 50 mM HEPES buffer (pH 7). Horse heart cytochrome c was added from a stock solution that was prepared by adding 50 μl of a 4.6 mM sodium dithionite solution to 1 ml of 50 mM HEPES buffer (pH 7) containing 2 mg of horse heart cytochrome c. The concentration of reduced horse heart cytochrome c in the sample cuvette was calculated by measuring the A552 (A552 = 29,500 M−1 · cm−1). The enzyme assay was started by adding whole cells or cell extracts of G. sulfurreducens (0.03 to 0.5 mg of protein) to both sample and reference cuvettes. The activity was monitored by measuring the increase in A562 (32) due to the formation of a Fe(II)-ferrozine complex (A562 = 28,000 M−1 · cm−1) against a reference where no electron donor was added. An activity of 1 mU corresponded to 1 nmol of Fe(II) formed per min. In the absence of ferrozine, the enzyme activity could be also monitored by measuring the decrease in A552 due to the reoxidation of the reduced horse heart cytochrome c as previously described (A552 = 29,500 M−1 · cm−1) (12).
UV-visible spectroscopy.
UV-visible spectra of KCl-extracted proteins were recorded on a U-2000 spectrophotometer (Hitachi, Tokyo, Japan) in a 1-cm quartz cuvette containing 0.1 to 0.3 mg of protein/ml. To record the redox difference spectra of KCl extracts, the protein solution was reduced with sodium dithionite and subsequently reoxidized by adding increasing amounts of an anaerobic solution of ferrihydrite to the reaction mixture in order to obtain final concentrations ranging from 5.4 to 13.7 mM.
Extraction of iron(III) reductase.
Membrane fractions, crude extracts or whole cells were stirred for 1 h at 4°C in a high-ionic-strength salt buffer (100 mM Tris, 0.1 mM DTE, 0.5 M KCl [pH 7.6]) in order to release the iron(III) reductase.
Native polyacrylamide gel electrophoresis (PAGE) stained for the iron(III) reductase.
For detection of enzymatic reduction of Fe(III) in the presence of ferrozine in native polyacrylamide gels, a procedure adapted from Moody and Dailey (21) was used. An aliquot of 40 μl of the KCl-extracted proteins containing 950 μg of protein after dialysis and concentration (Amicon ultrafiltration cell with PM-10 filter) was applied to a 1-mm, 12% Ready gel (Bio-Rad). The gel was run for 3 h at 10 mA. The gel was removed and washed for 1 min in 200 ml of 0.1 mM Tris buffer (pH 7.6). After the washing buffer was removed, the gel was placed in 170 ml of the same buffer containing 30 mg of NADH, 2 mg of reduced horse heart cytochrome c, and 107 mg of ferrozine. The reaction was started by adding 150 μl of a 300 mM Fe(III)-NTA solution. A purple band was observed where iron(III) reduction took place after 30 min of incubation at ambient temperature. Photographs could then be taken.
To isolate the protein of the activity band, a preparative gel was performed according to the following procedure. The dialyzed and concentrated KCl-extracted proteins were applied on the 8 lanes of the 1-mm, 12% Ready gel. To avoid disturbance due to iron for further processing, one lane was separated from the others by cutting the gel and then was stained for the iron(III) reductase activity. With this procedure, the position of the active band of the other lanes could be estimated and the bands could be excised. These bands were suspended in 5 ml of 50 mM HEPES (pH 7) containing 0.1 mM DTE and homogenized on ice with a potter. Sodium dodecyl sulfate (SDS) was added to a final concentration of 1%. The solution was incubated for 30 min at 50°C and then for 90 min at 37°C. The sample was centrifuged for 5 min at 12,000 × g. The proteins of the supernatant were precipitated by adding 11 ml of ethanol to 4 ml of supernatant followed by incubation at −20°C for 2 days. Precipitated proteins were collected by centrifugation at 12,000 × g for 10 min and then resuspended in 100 μl of H2O.
Denaturing SDS-PAGE analysis of KCl-extracted proteins.
SDS-PAGE was carried out as described by Laemmli (14) after the samples were desalted by dialysis. Proteins were visualized by a silver-staining procedure (27), and the cytochromes were stained by a method with o-dianisidine and hydrogen peroxide (10).
Chemicals.
Ferrozine, DNase I, horse heart cytochrome c, NADPH, safranin T, phenosafranin, DTE, and o-dianisidine-HCl tablets were purchased from Fluka (Buchs, Switzerland); NADH, neutral red, and bromophenol blue were obtained from Merck (Darmstadt, Germany); and benzyl viologen, methyl viologen, rotenone, and 2-n-heptyl-4-hydroxyquinoline N-oxide (HOQNO) were from Sigma (Munich, Germany).
RESULTS
Iron(III) reductase activity.
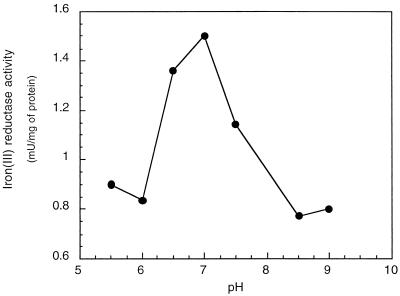
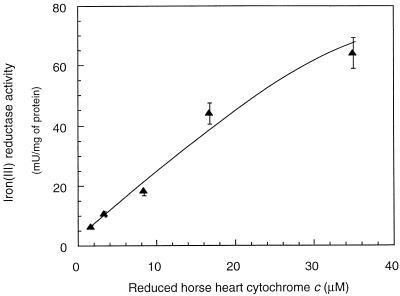
The iron(III) reductase activity of G. sulfurreducens could be measured with NADH but not with NADPH (Table 1). If Fe(III) NTA was used as an electron acceptor, the highest Fe(III)-reducing activities were measured (10.6 mU/mg of protein), whereas with Fe(III)-citrate and Fe(III)-EDTA, lower (2.1 mU/mg of protein) and no activities were detected, respectively. Cell extracts that were heated at 100°C for 5 min showed no iron(III) reductase activity. Exposure to air for 2 h decreased the activity by 80%, indicating an oxygen lability of the iron(III) reductase activity. The activity was highest at pH 7 (Fig. 1). In order to develop an NADH-independent assay, different artificial electron donors were tested (Table 1). The major difficulty was the rapid chemical reduction of Fe(III) by these artificial electron donors, which did not allow us to distinguish between an enzymatic and chemical reduction. Also, reducing agents such as cysteine, dithionite, and Ti(III) citrate could not be used to reduce the iron(III) reductase since they also reduced Fe(III) chemically. Only horse heart cytochrome c reduced by dithionite could be used as an alternative electron donor (Table 1). The iron(III) reductase activity increased with increasing concentrations of reduced horse heart cytochrome c (Fig. 2). However, at concentrations above 35 μM, activity measurements with ferrozine were not possible due to a background absorption by reduced horse heart cytochrome c at 562 nm that was too high. The iron(III) reductase activity could alternatively be determined by measuring the oxidation of reduced horse heart cytochrome c at 552 nm. In this case, due to the high absorbance of the reduced horse heart cytochrome c, it was not possible to use concentrations above 55 μM, and the highest iron(III) reductase activity measured was of 75 mU/mg of protein.
TABLE 1.
Electron donors tested for iron(III) reductase activitya
| Electron donor | Concn (mM) | Chemical reduction of Fe(III) | Iron(III) reductase activity (mU/mg of protein) |
|---|---|---|---|
| NADH | 1 | No | 35 |
| NADPH | 1 | No | 0 |
| Benzyl viologen | 0.5 | Yes | ND |
| Methyl viologen | 0.5 | Yes | ND |
| Phenosafranin | 0.5 | Yes | ND |
| Safranin T | 0.5 | Yes | ND |
| Neutral red | 0.5 | Yes | ND |
| Bromophenol blue | 0.5 | No | 0 |
| Horse heart cytochrome c | 0.004 | No | 30 |
Reactions were performed in 50 mM HEPES buffer (pH 7) containing 0.3 mM Fe(III)-NTA and 0.5 mM ferrozine. An activity of 1 mU corresponds to 1 nmol of Fe(II) formed per min. ND, not determined.
FIG. 1.
Iron(III) reductase activity as a function of pH. The assay was done in a solution containing HEPES, MES (morpholineethane sulfonic acid), PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], TAPS {N-[Tris-(hydroxymethyl)-methyl]-3-amino-propane sulfonic acid}, and KH2PO4 buffer, each constituent at a concentration of 20 mM. In addition, the reaction mixture contained 0.3 mM Fe(III)-NTA, 0.5 mM ferrozine, and 1 mM NADH.
FIG. 2.
Iron(III) reductase activity as a function of reduced horse heart cytochrome c concentration. The assay was done in 50 mM HEPES buffer (pH 7) containing 0.3 mM Fe(III)-NTA and 0.5 mM ferrozine.
Iron(III) reductase activity was not affected by 50 μM HOQNO and was partially inhibited (50 to 80%) by cyanide (KCN; 200 μM) with both NADH and horse heart cytochrome c as the electron donor. Rotenone completely inhibited the iron(III) reductase activity only with NADH.
Localization of the iron(III) reductase.
Breakage of the cells of G. sulfurreducens by EDTA-lysozyme treatment followed by sonication led to a 1.5-fold increase of iron(III) reductase activity in comparison with unbroken cells when NADH was used as an electron donor (Table 2). No significant increase caused by breaking the cells was observed when horse heart cytochrome c was used as the electron donor.
TABLE 2.
Localization of iron(III) reductase in different cellular fractions of G. sulfurreducens
| Fraction | Protein (mg) | Iron(III) reductase
activitya with:
|
|||||
|---|---|---|---|---|---|---|---|
| NADH
|
Horse heart cytochrome c
|
||||||
| Sp act (mU/mg of protein) | Total activity (mU) | % | Sp act (mU/mg of protein) | Total activity (mU) | % | ||
| Whole cells vs. crude extract | |||||||
| Whole cells | 110 ± 7 | 4.8 ± 0.2 | 528 ± 20 | 100 | 7.0 ± 0.7 | 770 ± 70 | 100 |
| Crude extract | 114 ± 10 | 7.7 ± 0.5 | 878 ± 50 | 166 | 7.4 ± 0.5 | 844 ± 50 | 110 |
| Periplasm vs. spheroplasts | |||||||
| Periplasm | 1.7 ± 0.2 | 9 ± 1 | 15.3 ± 2 | 23 | 9.5 ± 1 | 16.2 ± 2 | 32 |
| Spheroplasts | 8.7 ± 1.0 | 6.0 ± 1 | 52.0 ± 9 | 77 | 4.0 ± 0.5 | 35.0 ± 4 | 68 |
| Soluble fraction vs. membrane fraction | |||||||
| Soluble fraction | 7.5 ± 1 | 9.7 ± 0.5 | 72.7 ± 5 | 17 | 11.0 ± 1 | 82 ± 7.0 | 33 |
| Membrane fraction | 60.3 ± 0.5 | 6.0 ± 1 | 362 ± 6 | 83 | 2.7 ± 0.5 | 163 ± 3 | 67 |
Reactions were performed in 50 mM HEPES buffer (pH 7) containing 0.3 mM Fe(III)-NTA, 0.5 mM ferrozine, and either 1 mM NADH or 4 μM horse heart cytochrome c. An activity of 1 mU corresponds to 1 nmol of Fe(II) formed per min.
The spheroplast fraction contained 77 and 68% of iron(III) reductase activity, respectively, when horse heart cytochrome c and NADH were used as electron donors, whereas the periplasmic fractions contained 23 and 32% of the iron(III) reductase activity, respectively (Table 2). The soluble fraction of crude extracts accounted for approximately the same percentage of iron(III) reductase activity as the periplasmic fraction of the spheroplast preparation (17 and 33%, respectively). The membrane fraction contained 83 and 67% of the iron(III) reductase activity, respectively.
Separation of the outer and cytoplasmic membranes was achieved by using a highly concentrated sucrose gradient of 50 to 80%. The outer membrane, identified by its high KDO content of 77 ± 5 ng/mg of protein, contained 75 and 79% of the iron(III) reductase measured with horse heart cytochrome c and NADH, respectively (Table 3). The cytoplasmic fraction had a low KDO content of 2.2 ± 0.2 ng/mg of protein and contained 25 and 21% of the iron(III) reductase activity, respectively (Table 3).
TABLE 3.
Localization of the iron(III) reductase in the outer membrane fraction of G. sulfurreducens
| Fraction | Protein (mg) | KDO
content
|
Iron(III) reductase
activitya with:
|
||||
|---|---|---|---|---|---|---|---|
| Total (mg) | Specific (ng/mg of protein) | NADH
|
Horse heart cytochrome
c
|
||||
| Sp act (mU/mg of protein) | Total activity (mU) | Sp act (mU/mg of protein) | Total activity (mU) | ||||
| Low-density band | 0.85 ± 0.04 | 2.60 ± 0.04 | 77 ± 5 | 112 ± 12 | 95.2 ± 10 | 122 ± 15 | 103 ± 12 |
| High-density band | 1.02 ± 0.06 | 0.20 ± 0.02 | 2.2 ± 0.2 | 25 ± 4 | 25.5 ± 4 | 33 ± 3 | 33.6 ± 3 |
Reactions were performed in 50 mM HEPES buffer (pH 7) containing 0.3 mM Fe(III)-NTA, 0.5 mM ferrozine, and either 1 mM NADH or 4 μM horse heart cytochrome c. An activity of 1 mU corresponds to 1 nmol of Fe(II) formed per min.
Solubilization of the iron(III) reductase.
The activity present in the soluble fractions might have been due to iron(III) reductase that was only loosely associated with the membranes. This hypothesis was substantiated by the finding that the iron(III) reductase activity can be solubilized by incubation of the membrane fraction with KCl 0.5 M (Table 4). When horse heart cytochrome c was used as the electron donor, an iron(III) reductase activity of 45.2 ± 5 mU was found in the soluble fraction that corresponds to 188% activity compared to the membrane fraction before treatment (Table 4). With NADH as the electron donor, only 14% of the iron(III) reductase activity was present in the soluble fraction. The results obtained after solubilization with KCl also showed that with NADH as electron donor 59% less iron(III) reductase activity was recovered compared with untreated membrane fraction, whereas with horse heart cytochrome c an increase of total activity by a factor of 2.6 was found.
TABLE 4.
Solubilization of iron(III) reductase from membrane fractions of G. sulfurreducens
| Fraction | Protein (mg) | Iron(III)
reductase activitya with:
|
|||||
|---|---|---|---|---|---|---|---|
| NADH
|
Horse heart cytochrome c
|
||||||
| Sp act (mU/mg of protein) | Total activity (mU) | % | Sp act (mU/mg of protein) | Total activity (mU) | % | ||
| Membrane fraction before extraction | 6.0 ± 1.0 | 6.0 ± 1.0 | 36.0 ± 6.0 | 100 | 4.0 ± 0.5 | 24.0 ± 3.0 | 100 |
| Soluble fraction after extraction | 3.6 ± 0.2 | 1.43 ± 0.2 | 5.1 ± 0.6 | 14 | 12.5 ± 1.0 | 45.2 ± 5.0 | 188 |
| Membrane fraction after extraction | 2.1 ± 0.3 | 4.5 ± 0.5 | 9.5 ± 1.0 | 27 | 7.9 ± 1.0 | 17.0 ± 2.0 | 71 |
Reactions were performed in 50 mM HEPES buffer (pH 7) containing 0.3 mM Fe(III)-NTA, 0.5 mM ferrozine, and either 1 mM NADH or 4 μM horse heart cytochrome c. An activity of 1 mU corresponds to 1 nmol of Fe(II) formed per min.
The incubation of whole cells in Tris buffer containing 0.5 M KCl did not disrupt the cells, as verified by microscopy, and led to a solubilization of the iron(III) reductase activity. The soluble fraction obtained with the KCl treatment contained 54% of the iron(III) reductase activity (measured with horse heart cytochrome c), whereas no iron(III) reductase activity was present in the supernatant in the absence of KCl (Table 4). With NADH as electron donor, 34% of the iron(III) reductase activity was solubilized by KCl. The chelating agent EDTA, known to destabilize the outer membrane by complexing Ca2+ and Mg2+ ions present in the outer membrane, caused in combination with KCl a solubilization of up to 81% of the iron(III) reductase activity (Table 5).
TABLE 5.
Solubilization of iron(III) reductase from whole cells of G. sulfurreducensa
| Addition(s)b | Fraction | Protein (mg) | Iron(III) reductase
activityc with:
|
|||||
|---|---|---|---|---|---|---|---|---|
| NADH
|
Horse heart cytochrome c
|
|||||||
| Sp act (mU/mg of protein) | Total activity (mU) | Soluble fraction/cell ratio (%) | Sp act (mU/mg of protein) | Total activity (mU) | Soluble fraction/cell ratio (%) | |||
| None | Soluble fraction | 2.0 ± 0.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cells | 2.2 ± 0.1 | 16.6 ± 1.0 | 36.5 ± 2.0 | 100 | 17.5 ± 3.0 | 38.5 ± 5.0 | 100 | |
| KCl | Soluble fraction | 3.6 ± 0.2 | 2.1 ± 0.1 | 7.6 ± 0.2 | 34 | 4.4 ± 0.4 | 15.8 ± 1.0 | 54 |
| Cells | 2.6 ± 0.1 | 5.7 ± 0.5 | 14.8 ± 2.0 | 66 | 5.1 ± 0.5 | 14.0 ± 2.0 | 46 | |
| EDTA | Soluble fraction | 3.4 ± 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cells | 2.8 ± 0.4 | 11.7 ± 1.5 | 32.8 ± 4.0 | 100 | 9.5 ± 0.5 | 26.6 ± 1.0 | 100 | |
| KCl + EDTA | Soluble fraction | 3.3 ± 0.2 | 6.4 ± 0.5 | 21.1 ± 1.0 | 66 | 11.7 ± 2.0 | 38.6 ± 4.0 | 81 |
| Cells | 2.6 ± 0.1 | 4.2 ± 0.2 | 10.9 ± 0.5 | 34 | 3.4 ± 0.4 | 8.8 ± 1.0 | 19 | |
For solubilization experiments, cells were dissolved in 100 mM Tris buffer (pH 7.8).
KCl was added to a final concentration of 0.5 M; EDTA was added to a final concentration of 1 mM.
Reactions were performed in 50 mM HEPES buffer (pH 7) containing 0.3 mM Fe(III)-NTA, 0.5 mM ferrozine, and either 1 mM NADH or 4 μM horse heart cytochrome c. An activity of 1 mU corresponds to 1 nmol of Fe(II) formed per min.
Cytochromes and iron(III) reductase activity in KCl extracts.
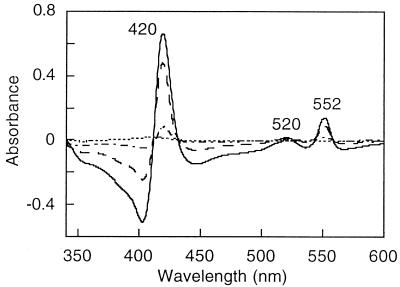
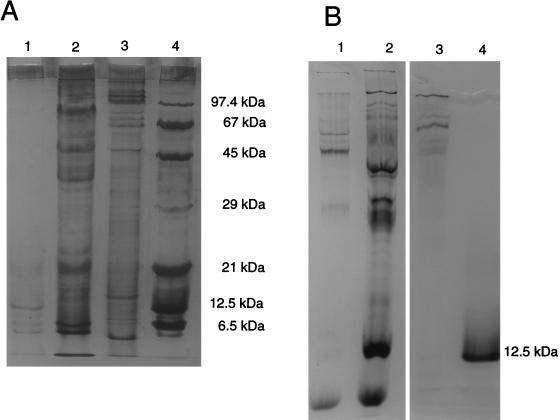
The UV-visible spectrum of the KCl extract obtained from whole cells exhibited a strong peak at 410 nm and shoulders centered at 520 and 580 nm (data not shown). These features are characteristic of hemoproteins. Redox difference spectra of this sample exhibited a Soret band at 420 nm and β and α bands at 522 and 552 nm, respectively, all of which are characteristic of c-type cytochromes. The addition of ferrihydrite to the cuvette containing the dithionite-reduced cytochrome c led to the disappearance of this spectrum (Fig. 3). This oxidation suggested that c-type cytochromes are involved in iron(III) reduction. Silver- and heme-stained SDS gels of the KCl fraction containing the iron(III) reductase revealed the presence of numerous proteins and several cytochromes (Fig. 4). Native PAGE of the KCl extract stained for the iron(III) reductase activity exhibited one purple band in the upper part of the gel, indicating that the iron(III) reductase could be a high-molecular-mass protein (data not shown). Analyzing the proteins obtained from a native preparative gel by SDS-PAGE revealed the presence of three cytochrome-containing proteins with molecular masses ranging from 67 to 97 kDa (Fig. 4).
FIG. 3.
UV-visible spectra of KCl extracts of whole cells. ——, redox difference spectrum, after addition of ferrihydrite; , 8.3 mM; . . , 10.8 mM; ----, 13.7 mM.
FIG. 4.
SDS-PAGE of different fractions of G. sulfurreducens extracts. The gels were stained for protein with silver (A) and heme (B). Lanes 1, whole cells after KCl extraction; lanes 2, KCl extract from whole cells; lanes 3, isolated band from a preparative iron(III) reductase activity gel; lanes 4, molecular mass standards including phosphorylase b (97.4 kDa), bovine serum albumin (67 kDa), egg albumin (45 kDa), carbonic hydrase (29 kDa), soybean trypsin inhibitor (21 kDa), horse heart cytochrome c (12.5 kDa), and trypsin inhibitor (6.5 kDa).
DISCUSSION
The iron(III) reductase activity assay with ferrozine and Fe(III) complexed with NTA and citrate, respectively, showed that the reduction of Fe(III)-NTA occurred five times faster than the reduction of Fe(III)-citrate. This result is in agreement with previous findings obtained with S. putrefaciens (2). The Fe(II) species formed with citrate may be kinetically less labile than those formed with NTA due to steric effects (9). Additional evidence for the effect of kinetic lability was obtained with Fe(III) complexed with the hexadentate ligand EDTA. In this case, no iron(III) reductase activity could be measured in the assay, which was probably caused by the inability of ferrozine to unbind Fe(II) from the Fe(II)-EDTA complex. A similar finding has been described by Dobbin et al. (9). However, Fe(III)-EDTA can act as an electron acceptor for the iron(III)-reducing bacteria as shown by the stimulation of benzene degradation by iron(III) reducers (20).
The iron(III) reductase activity of G. sulfurreducens could be measured with NADH as a physiological electron donor but not with NADPH. The same was also reported for S. putrefaciens (23). Since iron(III) reduction by G. sulfurreducens is a respiratory process, electrons derived from NADH oxidation are probably not directly transferred to Fe(III) but rather are transferred via a respiratory chain. This assumption is supported by the inhibition of NADH-dependent iron(III) reduction by rotenone that was also found for Geobacter metallireducens (11). In addition, the increase of activity after the cells were broken that gave NADH better access to the NADH dehydrogenase and the loss of activity upon solubilization of the iron(III) reductase by KCl that disrupted the respiratory chain also provided evidence for this assumption.
Here, for the first time, an artificial iron(III) reductase activity assay could be set up by using reduced horse heart cytochrome c as the electron donor. The electrons of the latter probably enter the respiratory chain to the iron(III) reductase on a different site than the electrons of NADH for two reasons. First, in contrast to NADH, there was no increase in activity with horse heart cytochrome c after the cells were broken. Second, disruption of the respiratory chain by solubilizing the iron(III) reductase did not result in a loss of activity as was found with NADH but rather an increased iron(III) reductase activity. No indications for a different site of electron transfer were obtained by the inhibitor experiments. The reactions with either NADH or horse heart cytochrome c showed the same inhibition pattern. Interestingly, the inhibition pattern of G. sulfurreducens was different from the patterns described for either S. putrefaciens (9, 23) or G. metallireducens (11). HOQNO had no effect on the iron(III) reductase activity of the two Geobacter species, but it did inhibit the iron(III) reduction by S. putrefaciens. Cyanide, on the other hand, inhibited the iron(III) reduction by S. putrefaciens and G. sulfurreducens, whereas no effect was observed for G. metallireducens.
Similar to other iron(III) reducers, such as S. putrefaciens and G. metallireducens, where the total iron(III) reductase activity was found exclusively in the membrane fraction (9, 11, 23), the major part of the iron(III) reductase activity of G. sulfurreducens was also found in the membrane fraction. The presence of iron(III) reductase activity in the outer membrane fraction has also been found for S. putrefaciens. For G. sulfurreducens, 75 to 79% of the iron(III) reductase activity of the membrane fraction was present in the outer membrane, whereas for S. putrefaciens it was 54 to 56%. The iron(III) reductase activity found in the cytoplasmic membrane fraction of G. sulfurreducens could be an artifact due to the sonication treatment since cell breakage by sonication can cause an extensive redistribution of membrane proteins between membranes (31). The minor part of the iron(III) reductase activity recovered in the soluble fractions after ultracentrifugation or preparation of spheroplasts is probably due to the release of the loosely membrane-associated iron(III) reductase during treatment. The solubilization of iron(III) reductase by KCl actually showed that this enzyme activity is loosely membrane associated since a high salt concentration treatment is a well-known method for detaching peripheral membrane proteins (25).
Because the major part of Fe(III) exists in the environment as insoluble Fe(III) oxides, a localization of the terminal reductase in the outer membrane is certainly conceivable. It has been proposed that direct contact of the cell surface with the iron oxides is needed to enable the bacteria to reduce Fe(III) (3, 4, 23). This would require an outer membrane iron(III) reductase facing the outside of the cells. Indications for such a localization of the iron(III) reductase in G. sulfurreducens were obtained with the solubilization of iron(III) reductase activity from whole cells by the KCl treatment. This treatment probably only detached peripheral outer membrane proteins located on the outside of the cells and did not detach proteins facing the periplasm. A recent study, however, showed that cell contact is not a requisite for reduction of solid Fe(III) oxides (6). In this study, an adhesion-deficient Shewanella alga strain reduced amorphous Fe(III) oxide at the same rates as a strain that strongly adhered to amorphous Fe(III) oxide. If the electrons are not directly transferred from the iron(III) reducers to the Fe(III) oxides, the electron transfer could be accomplished by diffusion of electron mediators or of free or complexed Fe(III). It has been shown that G. sulfurreducens excretes c-type cytochromes in the medium and that this cytochrome has iron(III) reductase activity (29). This suggested that this cytochrome could act as an electron mediator between the cells and the iron oxides. Indications for the production of a cell surface protein that efficiently chelate Fe(III) have been obtained for S. alga (6).
The presence of heme proteins in the protein fraction isolated from the iron(III) reductase activity band of a native gel, the solubilization of several c-type cytochromes by KCl from whole cells of G. sulfurreducens, and the possible involvement of these cytochromes in iron(III) reduction added further evidence for the hypothesized key role that periplasmic and outer membrane c-type cytochromes play in iron(III) reduction (18, 22, 24, 29). For S. putrefaciens it has been shown that the outer membrane of anaerobically grown cells contained, in addition to 54 to 56% iron(III) reductase activity (23), 80% of the membrane-bound cytochromes (22). Recently, the gene of a tetraheme cytochrome c of S. putrefaciens has been cloned and sequenced (24). This c-type cytochrome has been found in the cytoplasmic membrane as well as in the soluble fraction. It has been proposed that the tetraheme cytochrome c is involved in the transfer of electrons from the cytoplasmic membrane to acceptor proteins such as fumarate reductase located in the periplasm and iron(III) reductase located in the outer membrane (24). Finally, a c-type cytochrome that has been excreted into the medium by G. sulfurreducens and that has been purified is similar to the cytochrome c3 of other bacteria (29). This c-type cytochrome was also present in the membrane and soluble fraction and was able to reduce different electron acceptors including iron oxides. In contrast to the heme proteins present in the iron(III) reductase activity band that had a high molecular mass of 70 to 100 kDa, the excreted c-type cytochrome had a molecular mass of only 9.6 kDa (29). High-molecular-mass cytochromes were present in the outer membrane of Desulfovibrio vulgaris (Hildenborough) when cultivated in a medium with a high Fe2+ concentration (100 ppm) (33). A role in anaerobic biocorrosion has been postulated for these high-molecular-mass cytochromes. Whether the iron(III) reductase of G. sulfurreducens is indeed a high-molecular-mass cytochrome will be investigated in the near future.
ACKNOWLEDGMENTS
This work was financially supported by the European Environmental Research Organization (EERO) and the Swiss Federal Institute for Environmental Science and Technology (EAWAG).
We thank Derek Lovley for providing G. sulfurreducens; Wolfram Schumacher for helpful comments and discussions; Birgit Krause, Kornelia Zepp, and Mario Snozzi for critically reviewing the manuscript; and Bernhard Schink for giving us access to his data prior to publication.
REFERENCES
- 1.Albrechtsen H-J, Christensen T H. Evidence for microbial iron reduction in a landfill leachate-polluted aquifer (Vejen, Denmark) Appl Env Microbiol. 1994;60:3920–3925. doi: 10.1128/aem.60.11.3920-3925.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold R G, Olson T M, Hoffmann M R. Kinetics and mechanism of dissimilative Fe(III) reduction by Pseudomonassp 200. Biotechnol Bioeng. 1986;28:1657–1671. doi: 10.1002/bit.260281110. [DOI] [PubMed] [Google Scholar]
- 3.Arnold R G, Dichristina T J, Hoffmann M R. Reductive dissolution of Fe(III) oxides by Pseudomonassp 200. Biotechnol Bioeng. 1988;32:1081–1096. doi: 10.1002/bit.260320902. [DOI] [PubMed] [Google Scholar]
- 4.Caccavo F, Blakemore R P, Lovley D R. A hydrogen-oxidizing, Fe(III)-reducing microorganism from the Great Bay estuary, New Hampshire. Appl Environ Microbiol. 1992;58:3211–3216. doi: 10.1128/aem.58.10.3211-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caccavo F, Lonergan D J, Lovley D R, Davis M, Stolz J F, McInerney M J. Geobacter sulfurreducenssp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caccavo J R, Schamberger P C, Keiding K, Nielsen P H. Role of hydrophobicity in adhesion of the dissimilatory Fe(III)-reducing bacterium Shewanella algato amorphous Fe(III) oxide. Appl Environ Microbiol. 1997;63:3837–3843. doi: 10.1128/aem.63.10.3837-3843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canfield D E. Reactive iron in marine sediments. Geochim Cosmochim Acta. 1989;53:619–632. doi: 10.1016/0016-7037(89)90005-7. [DOI] [PubMed] [Google Scholar]
- 8.Coates J D, Phillips E J P, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacterspecies from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobbin P S, Powell A K, McEwan A G, Richardson D J. The influence of chelating agents upon the dissimilatory reduction of Fe(III) by Shewanella putrefaciens. Biometals. 1995;8:163–173. [Google Scholar]
- 10.Francis R T, Jr, Becker R R. Specific indication of hemoproteins in polyacrylamide gels using a double staining process. Anal Biochem. 1984;136:509–514. doi: 10.1016/0003-2697(84)90253-7. [DOI] [PubMed] [Google Scholar]
- 11.Gorby Y A, Lovley D R. Electron transport in the dissimilatory iron reducer, GS-15. Appl Environ Microbiol. 1991;57:867–870. doi: 10.1128/aem.57.3.867-870.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulse C H, Tiedje J M, Averill B A. A spectrophotometric assay for dissimilatory nitrite reductases. Anal Biochem. 1988;172:420–426. doi: 10.1016/0003-2697(88)90464-2. [DOI] [PubMed] [Google Scholar]
- 13.Karkhanis Y D, Zeltner J Y, Jackson J J, Carlo D J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gram-negative bacteria. Anal Biochem. 1978;85:595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Lascelles J, Burke K A. Reduction of ferric iron by l-lactate and dl-glycerol-3-phosphate in membrane preparations from Staphylococcus aureusand interactions with the nitrate reductase system. J Bacteriol. 1978;134:585–589. doi: 10.1128/jb.134.2.585-589.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lonergan D J J, Jenter H L, Coates J D, Phillips E J P, Schmidt T M, Lovley D R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J Bacteriol. 1996;178:2402–2408. doi: 10.1128/jb.178.8.2402-2408.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovley D R. Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev. 1991;55:259–287. doi: 10.1128/mr.55.2.259-287.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovley D R, Roden E E, Phillips E J P, Woodward J C. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar Geol. 1993;113:41–53. [Google Scholar]
- 19.Lovley D R, Phillips E J P. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988;54:1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovley D R, Woodward J C, Chapelle F H. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl Environ Microbiol. 1996;62:288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moody M D, Dailey H A. Aerobic ferrisiderophore reductase assay and activity stain for native polyacrylamide gels. Anal Biochem. 1983;134:235–239. doi: 10.1016/0003-2697(83)90290-7. [DOI] [PubMed] [Google Scholar]
- 22.Myers C R, Myers J M. Localization of cytochromes to the outer membrane of anaerobically grown Shewanella putrefaciensMR-1. J Bacteriol. 1992;174:3429–3438. doi: 10.1128/jb.174.11.3429-3438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myers C R, Myers J M. Ferric reductase is associated with the membrane of anaerobically grown Shewanella putrefaciensMR-1. FEMS Microbiol Lett. 1993;108:15–22. [Google Scholar]
- 24.Myers C R, Myers J M. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciensMR-1. J Bacteriol. 1997;179:1143–1152. doi: 10.1128/jb.179.4.1143-1152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohlendieck K. Extraction of membrane proteins. In: Doonan S, editor. Protein purification protocols. Totowa, N.J: Humana Press, Inc.; 1996. pp. 293–304. [Google Scholar]
- 26.Ponnamperuma F N. The chemistry of submerged soils. Adv Agron. 1972;24:29–96. [Google Scholar]
- 27.Rabilloud T. Mechanisms of protein silver staining in polyacrylamide gels: a 10 year synthesis. Electrophoresis. 1990;11:785–794. doi: 10.1002/elps.1150111003. [DOI] [PubMed] [Google Scholar]
- 28.Roden E E, Wetzel R G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr. 1996;41:1733–1748. [Google Scholar]
- 29.Seeliger S, Cord-Ruwisch R, Schink B. A periplasmic and extracellular c-type cytochrome of Geobacter sulfurreducensacts as a ferric iron reductase and as an electron carrier to other electron acceptors or to partner bacteria. J Bacteriol. 1998;180:3686–3691. doi: 10.1128/jb.180.14.3686-3691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Sprott G D, Koval S F, Schnaitman C A. Cell fractionation. In: Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. pp. 72–103. [Google Scholar]
- 32.Stookey L L. Ferrozine: a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 33.Van Ommen Kloeke F, Bryant R D, Laishley E J. Localization of cytochromes in the outer membrane of Desulfovibrio vulgaris(Hildenborough) and their role in anaerobic biocorrosion. Anaerobe. 1995;1:351–358. doi: 10.1006/anae.1995.1038. [DOI] [PubMed] [Google Scholar]