Abstract
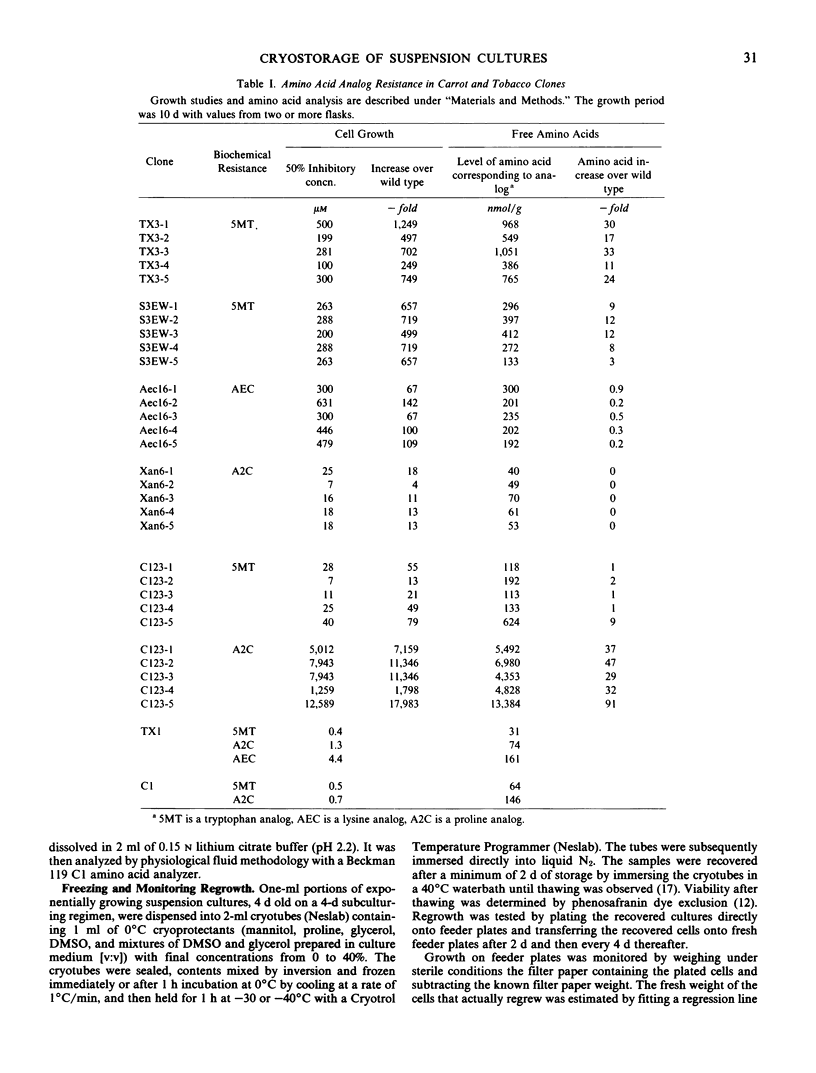
Five clones were isolated from five different amino acid analog-resistant Daucus carota L. var. Sativa and Nicotiana tabacum L. cv. Xanthi cell lines. The individual clones were similar in their resistance to dl-5-methyltryptophan, S-(2-aminoethyl)-l-cysteine, or azetidine-2-carboxylic acid, and in their corresponding free amino acid levels.
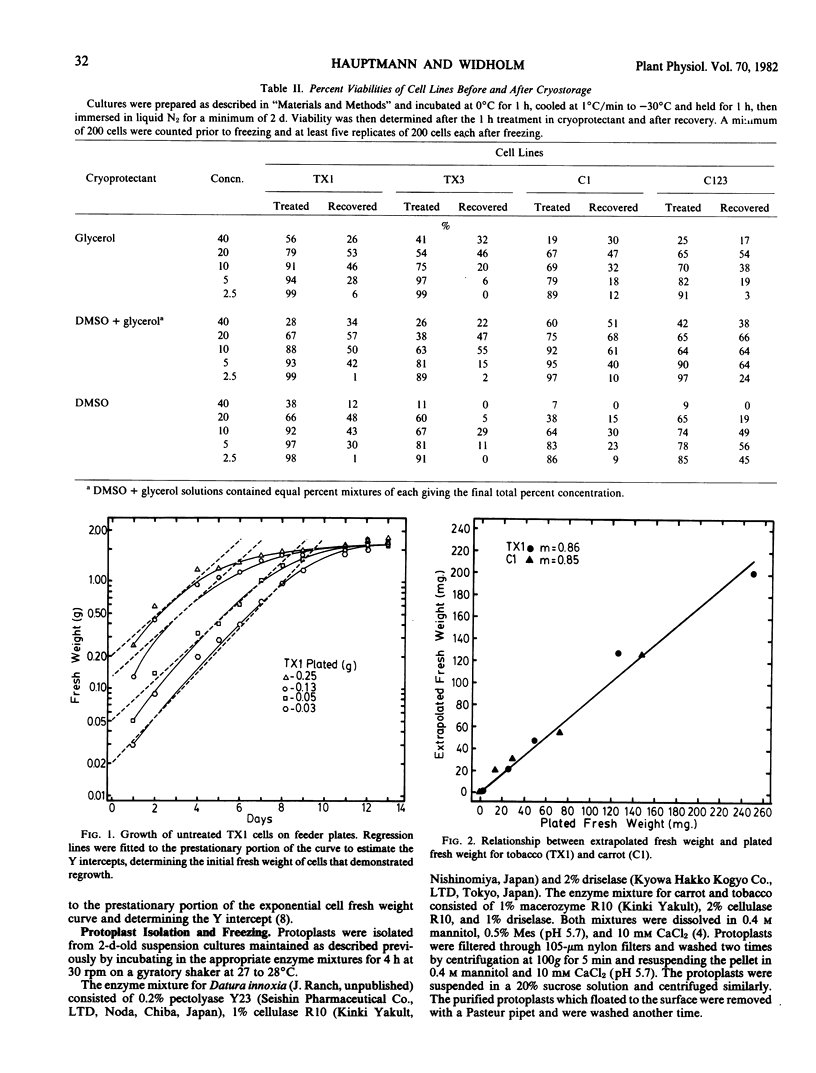
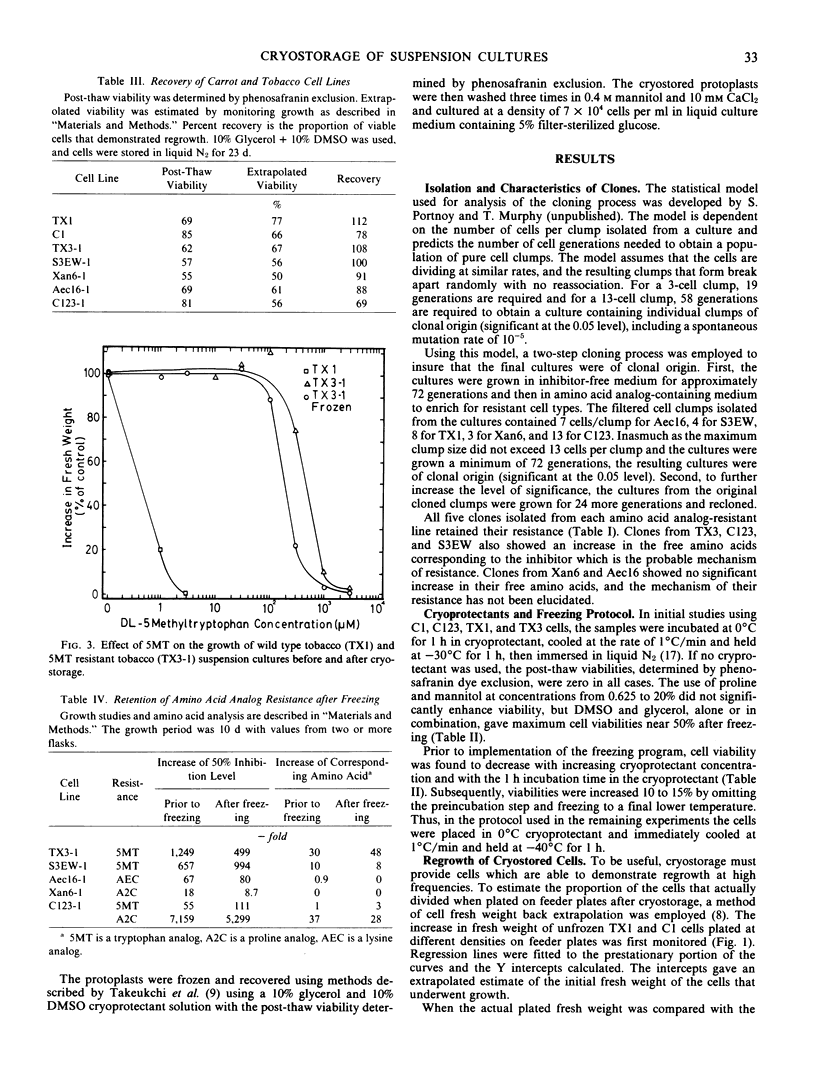
The cell suspensions were stored using a controlled freezing rate at −196°C with concentrations up to 40% of the four cryoprotectants: mannitol, proline, dimethylsulfoxide, glycerol, and combinations of dimethyl-sulfoxide and glycerol. No less than 55% post-thaw viability, determined by phenosafranin dye exclusion, was obtained after storage using a cryoprotectant mixture of 10% glycerol and 10% dimethylsulfoxide. Growth of the cryostored cells could be obtained consistently only by using feeder plate methodology with this combination of cryoprotectants. Post-thaw viability and percentage of cells demonstrating growth, as estimated by growth kinetics, were found to be similar. This indicates that little selection occurred during the freezing and recovery process. In addition, the amino acid analog-resistant traits were unaltered following cryostorage.
Suspension cultures of Datura innoxia Mill. were frozen similarly with maximum post-thaw viability of 38%, but subsequent growth was not obtained.
Protoplasts of D. innoxia, tobacco and carrot were also cryostored using a mixture of 10% dimethylsulfoxide and 10% glycerol as cryoprotectants. Viabilities of no less than 40% were obtained, however, only the carrot protoplasts regenerated cell walls and underwent cell division.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Sung Z. R. Mutagenesis of cultured plant cells. Genetics. 1976 Sep;84(1):51–57. doi: 10.1093/genetics/84.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm J. M. Cultured Nicotiana tabacum cells with an altered anthranilate synthetase which is less sensitive to feedback inhibition. Biochim Biophys Acta. 1972 Jan 28;261(1):52–58. doi: 10.1016/0304-4165(72)90312-1. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 1972 Jul;47(4):189–194. doi: 10.3109/10520297209116483. [DOI] [PubMed] [Google Scholar]
- Withers L. A., King P. J. Proline: A Novel Cryoprotectant for the Freeze Preservation of Cultured Cells of Zea mays L. Plant Physiol. 1979 Nov;64(5):675–678. doi: 10.1104/pp.64.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]


