Abstract
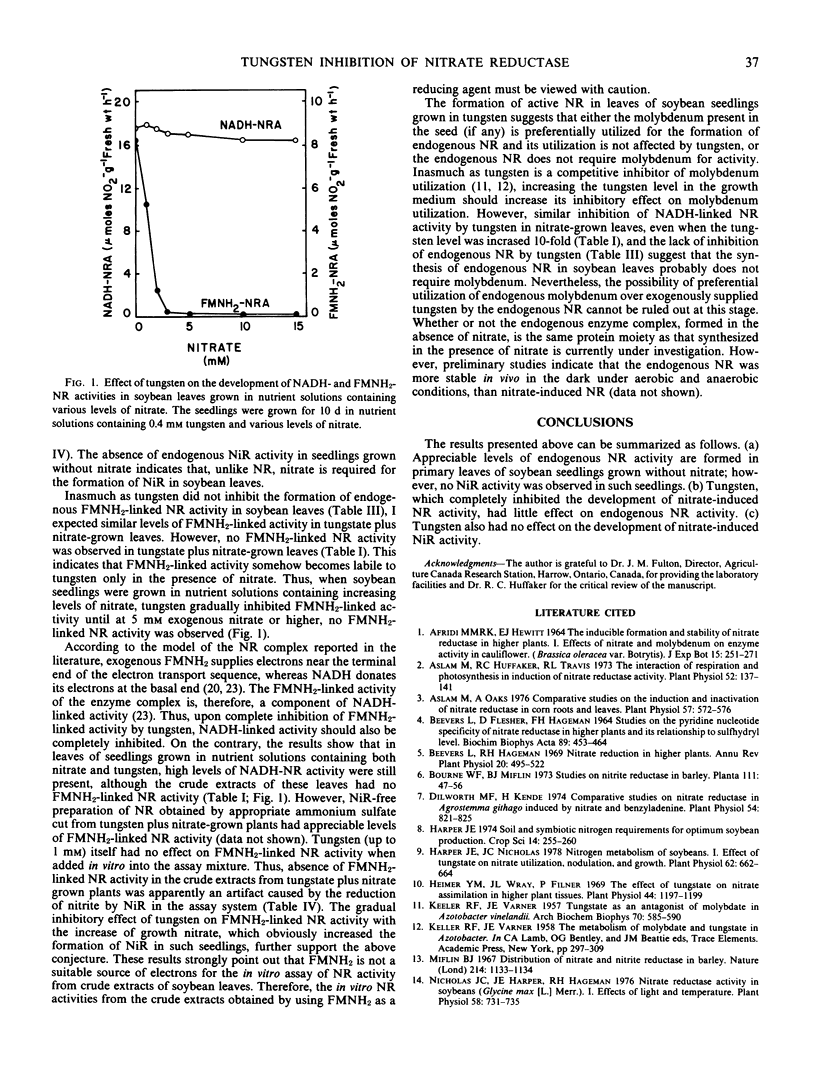
The effect of tungsten on the development of endogenous and nitrate-induced NADH- and FMNH2-linked nitrate reductase activities in primary leaves of 10-day-old soybean (Glycine max [L.] Merr.) seedlings was studied. The seedlings were grown with or without exogenous nitrate. High levels of endogenous nitrate reductase activities developed in leaves of seedlings grown without nitrate. However, no endogenous nitrite reductase activity was detected in such seedlings. The FMNH2-linked nitrate reductase activity was about 40% of NADH-linked activity. Tungsten had little or no effect on the development of endogenous NADH- and FMNH2-linked nitrate reductase activities, respectively. By contrast, in nitrate-grown seedlings, tungsten only inhibited the nitrate-induced portion of NADH-linked nitrate reductase activity, whereas the FMNH2-linked activity was inhibited completely. Tungsten had no effect on the development of nitrate-induced nitrite reductase activity. The complete inhibition of FMNH2-linked nitrate reductase activity by tungsten in nitrate-grown plants was apparently an artifact caused by the reduction of nitrite by nitrite reductase in the assay system. The results suggest that in soybean leaves either the endogenous nitrate reductase does not require molybdenum or the molybdenum present in the seed is preferentially utilized by the enzyme complex as compared to nitrate-induced nitrate reductase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslam M., Huffaker R. C., Travis R. L. The interaction of respiration and photosynthesis in induction of nitrate reductase activity. Plant Physiol. 1973 Aug;52(2):137–141. doi: 10.1104/pp.52.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M., Oaks A. Comparative studies on the induction and inactivation of nitrate reductase in corn roots and leaves. Plant Physiol. 1976 Apr;57(4):572–576. doi: 10.1104/pp.57.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEEVERS L., FLESHER D., HAGEMAN R. H. STUDIES ON THE PYRIDINE NUCLEOTIDE SPECIFICITY OF NITRATE REDUCTASE IN HIGHER PLANTS AND ITS RELATIONSHIP TO SULFHYDRYL LEVEL. Biochim Biophys Acta. 1964 Sep 18;89:453–464. doi: 10.1016/0926-6569(64)90071-9. [DOI] [PubMed] [Google Scholar]
- Dilworth M. F., Kende H. Comparative Studies on Nitrate Reductase in Agrostemma githago Induced by Nitrate and Benzyladenine. Plant Physiol. 1974 Dec;54(6):821–825. doi: 10.1104/pp.54.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. E., Nicholas J. C. Nitrogen metabolism of soybeans: I. Effect of tungstate on nitrate utilization, nodulation, and growth. Plant Physiol. 1978 Oct;62(4):662–664. doi: 10.1104/pp.62.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer Y. M., Wray J. L., Filner P. The effect of tungstate on nitrate assimilation in higher plant tissues. Plant Physiol. 1969 Aug;44(8):1197–1199. doi: 10.1104/pp.44.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- Nicholas J. C., Harper J. E., Hageman R. H. Nitrate Reductase Activity in Soybeans (Glycine max [L.] Merr.): I. Effects of Light and Temperature. Plant Physiol. 1976 Dec;58(6):731–735. doi: 10.1104/pp.58.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notton B. A., Hewitt E. J. The role of tungsten in the inhibition of nitrate reductase activity in spinach (spinacea oleracea L.) leaves. Biochem Biophys Res Commun. 1971 Aug 6;44(3):702–710. doi: 10.1016/s0006-291x(71)80140-7. [DOI] [PubMed] [Google Scholar]
- Paneque A., Del Campo F. F., Ramírez J. M., Losada M. Flavin nucleotide nitrate reductase from spinach. Biochim Biophys Acta. 1965 Sep 27;109(1):79–85. doi: 10.1016/0926-6585(65)90092-0. [DOI] [PubMed] [Google Scholar]
- Sanderson G. W., Cocking E. C. Enzymic Assimilation of Nitrate in Tomato Plants. I. Reduction of Nitrate to Nitrite. Plant Physiol. 1964 May;39(3):416–422. doi: 10.1104/pp.39.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader L. E., Ritenour G. L., Eilrich G. L., Hageman R. H. Some characteristics of nitrate reductase from higher plants. Plant Physiol. 1968 Jun;43(6):930–940. doi: 10.1104/pp.43.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI H., NASON A. Tungstate as competitive inhibitor of molybdate in nitrate assimilation and in N2 fixation by Azotobacter. Biochim Biophys Acta. 1957 Feb;23(2):433–435. doi: 10.1016/0006-3002(57)90351-7. [DOI] [PubMed] [Google Scholar]
- Thayer J. R., Huffaker R. C. Determination of nitrate and nitrite by high-pressure liquid chromatography: comparison with other methods for nitrate determination. Anal Biochem. 1980 Feb;102(1):110–119. doi: 10.1016/0003-2697(80)90325-5. [DOI] [PubMed] [Google Scholar]
- Wray J. L., Filner P. Structural and functional relationships of enzyme activities induced by nitrate in barley. Biochem J. 1970 Oct;119(4):715–725. doi: 10.1042/bj1190715. [DOI] [PMC free article] [PubMed] [Google Scholar]


