Abstract
The potent bactericidal activity of sodium nitroprusside {SNP; Na2[Fe(CN)5(NO)]} towards Clostridium sporogenes has been investigated. SNP inhibited cell growth in the concentration range of 10 to 40 μM. Concentrations above 80 μM caused irreversible loss of cell viability and cell lysis. Inhibition of cell growth was similar in complex and in defined media. SNP was found to be unreactive towards individual components of the defined medium, with the exception of cysteine. The chemical characteristics responsible for the potency of SNP were investigated by synthesizing analogs of SNP in which the Fe was replaced by different metals. The inhibitory potency of the pentacyanonitrosyl complexes decreased in the order Fe > Cr > V, which correlates with N-O stretching frequency (vNO). In contrast, the Ru complex which had a vNO comparable to that of Fe was a poor inhibitor. Electron paramagnetic resonance spectroscopy showed that SNP was rapidly reduced to the paramagnetic Fe(I) compound [Fe(CN)4(NO)]2− on contact with cells. Analysis of fractions from SNP-treated cells showed 90% oxidation of thiols in the cell walls compared with those in control cells. The toxicity of SNP involves S-nitrosation and reduction, the lack of toxicity of the Ru analog being consistent with the fact that it has poor reactivity towards thiols. When C. sporogenes cells were exposed to sublethal concentrations of SNP and viewed under the electron microscope, they showed blisters on the surface. These results point to the cell wall surface as a primary point of attack of the nitrosyl complex.
Inhibition by nitrite of microorganisms such as Clostridium botulinum in foods has been studied for more than 50 years (29). A landmark discovery was that nitrite may be converted in growth medium and in meat systems into other compounds that are considerably more toxic to the bacteria. Perigo et al. (27) observed that heating nitrite with growth medium increased its bacteriostatic effect. Species such as the anion of Roussin’s black salt (RBS) [Fe4S3(NO)7]− and the nitrosylcysteinylferrate anion, which are formed in heated growth media, were shown to be very effective inhibitors of the growth of clostridial spores (22). RBS is a selective and powerful inhibitor of clostridial growth at micromolar concentrations (2), compared with the millimolar concentrations required for nitrite. However, the question of the mechanism of inhibition of the bacteria remains open.
Several different hypotheses have been proposed for the mechanism of inhibition of clostridia by nitrite. A common view (3) is that it acts as a general nitrosating agent. It is significant that nitrosyl complexes such as RBS and sodium nitroprusside (SNP) contain NO groups in the formal oxidation state NO+ and can act as nitrosating agents at neutral pH values, whereas nitrite is ineffective as a nitrosating agent under these conditions.
A possible target for these inhibitors is the anaerobic metabolism of glucose. Woods et al. (34) noted that C. botulinum released pyruvate in the presence of nitrite and suggested that nitric oxide, formed from nitrite, inhibits the phosphoroclastic breakdown of pyruvate by reaction with the nonheme iron proteins ferredoxin, pyruvate-ferredoxin oxidoreductase, and hydrogenase. In support of this, Reddy et al. (28) observed the formation of iron-sulfur-nitrosyl complexes in extracts of C. botulinum treated with nitrite. They proposed that the antimicrobial activity of nitrite depends on disruption of respiration by destruction of natural iron-sulfur clusters of redox proteins. Carpenter et al. (9) also reported the loss of activity of ferredoxin and pyruvate-ferredoxin oxidoreductase during treatment with nitrite. However, Payne et al. (25, 26) examined the effect of nitrite and iron-sulfur-nitrosyl complexes on iron-sulfur clusters in Clostridium sporogenes cells (25) and on the isolated iron-sulfur proteins (26) and were unable to demonstrate a direct action of the compounds on the iron-sulfur proteins. A second possible effect is that of a general oxidizing agent. O’Leary and Solberg (24) noted the depletion of thiols in the cell cytoplasm after nitrite treatment.
We have recently found that SNP inhibited the growth of C. sporogenes cells at concentrations comparable to those of RBS, when expressed per NO group (11). Like nitrite, SNP is relatively nontoxic to human cells and has been used as a vasodilator during surgery (8). The reasons why it is toxic to bacterial cells are unclear and may be important in understanding the general toxicity of nitrosyl compounds to bacteria. The chemistry of SNP has been studied extensively. The nitrosyl ligand functions as a nitrosating agent and is readily attacked (at the nitrogen atom of the nitrosyl ligand) by a range of nucleophiles including amines (5, 20), azide (33), hydroxylamine (19), carbanions (6), and thiols (7, 23). These reactions parallel those of nitrous acid. Thus, the reactions of nitroprusside anion with secondary amines such as diethylamine give N-nitrosamines. Reactions of nitroprusside with thiols are usually extremely rapid and are just within the limits of conventional stopped-flow kinetic methods (15). As NO is 30-fold less active towards C. sporogenes than is SNP (11), it seems unlikely that NO derived from nitroprusside (by, for example, nitrosation of thiol and subsequent decomposition of the S-nitrosothiol) could be the main inhibitory agent.
We now report the results of a detailed investigation of the inhibitory effect of SNP on C. sporogenes. The experiments described in this paper were carried out in order to determine the mode of action of SNP at the cellular level, at the concentrations used for the growth inhibition experiments. A number of standard assay systems were defined. Standard methods of optical density and viable cell counts were used to measure cell growth in anaerobic culture. Light and transmission electron microscopy were used to view the morphology of cells. Chemical determination of the sulfhydryl groups in cell fractions was used to assess the effect of SNP on the -SH content of each fraction. Electron paramagnetic resonance (EPR) spectroscopy of whole cells and cell fractions was used to observe the quantities of iron-sulfur proteins and their redox state in the cells (25). EPR signals were also detected from various species formed by reduction of the nitroprusside ion. We have also surveyed the toxicity of a number of other pentacyanonitrosyl complexes, all formally containing the NO+ group.
MATERIALS AND METHODS
Organism.
Cultures of C. sporogenes NCIB 10696 were maintained on reinforced clostridial medium (RCM) by daily subculturing. Once monthly, a single colony was isolated on RCM agar plates and subcultured.
Growth and inhibition studies.
Complex medium, prepared from Oxoid nutrient broth no. 2 medium which had been autoclaved and supplemented with sterile glucose and thioglycolate to give final concentrations of 1 and 0.1%, respectively, was sparged with argon for 5 min in flasks stoppered with Subaseals. The flasks were inoculated from an overnight culture of C. sporogenes and incubated at 37°C for 2 to 3 h. When cell growth reached exponential phase, at an optical density value at 550 nm of 0.50 (approximately 5 × 105 CFU), a 1-ml sample of a freshly prepared solution of the test compound, in medium, was added to the culture.
Defined medium was prepared as described by Lovitt et al. (18). Cultures were grown as described above except that the optical density measurements were carried out at 680 nm.
Growth was estimated by the measurement of optical density at 550 or 680 nm, with appropriate dilution so that the optical density measured was less than 0.5. Samples were taken from flasks with the aid of a syringe connected to a two-way stopper valve to prevent entry of oxygen.
Viability was estimated at intervals throughout the experiment by diluting samples into low-phosphate basal medium (18) and mixing them well. Serial dilutions were made, and 20 μl of each dilution was dropped onto the RCM agar plates. The plates were then allowed to dry and placed in an anaerobic jar at 37°C for 24 h. Individual cell colonies were counted and extrapolated to original viable cell counts as CFU per milliliter. The concentration which would inhibit growth by 50% of the control (IC50) was estimated by measuring growth rates with at least six sublethal concentrations of inhibitor.
Electron microscopy.
For preparation of transmission electron microscopy samples, cells were harvested by centrifugation. They were fixed in 2.5% glutaraldehyde and 0.1 M sodium cacodylate buffer for 2 h, postfixed in 1% osmium tetroxide for a further 2 h, washed with 30% ethanol and embedded in agarose, and further dehydrated in 70, 90, and (three times) 100% ethanol. The samples were finally embedded in Spurr’s resin series and cut for electron microscopy.
Protein and thiol determinations.
Protein concentration was determined by the bicinchoninic acid protein assay kit (Pierce), with bovine serum albumin as standard. Free thiol groups were determined with 5,5′-dithiobis(2-nitrobenzoate) (12, 24).
Cellular fractionation.
For fractionation of C. sporogenes, transfers were carried out at 0 to 5°C in a Miller-Howe anaerobic cabinet in a nitrogen atmosphere containing less than 5 ppm of oxygen. Cells were pelleted by centrifugation, washed with argon-sparged low-phosphate basal medium, resuspended in phosphate buffer (50 mM, pH 7.4), and broken, either in a French press at 107 kPa of pressure or by sonication in an ice bath. The cell wall, membrane, and cytoplasm fractions were obtained essentially by the method of Levett (17) by centrifugation at 22,000 × g for 20 min and then at 120,000 × g for 2 h.
EPR spectroscopy.
EPR spectra of cells treated with SNP were recorded at room temperature in a quartz flat cell of 0.3 mm in thickness. Cells were harvested by centrifugation, washed, and finally resuspended in argon-saturated 50 mM phosphate buffer, pH 7.5. The initial amount of cells in the growth flasks was 108 cells/ml. Samples were taken and frozen for EPR spectroscopy of iron-sulfur proteins. These samples were transferred with syringes to quartz tubes and mixed with nitroprusside, dithionite, or other reagents under argon gas and then frozen after minimum time in liquid nitrogen. Spectra were recorded on a Bruker ESP300 spectrometer with an Oxford Instruments ESR900 liquid helium flow cryostat for low-temperature measurements.
Synthesis of pentacyanonitrosyl complexes.
Na2[Fe(CN)5NO] · H2O (SNP) was obtained from Merck. Li2[Ru(CN)5NO] · H2O, K3[Cr(CN)5NO] · H2O, and K3[V(CN)5NO] · H2O were synthesized by published methods (1, 4, 13, 14).
RESULTS
Bactericidal activity of SNP on C. sporogenes.
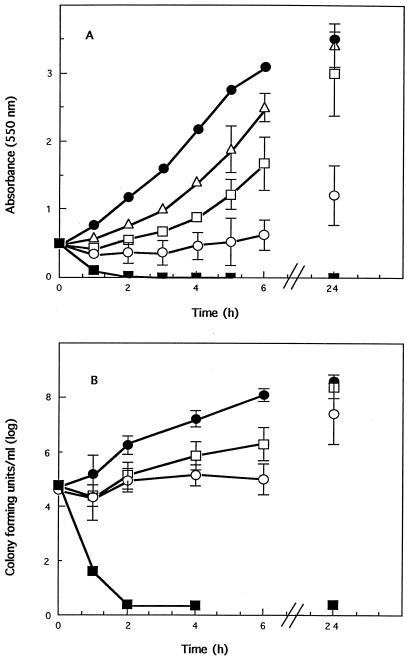
Time-kill studies were carried out on C. sporogenes with the inhibitor SNP, monitoring the growth of cultures by optical density (Fig. 1A) and viable cell counts (Fig. 1B). As SNP is photosensitive, experiments were carried out in darkness, although it was found that room light had no discernible influence on the inhibitory effects.
FIG. 1.
The effect of lethal and sublethal concentrations of SNP on actively growing cells of C. sporogenes. SNP was added to cultures that had reached an absorbance (550 nm) of 0.5, corresponding to 5 × 105 cells ml−1, at a final concentration of 10 (▵), 20 (□), 40 (○), or 80 (■) μM. The effect on growth was monitored at time intervals and compared to that for control cells (•). (A) Growth shown as change in absorbance at 550 nm. (B) Viability of cells shown as CFU per milliliter. Each datum point is the mean of at least three experiments. Absence of growth is given a value of 1.
Two distinct processes were observed during these inhibition studies. Immediately after SNP was added, aggregation of the cells to give darker-colored clumps was observed, especially at sublethal concentrations of SNP, while at higher SNP concentrations, lysis of the cells was observed. In the sublethal concentration range of 10 to 40 μM, the bacteriostatic effect increased with SNP concentration and decreased with increased inoculum size (data not shown). This must relate to the ratio of SNP to cells. Because of this observation, it was important to standardize the experiments in terms of inoculum size especially in the determination of IC50s for different compounds.
Concentrations of SNP above 80 μM were bactericidal, and under the conditions employed in this study, the effect was independent of inoculum size (data not shown). There was a rapid decrease in absorbance of the cultures (Fig. 1A) and total loss of cell viability within minutes which was not reversed (Fig. 1B). The speed at which lysis occurred strongly suggested that the cell wall was rapidly ruptured.
Possible reactions of SNP with growth medium.
Most growth and inhibition studies of C. sporogenes have been carried out in complex medium. It seems likely that medium constituents could react with nitrite and related compounds to give chemically different species. It has been shown that the bacteriostatic effect of nitrite is stimulated by heating of the growth medium containing the inhibitor (27). By contrast, when SNP (400 μM) was autoclaved with growth medium at 121°C for 15 min, it was no longer bacteriostatic. The color of the medium was observed to turn from yellow to blue after autoclaving, indicating possible chemical interaction between SNP and the medium during the autoclaving. One explanation for the blue color is the formation of Prussian blue, Fe4[Fe(CN)6]4, which was found not to be toxic to C. sporogenes cells.
To test the possibility that SNP can interact with unknown constituents of the medium to produce inhibitory species, growth inhibition studies were carried out in a defined medium (18). These showed the same effects as those observed in the complex medium. The IC50 for SNP was estimated to be 30 ± 10 mM (two determinations) in defined medium, compared with 20 ± 2 mM (five determinations) in nutrient broth. This failed to provide evidence for the production of additional inhibitory species in the complex medium.
The possibility of reactions occurring between SNP and constituents of the defined medium was investigated by UV-visible spectrophotometry, with repetitive scanning between 600 and 200 nm. No reaction was observed between any of the individual components and SNP at pH 7, over the periods of hours associated with growth experiments, except for the expected reaction with cysteine. A very slow reaction was observed between SNP and the complete medium, over several days, producing unidentified products, but this was unlikely to be of major importance within the time span of the growth experiments.
Activity of some analogs of SNP.
It was of interest to compare the activities of a range of pentacyanonitrosyl complexes in order to identify the property that makes SNP so potent an inhibitor. Various analogs of SNP in which the Fe was replaced by different metals were synthesized. IC50s for the compounds are summarized in Table 1. Analogous compounds, from which the nitrosyl group was absent, were tested and found to be nontoxic, clearly demonstrating that the nitrosyl group was responsible for activity. The inhibitory potency of the pentacyanonitrosyl complexes decreased in the order Fe > Cr > V. The Ru analog of SNP was a very poor inhibitor.
TABLE 1.
Values of vNO and IC50s for SNP and various analogsa
| Compound | vNO | IC50 (μM) |
|---|---|---|
| Na2[Fe(CN)5NO] · H2O | 1,944 | 20 |
| Li2[Ru(CN)5NO] · H2O | 1,926 | 1,000 |
| K3[Cr(CN)5NO] · H2O | 1,641 | 100 |
| K3[V(CN)5NO] · H2O | 1,575 | >2,000 |
Cells were grown to an absorbance of 0.5 at 550 nm with complex medium. The analogs were then added, and the effect on cell growth was monitored by changes in absorbance. In determining IC50, at least five concentrations of sublethal levels were used for each experiment. Each IC50 is the mean of results from at least three experiments.
EPR spectroscopy of cells treated with SNP.
At room temperature, EPR spectra of suspensions of SNP-treated C. sporogenes cells showed the formation of a paramagnetic species, with a three-line hyperfine splitting typical of a 14N nucleus. This spectrum is similar to that produced by reaction of SNP with dithionite (7). It is presumed to represent a paramagnetic species derived from reaction of the cells with SNP. The signal increased with time of exposure to SNP, indicating that the SNP was reduced by some cell constituent. This signal was not observed in the absence of cells.
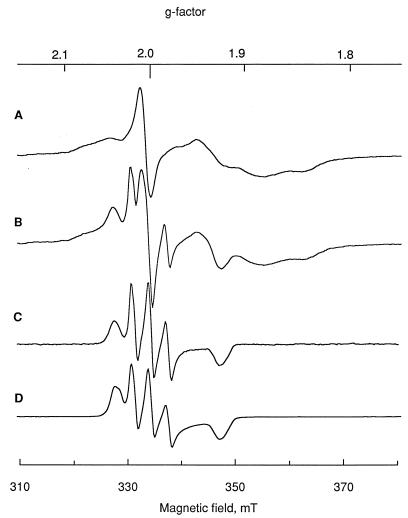
Low-temperature EPR spectra of C. sporogenes cells treated with SNP were also recorded. Spectra of the frozen samples at temperatures below 30 K showed the characteristic signals of reduced [4Fe-4S] iron-sulfur clusters, centered around g = 1.94 (Fig. 2A). These signals are expected to arise largely from ferredoxin, but comparison of line shape with that of purified ferredoxin (not shown) indicates the presence of other iron-sulfur clusters, probably pyruvate-ferredoxin reductase (25). On addition of SNP, the signals due to [4Fe-4S] clusters were relatively unaffected (Fig. 2B). However, it was noticed that a new EPR signal appeared. This can be seen more clearly by subtraction of the signals due to the [4Fe-4S] clusters from the spectrum (Fig. 2C). The spectrum has approximately axial symmetry. There is a three-line hyperfine splitting around g = 1.999 and another feature at g = 1.927. This species has a different average g factor from that observed at room temperature and presumably represents another compound derived from SNP. A similar spectrum was observed on reaction of SNP with a thiol compound (Fig. 2D).
FIG. 2.
EPR spectra of C. sporogenes cells. Cells were harvested in late exponential phase, resuspended in a minimum volume of phosphate buffer, placed in EPR quartz tubes, and frozen at liquid nitrogen temperature. (A) EPR spectrum of cell suspension in buffer. (B) EPR spectrum of cell suspension treated with SNP. (C) Spectrum of difference between panels A and B. (D) EPR spectrum of SNP treated with 2-mercaptoethanol. Measurement conditions: temperature, 16 K; microwave frequency, 9.355 GHz; power, 20 mW; gain, 3.2 × 103; modulation amplitude, 1 mT.
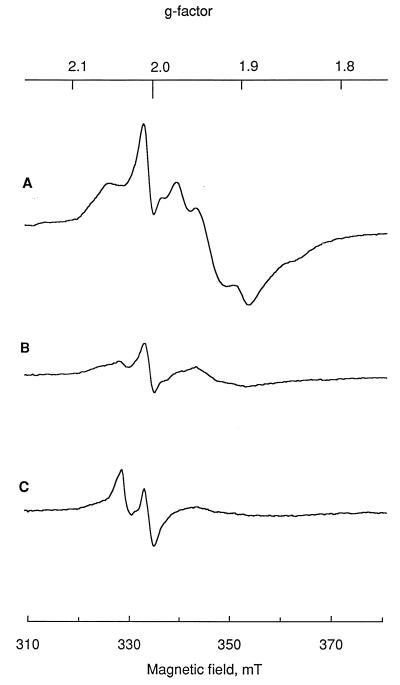
Cells grown with 40 μM SNP showed the appearance of yet another signal, centered at g = 2.033 (Fig. 3). This signal has been frequently observed in biological systems exposed to NO (16, 31) and is assigned to dinitrosyl iron complexes (DNIC). The amplitude of this signal appears small in the spectrum recorded at 16 K (Fig. 3), due to microwave power saturation, but was more prominent when measured at 50 K, at which the iron-sulfur clusters were not detectable (data not shown). After cells were grown for 6 h in the presence of SNP, the EPR spectra (not shown) derived from reduced SNP were no longer detectable. The signals from reduced [4Fe-4S] clusters were less intense in SNP-treated cells than in the control cells. The decrease was in parallel with the cell density in the samples, indicating that the content of iron-sulfur proteins per cell was relatively unaffected.
FIG. 3.
EPR spectra of C. sporogenes cells grown in the presence of SNP. (A) Control cells, recorded at a temperature of 16 K. (B) Cells grown with 20 μM SNP at 16 K. (C) Cells grown with 40 μM SNP at 16 K. Cells were harvested 6 h after SNP was added. Measurement conditions: microwave frequency, 9.365 GHz; power, 20 mW; gain, 2 × 104; modulation amplitude, 0.2 mT.
Effects on cell wall morphology.
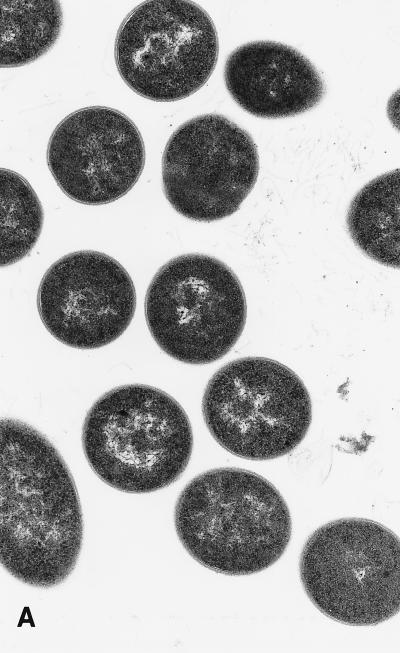
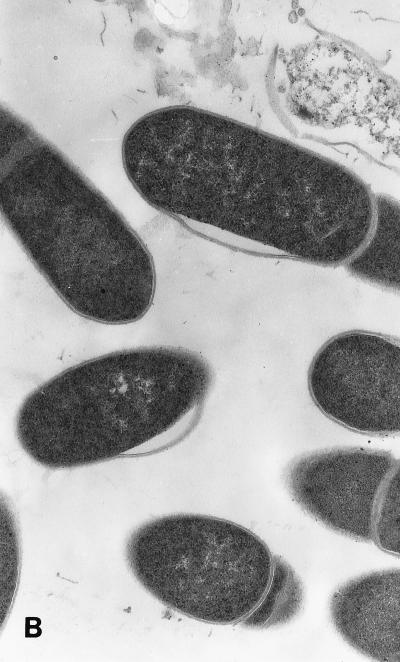
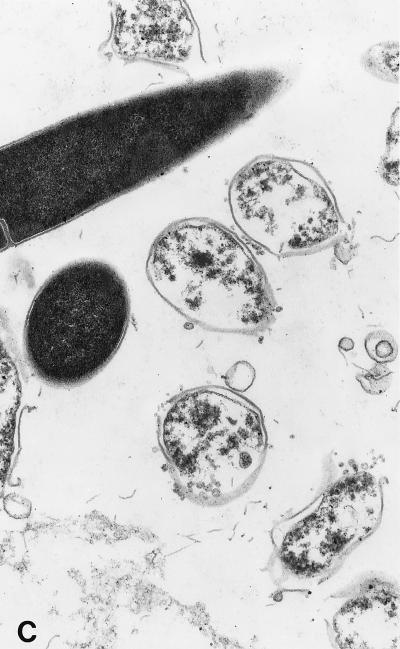
When examined by transmission electron microscopy, C. sporogenes cells exposed to 20 and 40 μM SNP showed an altered morphology with the appearance of blisters in the cell wall which were clearly visible at 40 μM (Fig. 4). After 240 min of incubation, a large amount of cell debris was present, with 94% of cells showing damage. These effects are consistent with a primary attack at the cell wall. The blistering of the outer cell membrane and the rapid lysis of cells are similar in appearance to those induced by a variety of antimicrobial proteins (35).
FIG. 4.
Transmission electron micrographs of cells grown in the presence of SNP. SNP was added to actively growing cells as described for Fig. 1. (A) Control cells. (B) Cells treated with a final concentration of 20 μM SNP for 240 min. (C) Cells treated with 40 μM SNP for 10 min. Magnification, ca. ×30,000.
Damage to cells by each concentration of SNP was quantified by examining 600 cells in a representative grid section and counting those that showed recognizable blistering. The proportions of damaged cells in the controls and cells treated with 20 and 40 μM SNP were 3, 11, and 20%, respectively, after 10 min and 4, 52, and 94%, respectively, after 240 min.
Determination of thiol residues in SNP-treated cells.
The thiol composition of cells was determined by Ellman’s reagent. Whole cells treated with 100 μM SNP and immediately harvested showed a decrease of (58 ± 5)% of the available thiol groups. This is probably due to the reaction of thiol groups at the cell surface, but the possibility that there was a contribution from lysed cells could not be excluded. Cells were broken and separated under a nitrogen atmosphere into cytoplasm, membrane, and outer cell wall-membrane fractions. Comparison of control cells with cells grown in the presence of SNP showed a 90% decrease of thiols in the cell wall fraction (Table 2). Similar decreases of the thiol content in cell extract have been observed in Clostridium perfringens treated with bacteriostatic concentrations of nitrite by O’Leary and Solberg (24). In an analogous experiment in which the cells were fractionated and then treated with 100 μM SNP, the reduction in thiol content for the cell wall was 26%, that for the cytoplasmic fraction was 70%, and that for the membrane was 50% of that of control. This suggests that SNP will attack a number of cellular thiol constituents and that its action is nonspecific. The analysis of these fractions by EPR showed complex spectra, indicating that a number of species were being formed (results not shown).
TABLE 2.
Titration of free thiols from cells treated with 100 μM SNP and fractionated to give cell wall, membrane, and cytoplasma
| Fraction | μmol of SH (g of protein−1)
|
% Decrease | |
|---|---|---|---|
| Control cells | Cells treated with 100 μM SNP | ||
| Cell wall | 90 | 9 | 90 |
| Membrane | 60 | 53 | 10 |
| Cytoplasm | 157 | 86 | 50 |
C. sporogenes was grown in 3 liters of medium to give an optical density value at 550 nm of 1; SNP was then added to give a final concentration of 100 mM. The cells were harvested and fractionated into cell wall, membrane, and cytoplasm. For each fraction, the amount of free thiols was estimated as described in Materials and Methods.
DISCUSSION
Treatment of cultures of C. sporogenes with SNP caused inhibition of cell growth (Fig. 1). SNP has a number of chemical properties that might be relevant to its bacteriostatic effect. It is a good nitrosating agent, reacting rapidly with thiol groups to form S-nitrosothiols which release nitric oxide, NO. Complexes such as SNP, which contain nitrosyl groups in the formal oxidation state NO+, are able to function as nitrosating agents at neutral pH values, whereas nitrite-nitrous acid is effective only at acid pH. The chemistry of NO+ is characterized by addition and substitution reactions with nucleophiles such as electron-rich bases and aromatic compounds. Nitrosation in aqueous phase can occur at -S, -N, -O, and -C centers in organic molecules and appears to involve NO+ or NO+ carriers (32), depending on the conditions.
Reaction of nitroprusside with a thiol such as cysteine leads to a one-electron reduction of the complex (10). This initial step involves the rapid attack of the thiolate anion on the nitrogen atom of the nitrosyl ligand to give a highly colored species, followed by loss of the thiol radical RS· and formation of [Fe(CN)5NO]3-. This species is paramagnetic, with the unpaired electron centered largely on the NO group (vNO = 1,580 cm−1; EPR g values: gx = 1.9993, gy = 1.9282, and gz = 2.008). Subsequent reactions involve internal electron transfer and the formation of [Fe(CN)4NO]2−, in which the unpaired electron is now on the iron rather than on the nitrosyl group, giving an Fe(I) complex with an NO+ group (gx = 2.036, gy = 2.0325, gz = 2.0054; vNO = 1,755 cm−1). It is likely that electron transfer from the NO group to Fe(II) in [Fe(CN)5NO]3− precedes loss of cyanide, because Fe(I) complexes are substitution labile. The species [Fe(CN)4NO]2− then decomposes to a variety of products, including NO and, if an excess of thiol is present, mononuclear DNIC (g = 2.035). Thus, SNP, reacting with cell walls, would be expected to cause oxidation of thiols and local release of NO.
EPR spectra of C. sporogenes cells treated with SNP recorded at both liquid helium and room temperatures are consistent with a reaction between SNP and thiols. Signals at g = 1.999 and g = 2.028 are similar to published spectra of [Fe(CN)5NO]3− and [Fe(CN)4NO]2−, respectively (10). The g = 1.999 signal was observed before the g = 2.028 signal, and the intensity of both signals increased with time in the period from 20 to 50 min. A third paramagnetic species with a signal at g = 2.035 was subsequently observed and assigned to the DNIC species.
The results of analysis of thiols (Table 2) suggest a specific oxidizing action of SNP at the cell wall. This occurred at a concentration of 0.1 mM SNP, compared with 14.4 mM nitrite as used in a previous study (24). C. sporogenes is a gram-positive bacterium, whose cell walls have a relatively thick peptidoglycan layer which acts as the primary protector of the cell wall. The thiols that were measured were presumably in proteins associated with peptidoglycan, termed S-layer proteins (30). The damage to the cell wall can clearly be seen from the electron micrographs. Compounds that act at the cell wall, such as cationic peptides, cause blistering, resulting in loss of structural integrity leading to rapid cell lysis (35). It is conceivable that oxidation (via nitrosation) by SNP of thiol groups at the cell surface could cause a breakdown in the integrity of the cell, causing blistering and lysis.
Further evidence is provided by comparison of the cytotoxicity of SNP with that of the related transition metal nitrosyl complexes (Table 1). These were all anionic, pentacyano species: K3[V(CN)5NO] · H2O, a 16-electron complex; K3[Cr(CN)5NO] · H2O, a 17-electron complex; and Li2[Ru(CN)5NO] · H2O, an 18-electron complex. Nitrosyl complexes may be classified by structure and by the value of the N-O stretching frequency in the infrared spectrum. The nitrosyl ligand in transition metal nitrosyl complexes may formally be classified as linear M-NO+ or bent M-NO− species (and occasionally with neutral NO). The NO+ character increases with vN-O for a homologous series of compounds (for example, with the same charge). In general, complexes with higher vN-O are expected to function more effectively as nitrosating agents. Thus, if the bactericidal effects of nitrite result from a nitrosation reaction, then the toxic effects of a series of chemically and structurally similar nitrosyl compounds should correlate with the N-O stretching frequency. This correlation appears to hold good for complexes of the first-row transition elements iron, chromium, and vanadium (Table 1). However, for the second-row transition element ruthenium, other chemical factors come into play. The ruthenium complex differs from the other compounds in its lack of reactivity towards thiols and its inability to undergo reduction to Ru(I). Thus, the lack of toxicity of the ruthenium analog of nitroprusside, despite its similarity in N-O stretching frequency, is consistent with a toxicity mechanism requiring S-nitrosation and reduction of the metal.
The toxic effect of the nitroprusside anion towards C. sporogenes results from nitrosation of the thiol group in the cell wall, with resulting structural damage. This is supported by electron microscopy, analysis of thiol groups in control and treated cells, EPR spectroscopy, and comparison with the reactivities of a number of other pentacyanonitrosyl complexes. Effects on metabolism appear to be secondary, possibly resulting from entry of SNP subsequent to damage to the cell wall.
ACKNOWLEDGMENTS
The work was supported by a grant from the Agricultural and Food Research Council. X.-Y.C. acknowledges a K. C. Wong scholarship and support from the Chinese Government; C.L.T.M. acknowledges financial support from Conacyt, Mexico.
We thank John Pacy for the preparation of the transmission electron micrographs, Andrew White for assistance with the EPR measurements, and Shaun Maraj for his help in the synthesis of the pentacyanonitrosyl complexes.
REFERENCES
- 1.Armor J N, Scheidegger H, Taube H. A bimolecular mechanism for substitution. J Am Chem Soc. 1968;90:5928–5929. [Google Scholar]
- 2.Ashworth J, Didcock A, Hargreaves L L, Jarvis B, Walters C L, Larkworthy L F. Chemical and microbiological comparisons of inhibitors derived thermally from nitrite with an iron thionitrosyl. J Gen Microbiol. 1974;84:403–408. doi: 10.1099/00221287-84-2-403. [DOI] [PubMed] [Google Scholar]
- 3.Benedict R C. Biochemical basis for nitrite-inhibition of Clostridium botulinum in cured meat. J Food Prot. 1980;43:877–891. doi: 10.4315/0362-028X-43.11.877. [DOI] [PubMed] [Google Scholar]
- 4.Butler A R, Glidewell C, Hyde A R, McGinnis J, Seymour J E. Ligand exchange processes in some Fe-S-CO and NO complexes. Polyhedron. 1983;2:1045–1049. [Google Scholar]
- 5.Butler A R, Glidewell C, Reglinsk J, Waddon A. The pentacyanonitrosylferrate ion. Part 1. Reactions with various amines. J Chem Res (Synop) 1984;1984:279. [Google Scholar]
- 6.Butler A R, Glidewell C, Chaipanich V, McGinnis J. The pentacyanonitrosylferrate ion. Part 2. Reactions with various carbanions. J Chem Soc Perkin Trans. 1986;II:7–9. [Google Scholar]
- 7.Butler A R, Calsy-Harrison A M, Glidewell C. The pentacyanonitrosylferrate ion. V. The course of the reactions of nitroprusside with a range of thiols. Polyhedron. 1988;7:1197–1202. [Google Scholar]
- 8.Butler A R, Williams D L H. The physiological role of nitric oxide. Chem Soc Rev. 1993;1993:233–241. [Google Scholar]
- 9.Carpenter C E, Reddy D S, Cornforth D P. Inactivation of clostridial ferredoxin and pyruvate-ferredoxin oxidoreductase by sodium nitrite. Appl Environ Microbiol. 1987;53:549–552. doi: 10.1128/aem.53.3.549-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke M J, Gaul J B. Chemistry relevant to the biological effects of nitric oxide and metallonitrosyls. Struct Bonding. 1993;81:147–181. [Google Scholar]
- 11.Cui X, Joannou C L, Hughes M N, Cammack R. The bacteriocidal effects of transition metal complexes containing the NO+ group on the food-spoilage bacterium Clostridium sporogenes. FEMS Microbiol Lett. 1992;98:67–70. doi: 10.1016/0378-1097(92)90133-9. [DOI] [PubMed] [Google Scholar]
- 12.Ellman G L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 13.Griffith W P, Lewis J, Wilkinson G. Infrared spectra of transition metal-nitric oxide complexes. Part IV. The pentacyanonitrosyl-complexes of chromium and molybdenum. J Chem Soc. 1959;1959:872–875. [Google Scholar]
- 14.Jagner S, Vannerberg N G. Crystal structure of potassium pentacyanonitrosylvanadate(I) Acta Chem Scand. 1968;22:3330–3331. [Google Scholar]
- 15.Johnson M D, Wilkins R G. Kinetics of the primary interaction of pentacyanonitrosylferrate(2-) (nitroprusside) with aliphatic thiols. Inorg Chem. 1984;23:231–235. [Google Scholar]
- 16.Lancaster J R, Jr, Hibbs J B. EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci USA. 1990;87:1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levett P N. Anaerobic microbiology: a practical approach. Oxford, United Kingdom: IRL Press; 1991. [Google Scholar]
- 18.Lovitt R W, Morris J G, Kell D B. The growth and nutrition of Clostridium sporogenes NCIB 8053 in defined media. J Appl Bacteriol. 1987;62:71–80. doi: 10.1111/j.1365-2672.1987.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 19.Lunak S, Veprek-Siska J. Reaction of hydroxylamine with nitroprusside ions. Collect Czech Chem Commun. 1974;39:2719–2723. [Google Scholar]
- 20.Maltz H, Grant M A, Navaroli M C. Reactions of nitroprusside with amines. J Org Chem. 1971;36:363–369. [Google Scholar]
- 21.Meyer C L, Roos J W, Papoutsakis E T. Carbon monoxide gassing leads to alcohol production and butyrate uptake without acetone formation in continuous cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol. 1986;24:159–167. [Google Scholar]
- 22.Moran D M, Tannenbaum S R, Archer M C. Inhibitor of Clostridium perfringens formed by heating sodium nitrite in a chemically defined medium. Appl Microbiol. 1975;30:838–843. doi: 10.1128/am.30.5.838-843.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morando P J, Borghi E B, de Schteingart L M, Blesa M A. The reaction of cysteine with the pentacyanonitrosylferrate(2-) ion. J Chem Soc Dalton Trans. 1981;1981:435–440. [Google Scholar]
- 24.O’Leary V, Solberg M. Effect of sodium nitrite inhibition on intercellular thiol groups and on activity of certain glycolytic enzymes in Clostridium perfringens. Appl Environ Microbiol. 1976;31:208–212. doi: 10.1128/aem.31.2.208-212.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payne M J, Woods L F J, Gibbs P, Cammack R. Electron paramagnetic resonance spectroscopic investigation of the inhibition of the phosphoroclastic system of Clostridium sporogenes by nitrite. J Gen Microbiol. 1990;136:2067–2076. doi: 10.1099/00221287-136-10-2067. [DOI] [PubMed] [Google Scholar]
- 26.Payne M J, Glidewell C, Cammack R. Interactions of iron-thiol-nitrosyl compounds with the phosphoroclastic system of Clostridium sporogenes. J Gen Microbiol. 1990;136:2077–2087. doi: 10.1099/00221287-136-10-2077. [DOI] [PubMed] [Google Scholar]
- 27.Perigo J A, Whiting E, Bashford T E. Observations on the inhibition of vegetative cells of Clostridium sporogenes by nitrite which has been autoclaved in a laboratory medium, discussed in the context of sub-lethally processed cured meats. J Food Technol. 1967;2:377–397. [Google Scholar]
- 28.Reddy D, Lancaster J R, Cornforth D P. Nitrite inhibition of Clostridium botulinum: electron spin resonance detection of iron-nitric oxide complexes. Science. 1983;221:769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- 29.Roberts T A, Gibson A M. Chemical methods for controlling Clostridium botulinum in processed meats. Food Technol. 1986;1986(April):163–171. [Google Scholar]
- 30.Sleytr U B, Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988;170:2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vanin A F, Varich V Y A. Nitrosyl non-heme iron complexes in animal tissues. Stud Biophys. 1981;86:177–185. [Google Scholar]
- 32.Williams D L H. Nitrosation. Cambridge, United Kingdom: Cambridge University Press; 1988. pp. 1–214. [Google Scholar]
- 33.Wolfe S K, Andrade C, Swineheart J H. Kinetic studies of the pentacyanonitrosylferrate(2-)-azide and -hydroxylamine reactions. Inorg Chem. 1974;13:2567–2572. [Google Scholar]
- 34.Woods L F J, Wood J M, Gibbs P A. The involvement of nitric oxide in the inhibition of the phosphoroclastic system in Clostridium sporogenes by sodium nitrite. J Gen Microbiol. 1981;125:399–406. doi: 10.1099/00221287-125-2-399. [DOI] [PubMed] [Google Scholar]
- 35.Yamauchi K, Tomita M, Giehl T J, Ellison R T., III Antibacterial activity of lactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect Immun. 1993;61:719–728. doi: 10.1128/iai.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]