Abstract
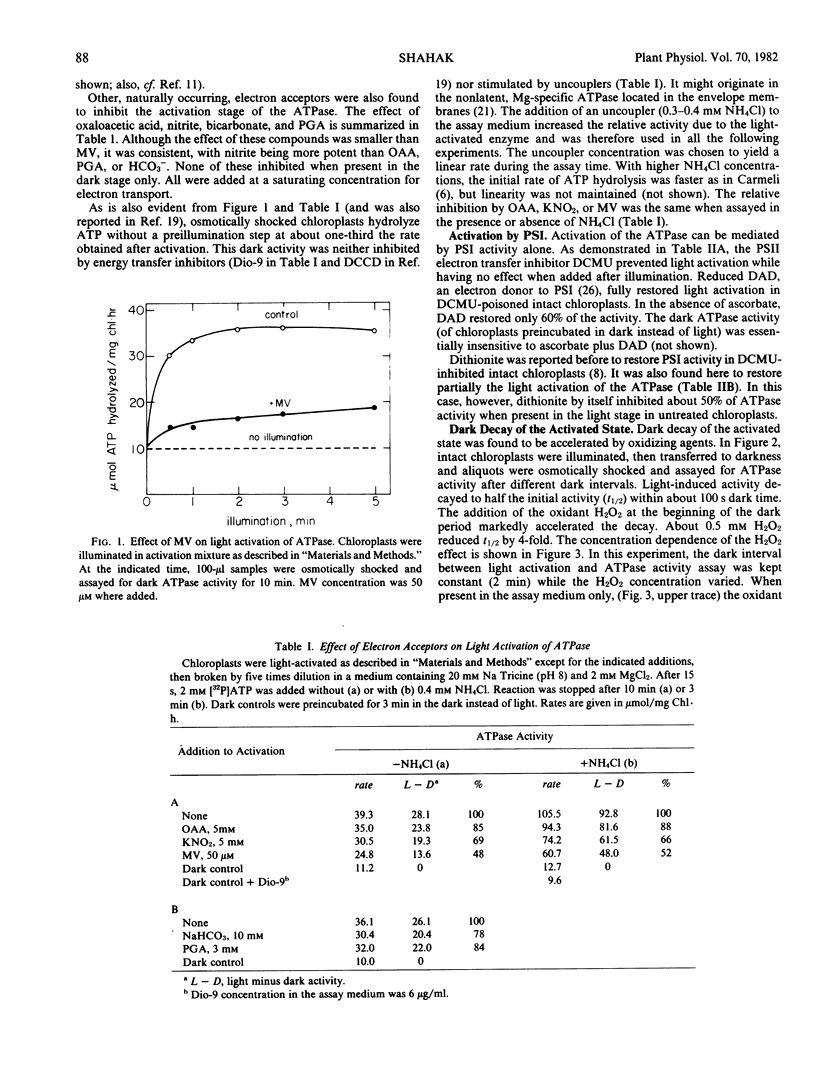
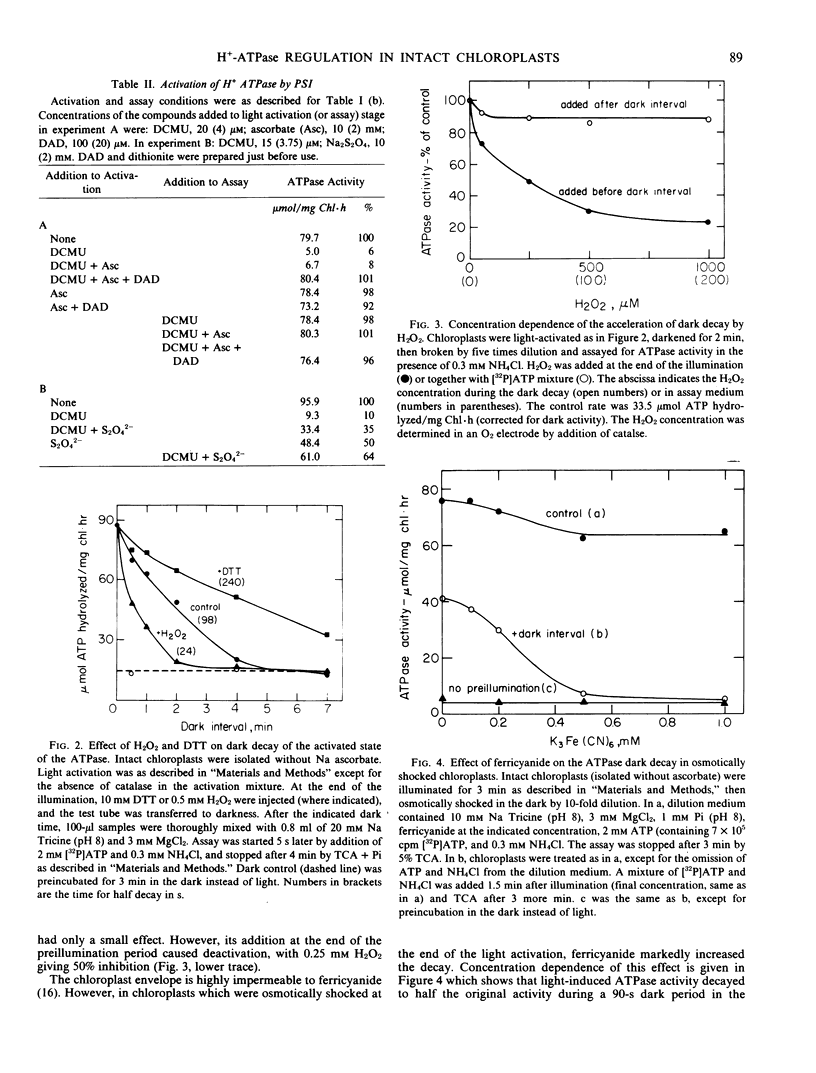
The light activation mechanism of the latent H+-ATPase was investigated in intact spinach (Spinacia oleracea, Hybrid 424) chloroplasts. The following observations were made. (a) Photosystem I electron acceptors such as methyl viologen, nitrite, oxaloacetate, etc., inhibit the light activation of the enzyme. (b) The electron transfer inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) fully inhibits the process. (c) Ascorbate plus diaminodurene or dithionite can restore light activation in DCMU-poisoned chloroplasts. (d) The activated state of the enzyme decays rather slowly (within a few minutes) after illumination of the intact chloroplasts. (e) The rate of dark decay is accelerated by oxidants (H2O2 or ferricyanide) and slowed down by dithiothreitol.
It is suggested that the physiological mechanism for regulation of the H+-ATPase involves oxidation and reduction reactions in a manner which resembles the regulation of the light-activated carbon cycle enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli C., Lifshitz Y. Effects of P 1 and ADP on ATPase activity in chloroplasts. Biochim Biophys Acta. 1972 Apr 20;267(1):86–95. doi: 10.1016/0005-2728(72)90140-5. [DOI] [PubMed] [Google Scholar]
- Carmeli C. Properties of ATPase in chloroplasts. Biochim Biophys Acta. 1969 Oct 21;189(2):256–266. doi: 10.1016/0005-2728(69)90052-8. [DOI] [PubMed] [Google Scholar]
- Crowther D., Mills J. D., Hind G. Protonmotive cyclic electron flow around photosystem I in intact chloroplasts. FEBS Lett. 1979 Feb 15;98(2):386–390. doi: 10.1016/0014-5793(79)80223-9. [DOI] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Kobayashi Y., Shibata K., Heber U. Synthesis and hydrolysis of ATP by intact chloroplasts under flash illumination and in darkness. Biochim Biophys Acta. 1978 Oct 11;504(1):142–152. doi: 10.1016/0005-2728(78)90013-0. [DOI] [PubMed] [Google Scholar]
- Magnusson R. P., Portis A. R., Jr, McCarty R. E. Quantitative, analytical separation of adenine nucleotides by column chromatography on polyethyleneimine-coated cellulose. Anal Biochem. 1976 May 7;72:653–657. doi: 10.1016/0003-2697(76)90580-7. [DOI] [PubMed] [Google Scholar]
- McKinney D. W., Buchanan B. B., Wolosiuk R. A. Association of a thioredoxin-like protein with chloroplaast coupling factor (CF1). Biochem Biophys Res Commun. 1979 Feb 28;86(4):1178–1184. doi: 10.1016/0006-291x(79)90241-9. [DOI] [PubMed] [Google Scholar]
- Mills J. D., Hind G. Light-induced Mg2+ ATPase activity of coupling factor in intact chloroplasts. Biochim Biophys Acta. 1979 Sep 11;547(3):455–462. doi: 10.1016/0005-2728(79)90026-4. [DOI] [PubMed] [Google Scholar]
- Poincelot R. P., Day P. R. An improved method for the isolation of spinach chloroplast envelope membranes. Plant Physiol. 1974 Nov;54(5):780–783. doi: 10.1104/pp.54.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshan V., Selman B. R. The relationship between light-induced adenine nucleotide exchange and ATPase activity in chloroplast thylakoid membranes. J Biol Chem. 1979 Sep 25;254(18):8801–8807. [PubMed] [Google Scholar]


