Abstract
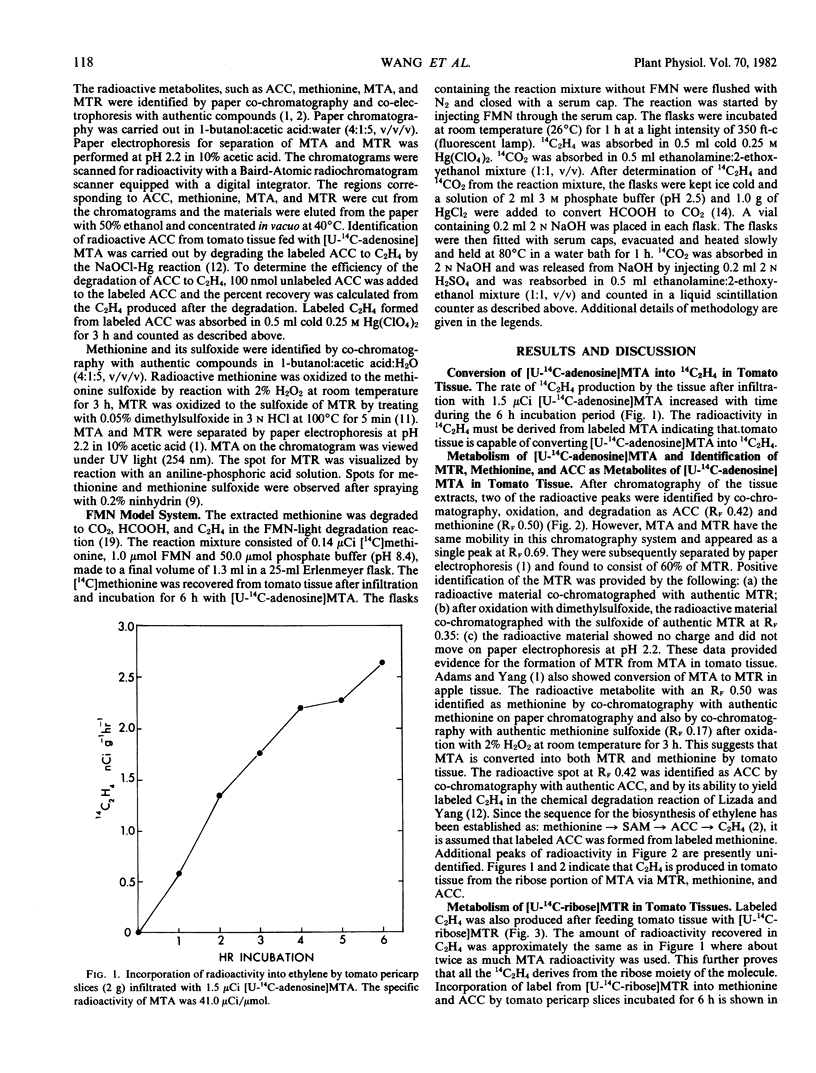
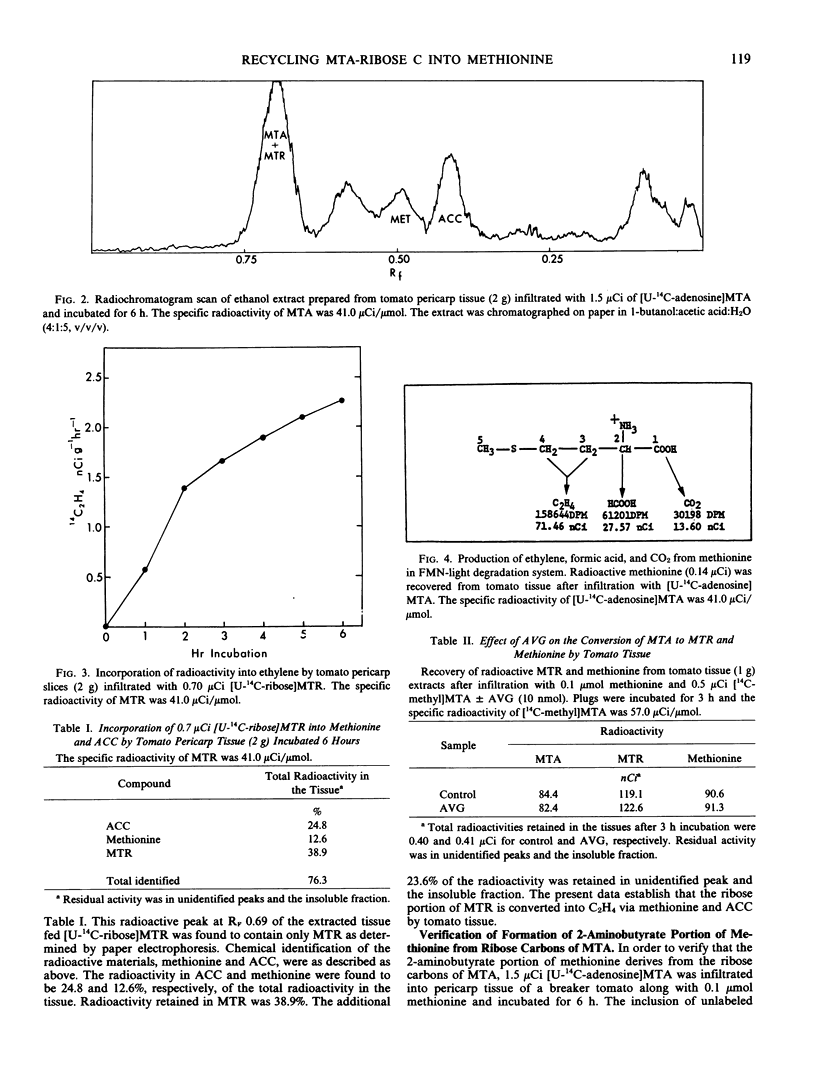
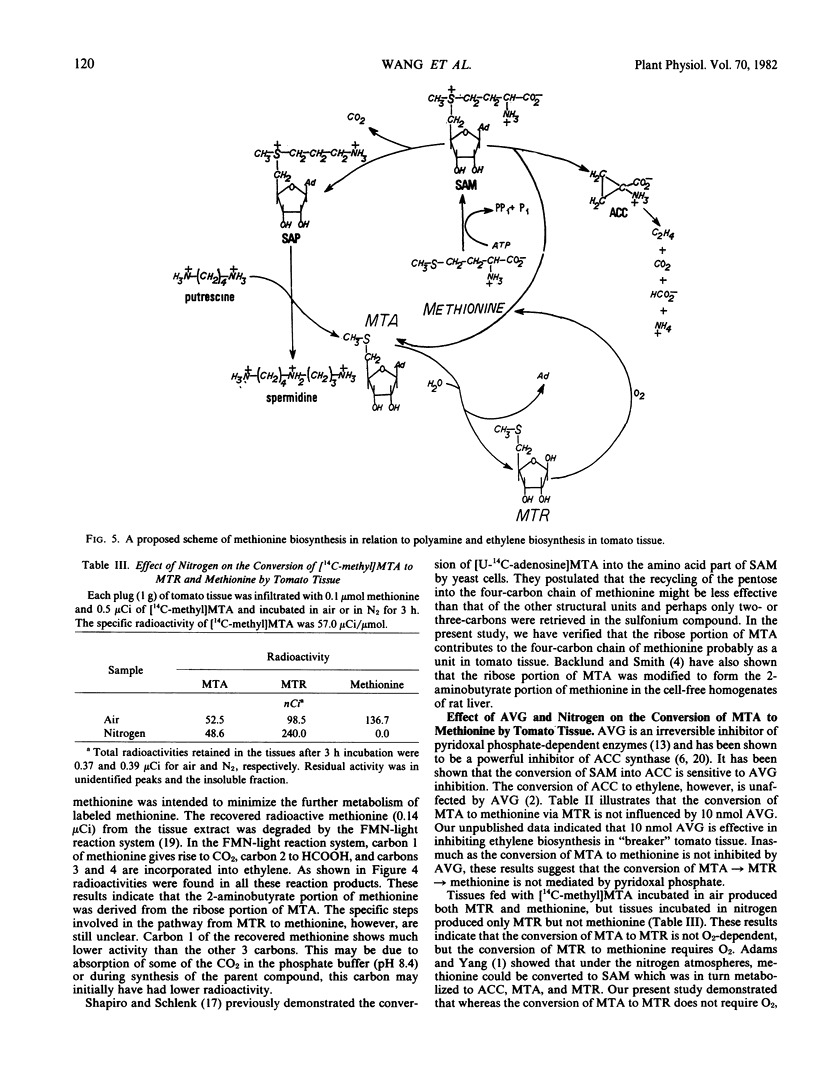
The ribose moiety of 5′-methylthioadenosine (MTA) is metabolized to form the four-carbon unit (2-aminobutyrate) of methionine in tomato tissue (Lycopersicon esculentum Mill., cv. Pik Red). When [U-14C-adenosine] MTA was administered to tomato tissue slices, label was recovered in 5-methylthioribose (MTR), methionine, 1-aminocyclopropane-1-carboxylic acid (ACC), C2H4 and other unidentified compounds. However, when [U-14C-ribose]MTR was administered, radioactivities were recovered in methionine, ACC and C2H4, but not MTA. This suggests that C2H4 formed in tomato pericarp tissue may be derived from the ribose portion of MTA via MTR, methionine and ACC. The conversion of MTR to methionine is not inhibited by aminoethoxyvinylglycine (AVG), but is O2 dependent. These data present a new salvage pathway for methionine biosynthesis which may be important in relation to polyamine and ethylene biosynthesis in tomato tissue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Methionine metabolism in apple tissue: implication of s-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol. 1977 Dec;60(6):892–896. doi: 10.1104/pp.60.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backlund P. S., Jr, Smith R. A. Methionine synthesis from 5'-methylthioadenosine in rat liver. J Biol Chem. 1981 Feb 25;256(4):1533–1535. [PubMed] [Google Scholar]
- Baur A. H., Yang S. F. Precursors of ethylene. Plant Physiol. 1969 Sep;44(9):1347–1349. doi: 10.1104/pp.44.9.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang P. K., Cantoni G. L. Activation of methionine for transmethylation. Purification of the S-adenosylmethionine synthetase of bakers' yeast and its separation into two forms. J Biol Chem. 1977 Jul 10;252(13):4506–4513. [PubMed] [Google Scholar]
- Lipton S. H., Bodwell C. E. Specific oxidation of methionine to methionine sulfoxide by dimethyl sulfoxide. J Agric Food Chem. 1976 Jan-Feb;24(1):26–31. doi: 10.1021/jf60203a025. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Ming Chu T., Mallette M. F., Mumma R. O. Isolation and characterization of 5'-S-methyl-5'-thioadenosine from Escherichia coli. Biochemistry. 1968 Apr;7(4):1399–1406. doi: 10.1021/bi00844a023. [DOI] [PubMed] [Google Scholar]
- Rando R. R. Chemistry and enzymology of kcat inhibitors. Science. 1974 Jul 26;185(4148):320–324. doi: 10.1126/science.185.4148.320. [DOI] [PubMed] [Google Scholar]
- SCHLENK F., EHNINGER D. J. OBSERVATIONS ON THE METABOLISM OF 5'-METHYLTHIOADENOSINE. Arch Biochem Biophys. 1964 Jul 20;106:95–100. doi: 10.1016/0003-9861(64)90161-4. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Barrett A. 5-Methylthioribose as a precursor of the carbon chain of methionine. Biochem Biophys Res Commun. 1981 Sep 16;102(1):302–307. doi: 10.1016/0006-291x(81)91521-7. [DOI] [PubMed] [Google Scholar]
- Shapiro S. K., Schlenk F. Conversion of 5'-methylthioadenosine into S-adenosylmethionine by yeast cells. Biochim Biophys Acta. 1980 Dec 1;633(2):176–180. doi: 10.1016/0304-4165(80)90403-1. [DOI] [PubMed] [Google Scholar]
- Yang S. F., Ku H. S., Pratt H. K. Photochemical production of ethylene from methionine and its analogues in the presence of flavin mononucleotide. J Biol Chem. 1967 Nov 25;242(22):5274–5280. [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]


