Abstract
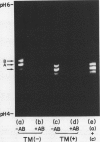
The biosynthetic mechanism of α-amylase synthesis in germinating rice (Oryza sativa L. cv. Kimmazé) seeds has been studied both in vitro and in vivo. Special attention has been focused on the glycosylation of the enzyme molecule. Tunicamycin was found to inhibit glycosylation of α-amylase by 98% without significant inhibition of enzyme secretion. The inhibitory effect exerted by the antibiotic on glycosylation did not significantly alter enzyme activity.
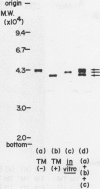
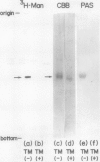
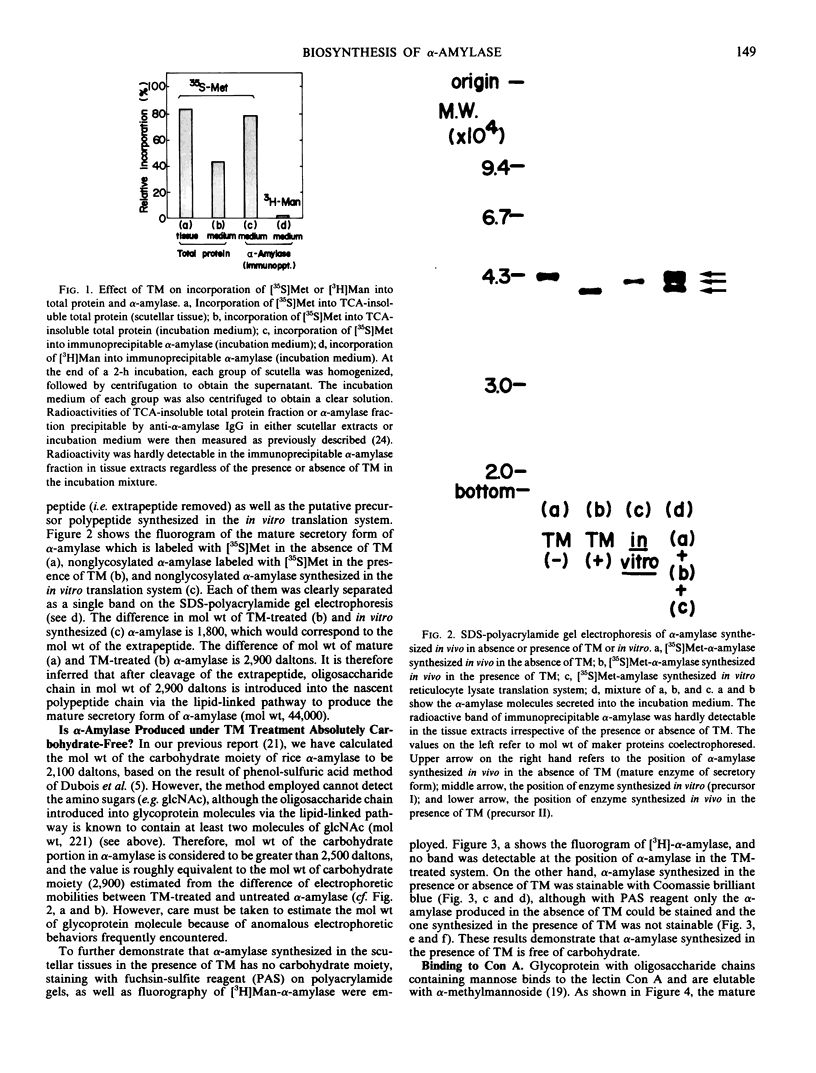
In an in vitro system using poly-(A) RNA isolated from rice scutellum and the reticulocyte lysate translation system, a precursor form of α-amylase (precursor I) is formed. Inhibition of glycosylation by Tunicamycin allowed detection of a nonglycosylated precursor (II) of α-amylase. The molecular weight of the nonglycosylated precursor II produced in the presence of Tunicamycin was 2,900 daltons less than that of the mature form of α-amylase (44,000) produced in the absence of Tunicamycin, and 1,800 daltons less than the in vitro synthesized molecule.
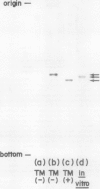
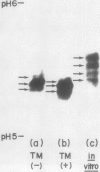
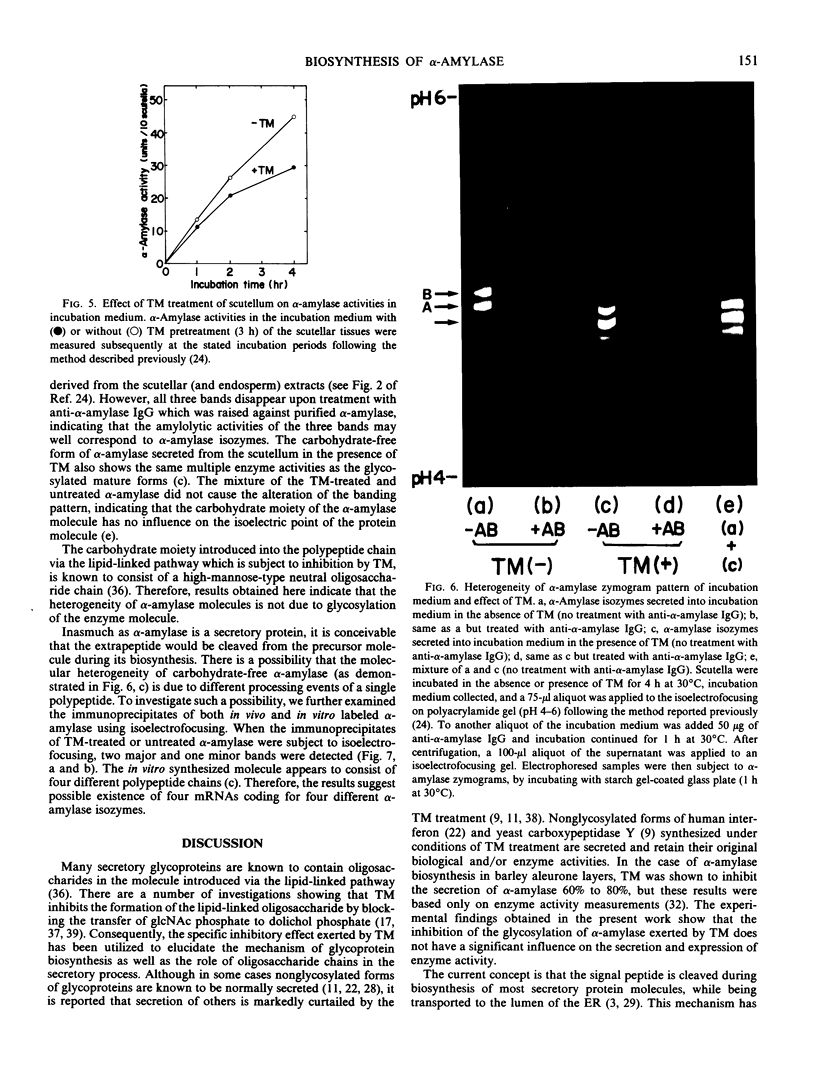
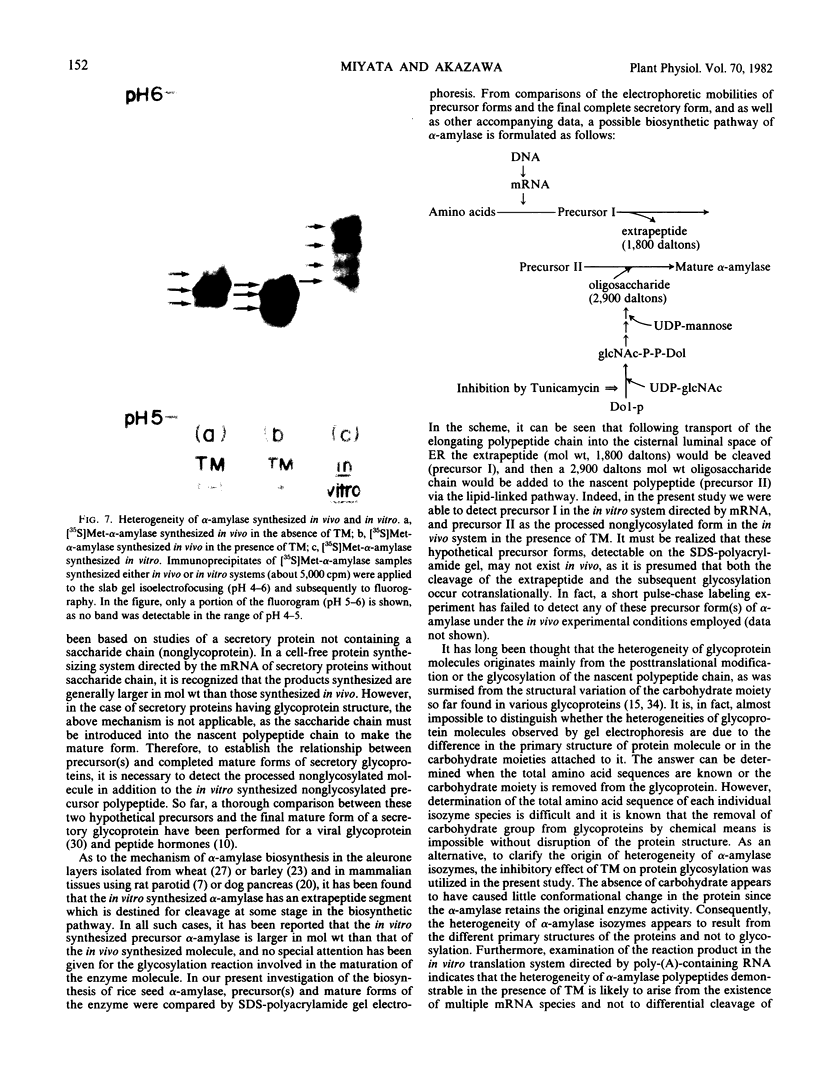
The inhibition of glycosylation by Tunicamycin as well as in vitro translation helped clarify the heterogeneity of α-amylase isozymes. Isoelectrofocusing (pH 4-6) of the products, zymograms, and fluorography were employed on the separated isozyme components. The mature and Tunicamycin-treated nonglycosylated forms of α-amylase were found to consist of three isozymes. The in vitro translated precursor forms of α-amylase consisted of four multiple components. These results indicate that heterogeneity of α-amylase isozymes is not due to glycosylation of the enzyme protein but likely to differences in the primary structure of the protein moiety, which altogether support that rice α-amylase isozymes are encoded by multiple genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Addition of glucosamine and mannose to nascent immunoglobulin heavy chains. Biochemistry. 1977 Oct 4;16(20):4490–4497. doi: 10.1021/bi00639a025. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1520–1526. doi: 10.1073/pnas.58.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOTTSCHALK A., FAZEKAS DE S. T., GROTH S. Studies on mucoproteins. III. The accessibility to trypsin of the susceptible bonds in ovine submaxillary gland mucoprotein. Biochim Biophys Acta. 1960 Oct 7;43:513–519. doi: 10.1016/0006-3002(60)90473-x. [DOI] [PubMed] [Google Scholar]
- Gorecki M., Zeelon E. P. Cell-free synthesis of rat parotid preamylase. J Biol Chem. 1979 Jan 25;254(2):525–529. [PubMed] [Google Scholar]
- Hasilik A., Tanner W. Carbohydrate moiety of carboxypeptidase Y and perturbation of its biosynthesis. Eur J Biochem. 1978 Nov 15;91(2):567–575. doi: 10.1111/j.1432-1033.1978.tb12710.x. [DOI] [PubMed] [Google Scholar]
- Herbert E., Budarf M., Phillips M., Rosa P., Policastro P., Oates E., Roberts J. L., Seidah N. G., Chrétien M. Presence of a pre-sequence (signal sequence) in the common precursor to ACTH and endorphin and the role of glycosylation in processing of the precursor and secretion of ACTH and endorphin. Ann N Y Acad Sci. 1980;343:79–93. doi: 10.1111/j.1749-6632.1980.tb47244.x. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Karn R. C., Shulkin J. D., Merritt A. D., Newell R. C. Evidence for post-transcriptional modification of human salivary amylase (amyl) isozymes. Biochem Genet. 1973 Dec;10(4):341–350. doi: 10.1007/BF00485989. [DOI] [PubMed] [Google Scholar]
- Kiely M. L., McKnight G. S., Schimke R. T. Studies on the attachment of carbohydrate to ovalbumin nascent chains in hen oviduct. J Biol Chem. 1976 Sep 25;251(18):5490–5495. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem. 1973;42(0):541–574. doi: 10.1146/annurev.bi.42.070173.002545. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Przybyla A. E., Rutter W. J. Isolation and in vitro translation of the messenger RNA coding for pancreatic amylase. J Biol Chem. 1977 Aug 10;252(15):5522–5528. [PubMed] [Google Scholar]
- Miyata S., Okamoto K., Watanabe A., Akazawa T. Enzymic Mechanism of Starch Breakdown in Germinating Rice Seeds: 10. IN VIVO AND IN VITRO SYNTHESIS OF alpha-AMYLASE IN RICE SEED SCUTELLUM. Plant Physiol. 1981 Dec;68(6):1314–1318. doi: 10.1104/pp.68.6.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi A., O'Malley J. A., Carter W. A., Takatsuki A., Tamura G., Sulkowski E. Glycosylation of interferons. Effects of tunicamycin on human immune interferon. J Biol Chem. 1978 Nov 10;253(21):7612–7615. [PubMed] [Google Scholar]
- Mozer T. J. Partial purification and characterization of the mRNA for alpha-amylase from barley aleurone layers. Plant Physiol. 1980 May;65(5):834–837. doi: 10.1104/pp.65.5.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Akazawa T. Enzymic mechanisms of starch breakdown in germinating rice seeds: 7. Amylase formation in the epithelium. Plant Physiol. 1979 Feb;63(2):336–340. doi: 10.1104/pp.63.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Decaleya R., Rappaport L. Synthesis of a possible precursor of alpha-amylase in wheat aleurone cells. Plant Physiol. 1979 Jan;63(1):195–200. doi: 10.1104/pp.63.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Persson H., Jörnvall H., Zabielski J. Multiple mRNA species for the precursor to an adenovirus-encoded glycoprotein: identification and structure of the signal sequence. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6349–6353. doi: 10.1073/pnas.77.11.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaiger H., Tanner W. Effects of gibberellic acid and of tunicamycin on glycosyl-transferase activities and on alpha-amylase secretion in barley. Eur J Biochem. 1979 Dec 17;102(2):375–381. doi: 10.1111/j.1432-1033.1979.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Speake B. K., Hemming F. W., White D. A. The effects of tunicamycin on protein glycosylation in mammalian and fungal systems. Biochem Soc Trans. 1980 Apr;8(2):166–168. doi: 10.1042/bst0080166. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Annu Rev Biochem. 1970;39:599–638. doi: 10.1146/annurev.bi.39.070170.003123. [DOI] [PubMed] [Google Scholar]
- Staneloni R. J., Tolmasky M. E., Petriella C., Ugalde R. A., Leloir L. F. Presence in a plant of a compound similar to the dolichyl diphosphate oligosaccharide of animal tissue. Biochem J. 1980 Oct 1;191(1):257–260. doi: 10.1042/bj1910257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer M. L., Rowland F. N., Murray L. W., Kaplan J. Inhibitory effects of tunicamycin on procollagen biosynthesis and secretion. Biochim Biophys Acta. 1977 Nov 7;500(1):187–196. doi: 10.1016/0304-4165(77)90058-7. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]