Abstract
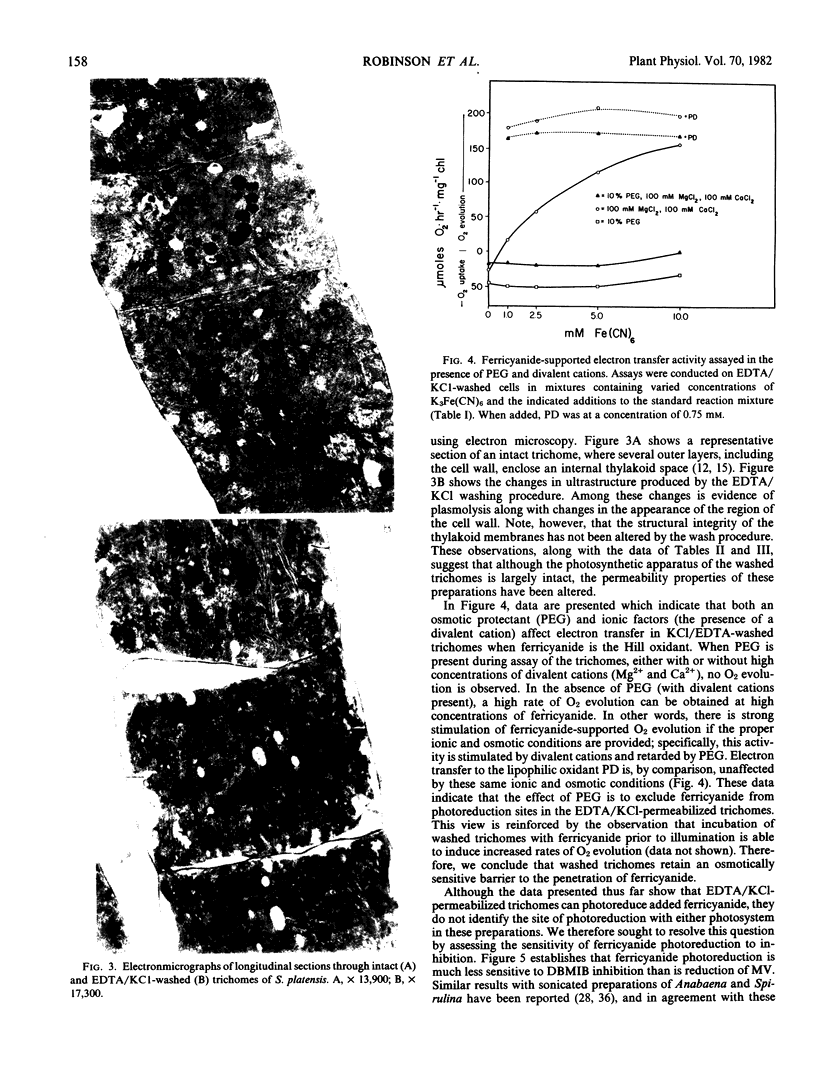
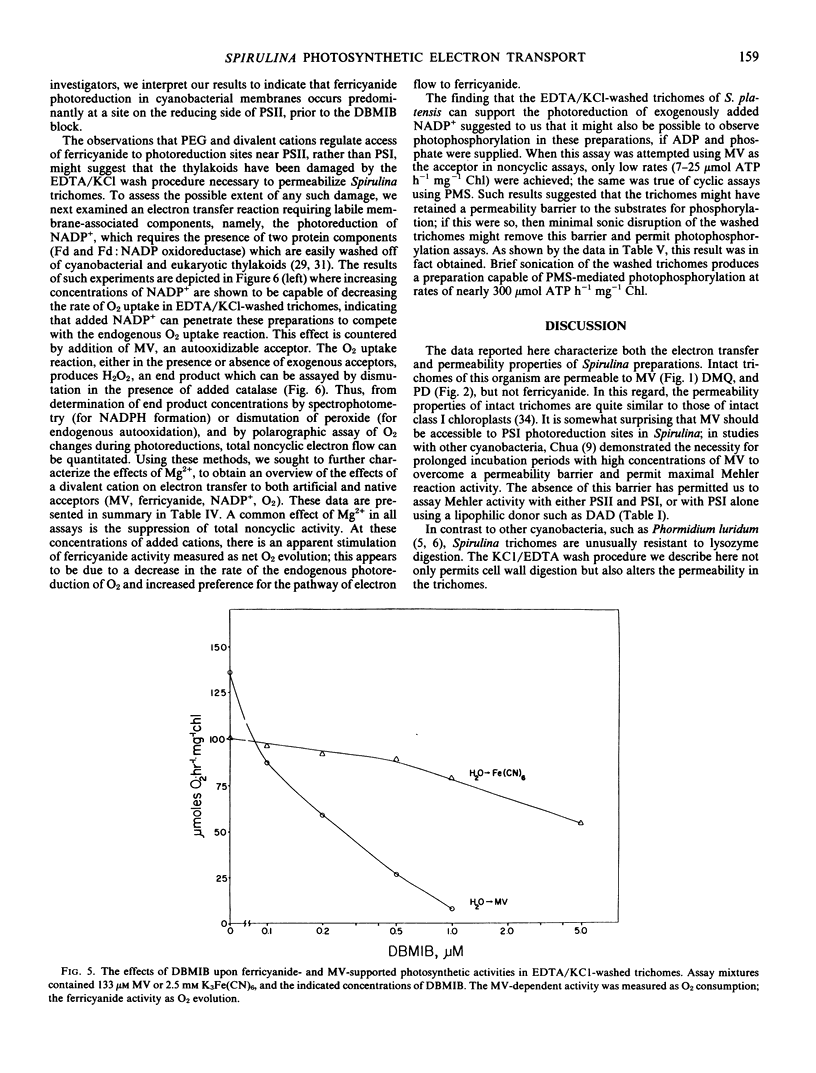
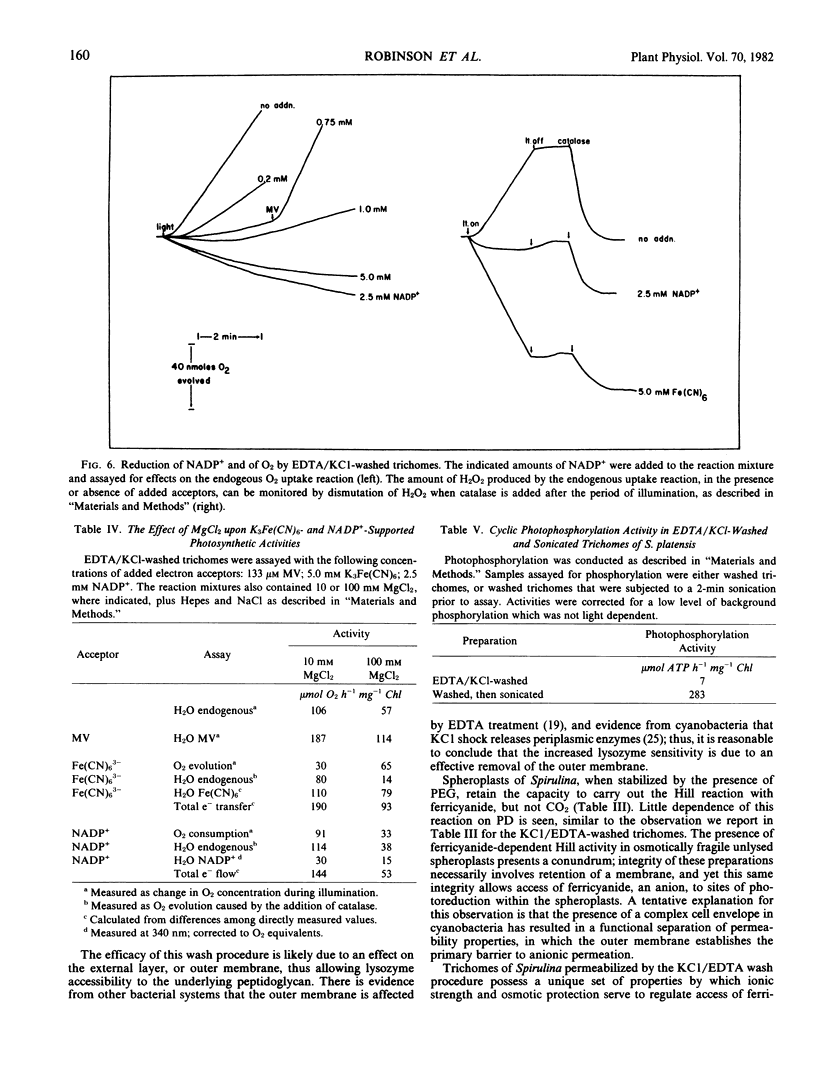
Electron transfer activity in intact trichomes of Spirulina platensis (Nordst.) Geitl. can be observed with either CO2 or methylviologen as the Hill acceptor. Ferricyanide cannot penetrate the intact trichomes, but photoreduction of this oxidant can be observed when mediated by lipophilic oxidants such as p-phenylenediamine or 2,5-dimethyl-p-benzoquinone. The insensitivity of these reactions to dibromothymoquinone indicates that they are due largely to the activity of photosystem II. Direct photoreduction of ferricyanide can be observed in spheroplasts of Spirulina, indicating that such preparations have altered permeability properties when compared with intact trichomes. Preparation of these spheroplasts, which are osmotically fragile, requires that intact trichomes be washed with KCl and EDTA to induce lysozyme sensitivity and thereby allow digestion of the cell wall. The KCl/EDTA washing procedure used for spheroplast preparation alters the permeability of Spirulina trichomes, as evidenced by the ability of these preparations to photoreduce ferricyanide. This photoreduction reaction is insensitive to dibromothymoquinone, and is stimulated by high concentrations of divalent cations. During assays, the reaction is inhibited by the inclusion of polyethyleneglycol as an osmotic protectant. Photoreduction of methylviologen and NADP+ is also observed in the washed trichomes, along with an endogenously catalyzed photoreduction of O2 to H2O2. Photophosphorylation cannot be observed in the washed preparations, but cyclic photophosphorylation with phenazinemethosulfate is observed after mild sonication. These results indicate that KCl/EDTA-washed trichomes of S. platensis retain the full range of energy transducing capacities associated with thylakoid membranes of the intact trichomes; the washing procedure facilitates spheroplast formation and alters, but does not abolish, permeability barriers in these preparations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., HARTMAN P. E., JACOB F. Chromosomal alterations affecting the regulation of histidine biosynthetic enzymes in Salmonella. J Mol Biol. 1963 Jul;7:23–42. doi: 10.1016/s0022-2836(63)80016-9. [DOI] [PubMed] [Google Scholar]
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Bartsch R. G. Amino acid sequence similarity between cytochrome f from a blue-green bacterium and algal chloroplasts. Nature. 1975 Jan 24;253(5489):285–288. doi: 10.1038/253285a0. [DOI] [PubMed] [Google Scholar]
- Biggins J. Photosynthetic Reactions by Lysed Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1447–1456. doi: 10.1104/pp.42.10.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins J. Preparation of Metabolically Active Protoplasts and Particle Preparations from the Blue-Green Alga, Phormidium luridum. Plant Physiol. 1967 Oct;42(10):1442–1446. doi: 10.1104/pp.42.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder A., Tel-or E., Avron M. Photosynthetic activities of membrane preparations of the blue-green alga Phormidium luridum. Eur J Biochem. 1976 Aug 1;67(1):187–196. doi: 10.1111/j.1432-1033.1976.tb10648.x. [DOI] [PubMed] [Google Scholar]
- Chua N. H. The methyl viologen-catalyzed Mehler reaction and catalase activity in blue-green algae and Chlamydomonas reinhardi. Biochim Biophys Acta. 1971 Sep 7;245(2):277–287. doi: 10.1016/0005-2728(71)90146-0. [DOI] [PubMed] [Google Scholar]
- DeRoo C. L., Yocum C. F. Cation-induced, inhibitor-resistant photosystem II reactions in cyanobacterial membranes. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1025–1031. doi: 10.1016/0006-291x(81)91926-4. [DOI] [PubMed] [Google Scholar]
- FREDRICKS W. W., JAGENDORF A. T. A SOLUBLE COMPONENT OF THE HILL REACTION IN ANACYSTIS NIDULANS. Arch Biochem Biophys. 1964 Jan;104:39–49. doi: 10.1016/s0003-9861(64)80032-1. [DOI] [PubMed] [Google Scholar]
- Golecki J. R. Studies on ultrastructure and composition of cell walls of the cyanobacterium Anacystis nidulans. Arch Microbiol. 1977 Jul 26;114(1):35–41. doi: 10.1007/BF00429627. [DOI] [PubMed] [Google Scholar]
- Gould J. M. The phosphorylation site associated with the oxidation of exogenous donors of electrons to photosystem I. Biochim Biophys Acta. 1975 Apr 14;387(1):135–148. doi: 10.1016/0005-2728(75)90058-4. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Rao K. K., Cammack R. A stable and easily extractable plant-type ferredoxin from the blue-green alga Spirulina maxima. Biochem Biophys Res Commun. 1972 May 26;47(4):798–802. doi: 10.1016/0006-291x(72)90562-1. [DOI] [PubMed] [Google Scholar]
- Honeycutt R. C., Krogmann D. W. A light-dependent oxygen-reducing system from Anabaena variabilis. Biochim Biophys Acta. 1970 Mar 3;197(2):267–275. doi: 10.1016/0005-2728(70)90037-x. [DOI] [PubMed] [Google Scholar]
- Izawa S., Gould J. M., Ort D. R., Felker P., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. 3. A dibromothymoquinone-insensitive phosphorylation reaction associated with photosystem II. Biochim Biophys Acta. 1973 Apr 27;305(1):119–128. doi: 10.1016/0005-2728(73)90237-5. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Ono T., Murata N. Photosynthetic electron transport and phosphorylation reactions in thylakoid membranes from the blue-green alga Anacystis nidulans. Biochim Biophys Acta. 1978 Jun 8;502(3):477–485. doi: 10.1016/0005-2728(78)90080-4. [DOI] [PubMed] [Google Scholar]
- Owers-Narhi L., Robinson S. J., DeRoo C. S., Yocum C. F. Reconstitution of cyanobacterial photophosphorylation by a latent Ca2+-ATPase. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1025–1031. doi: 10.1016/0006-291x(79)91929-6. [DOI] [PubMed] [Google Scholar]
- Patterson C. O., Myers J. Photosynthetic Production of Hydrogen Peroxide by Anacystis nidulans. Plant Physiol. 1973 Jan;51(1):104–109. doi: 10.1104/pp.51.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Ouitrakul R., Izawa S., Good N. E. Electron transport and photophosphorylation in chloroplasts as a function of the electron acceptor. J Biol Chem. 1971 May 25;246(10):3204–3209. [PubMed] [Google Scholar]
- Susor W. A., Krogmann D. W. Triphosphopyridine nucleotide photoreduction with cell-free preparations of Anabaena variabilis. Biochim Biophys Acta. 1966 May 12;120(1):65–72. doi: 10.1016/0926-6585(66)90277-9. [DOI] [PubMed] [Google Scholar]
- THOMAS J. B., DE ROVER W. On phycocyanin participation in the Hill reaction of the blue-green alga Synechococcus cedrorum. Biochim Biophys Acta. 1955 Mar;16(3):391–395. doi: 10.1016/0006-3002(55)90243-2. [DOI] [PubMed] [Google Scholar]
- Trebst A. Measurement of Hill reactions and photoreduction. Methods Enzymol. 1972;24:146–165. doi: 10.1016/0076-6879(72)24065-4. [DOI] [PubMed] [Google Scholar]
- Ward B., Myers J. Photosynthetic properties of permaplasts of anacystis. Plant Physiol. 1972 Nov;50(5):547–550. doi: 10.1104/pp.50.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise G., Drews G., Jann B., Jann K. Identification and analysis of a lipopolysaccharide in cell walls of the blue-green alga Anacystis nidulans. Arch Mikrobiol. 1970;71(1):89–98. doi: 10.1007/BF00412238. [DOI] [PubMed] [Google Scholar]
- Witholt B., Boekhout M., Brock M., Kingma J., Heerikhuizen H. V., Leij L. D. An efficient and reproducible procedure for the formation of spheroplasts from variously grown Escherichia coli. Anal Biochem. 1976 Jul;74(1):160–170. doi: 10.1016/0003-2697(76)90320-1. [DOI] [PubMed] [Google Scholar]