Nicotine is commonly used as a biomarker for tobacco smoke, and its concentration can be determined in various biologic specimens including blood [1], hair [2, 3], and tissues [4] including brain [5]. The presence of nicotine in these different sample types can reveal information about acute (plasma, brain) and chronic (hair) exposure to tobacco smoke and can help to reveal information about the effects and mechanisms of this exposure. Hair grows 1 cm per month and nicotine is deposited in hair proportional to blood nicotine levels so a 5 cm sample can provide average exposure levels over months [6, 7]. The most dependable method for nicotine analysis is mass spectrometry (MS) as it provides superior specificity over immunoassays [2]. While liquid chromatography-tandem triple quadrupole mass spectrometry (LC-MS/MS) methods have been validated that can assay nicotine concentrations in as little 1 mg of hair [3, 8], we have developed a method in which we can quantify nicotine in as little as 0.44 mg of hair. In addition, this method can be used to analyze small volumes of plasma (25 μL) and brain tissue (<200 mg) with just minor sample-specific alterations to the sample preparation procedure.
We pipetted 30 μL of standards or quality control (QC) samples and 25 μL of plasma samples into microtiter plates with 220 μL of water + 4 ng/mL of nicotine-d4 (internal standard; Cerilliant, Round Rock, TX, USA). The contents of each well were transferred to a 96-well supported liquid extraction (SLE) plate (Biotage, Uppsala, Sweden) and eluted with 2 × 500 μL of 95:5 dichloromethane (DCM):isopropanol (v:v) into a 96-well plate containing 100 μL of 300 mM methanolic hydrochloric acid (HCl) and dried in a Biotage TurboVap (15–20 linear feet/minute (lfm) nitrogen, 40°C). After reconstitution in 100 μL of 10:90 methanol:water, the contents of each well were filtered and transferred to a new 96-well microtiter plate. Minor additions to the above procedure were required for hair and brain samples. Hair samples (2–10 strands, up to 5 cm in length, target weight of 1–10 mg) and brain samples (0.291 ± 0.027 g) were digested overnight in 2 mL of 1M sodium hydroxide (NaOH) + 20 ng/tube nicotine-d4, and extracted with SLE as described above. Hair samples were washed twice by incubation in 4 mL of DCM for 2 hours at room temperature prior to digestion with NaOH to remove environmental nicotine contamination. While many nicotine analysis methods add the internal standard after digestion or homogenization [2, 3, 5], we added it after washing and prior to the digestion process in order to subject the internal standard to the same conditions as endogenous nicotine in the samples. In our opinion, this provides a more accurate assessment of matrix effects and nicotine recovery than adding the internal standard after sample digestion or homogenization. Calibration curves for hair and brain analysis were created by spiking unlabeled nicotine standard (Cerilliant) into 1 M NaOH in methanol and diluting serially to final concentrations between 0.012 and 100 ng/mL in a 10-point curve including a blank (0 standard). The spiked standards were then subjected to the SLE extraction procedure. Calibration curves for plasma analysis were created by spiking the same unlabeled nicotine standard into charcoal-stripped serum, and performing serial dilutions to final concentrations between 0.77 and 1500 ng/mL in a 10-point curve including a blank.
Nicotine was quantified using a Shimadzu Nexera-LCMS-8050 ultra-high performance LC-MS/MS instrument (Shimadzu Scientific, Kyoto, Japan). Separation was performed using reversed-phase chromatography on an Ace Excel 2 Super C18 50 mm × 2.1 mm column (Advanced Chromatography Technologies, Aberdeen, United Kingdom) with a 2 μm particle size at a flow rate of 0.4 mL/min. Mobile phase A was 26 mM ammonium acetate in water; mobile phase B was 0.006% formic acid in methanol. The formic acid addition to the methanol was required to ensure long-term stability of the chromatography while maintaining good retention with minimal tailing. The chromatography was run in a linear gradient from 10–74% B over 4 minutes, with an additional 2.3 minutes for column re-equilibration at 10% B. Multiple reaction monitoring (MRM) transitions for nicotine were: m/z 163.10 to 117.15 (quantifying ion); m/z 163.10 to 130.05 (reference ion); m/z 163.10 to 132.20 (reference ion); retention time, 1.76 min. MRM transitions for nicotine-d4 were: m/z 167.15 to 121.05 (quantifying ion); m/z 167.15 to 134.05 (reference ion); retention time, 1.75 min. The analytical column showed a loss of 0.03 min (~1.8 sec) in retention time over the life of the column (3100 injections) and inter-lot variation in retention time of up to 0.06 min (~3.6 sec). For this reason, MRMs were scheduled over 1.2 min. These minor differences in retention time are apparent in Figure 1. The total time for analysis was 6.3 min/sample. The interface temperature was 400°C, desolvation line temperature was 150°C, and the heat block temperature was 500°C. Gas was supplied by a Genius 1051 nitrogen and air generator (Peak Scientific, Inchinnan, United Kingdom). Nitrogen gas was used for nebulizing (3 L/min) and drying (10 L/min) gases, and air was used for heating (5 L/min) gas. Data were processed and analyzed using LabSolutions Software, V5.72 (Shimadzu). The lower limit of quantification (LLOQ) was 770 pg/mL (4.6 pg on the column) for plasma and 48 pg/mL for hair and brain samples (3.8 pg on the column). This resulted in adjusted LLOQs of 3.6 pg/mg nicotine in hair samples and 0.2 pg/mg nicotine in brain samples. The upper limit of quantification (ULOQ) for hair and brain samples was 100 ng/mL and for plasma was 1500 ng/mL. These limits were determined by the highest and lowest calibrators that were accurate within 80–120% [9]. All calibration curves were linear between the LLOQ and ULOQ (R2=0.9999).
Figure 1.
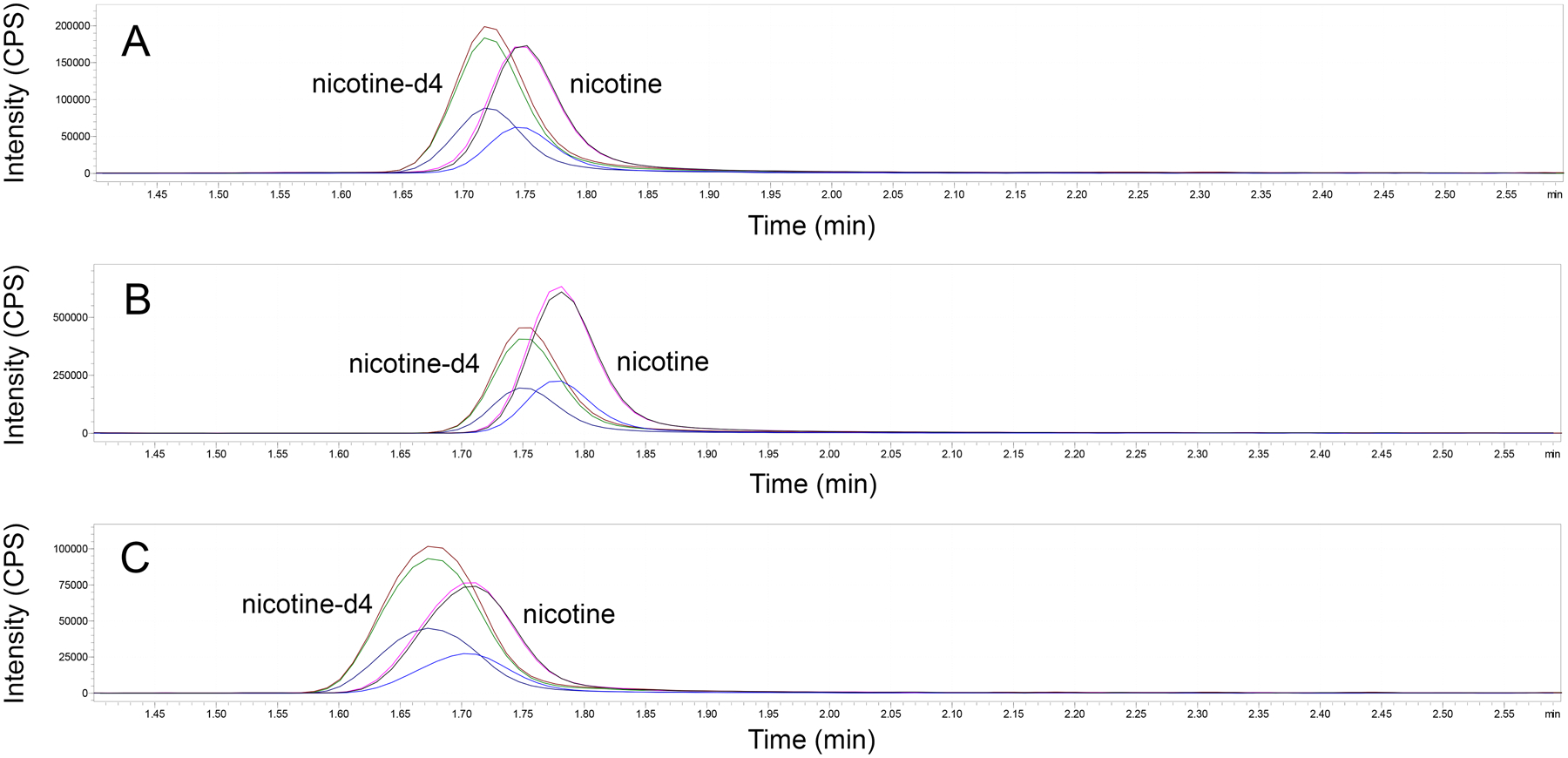
Representative chromatograms of nicotine and nicotine-d4 in mouse plasma (A; 80.94 ng/ml), human hair (B; 8.61 ng/ml), and mouse brain (C; 5.38 ng/ml) samples.
Precision and accuracy in the hair analysis were monitored using a pool of human hair obtained from nonsmokers and spiked with 20 ng of nicotine/tube prior to digestion. Precision as expressed by intra- and inter-assay CV were 2.4% and 4.8%, respectively; accuracy was 82.3% (n=8 assays, 4 replicates per assay). Normal human plasma spiked with unlabeled nicotine standard at three concentrations (2, 20, and 180 ng/mL) served as QCs. Intra-assay CVs were 8.0% at 2 ng/mL, 2.6% at 20 ng/mL, and 2.3% at 180 ng/mL; inter-assay CVs were 11.7% at 2 ng/mL, 6.6% at 20 ng/mL, and 9.1% at 180 ng/mL. Accuracies were 91.0%, 110.4%, and 89.8% for the 2, 20, and 180 ng/mL QC samples, respectively (n=5 assays, 4 replicates per assay). Precision and accuracy in the brain sample analysis were evaluated using a QC sample consisting of 5 ng/mL of unlabeled nicotine standard spiked into 1 M NaOH as sufficient brain tissue to serve as a matrix control was not available for this purpose. Intra-assay CV was 4.2%, inter-assay CV was 7.4%, and accuracy was 92.1% (n=7 assays, 4 replicates per assay).
Recoveries (extraction efficiencies) were evaluated by comparing pooled plasma or hair samples spiked with 0.5 ng of unlabeled nicotine standard before extraction and plasma or hair samples spiked with standard (0.5 ng) after extraction. Recovery in hair was 50%; recovery in plasma was 72%. Matrix effects were assessed by analysis of unlabeled nicotine standard (0.5 ng) spiked into plasma or hair samples after extraction compared to unlabeled nicotine standard (0.5 ng) spiked into 10:90 methanol:water without extraction. Matrix effects in hair were 100% and matrix effects in plasma were 90%. The nicotine spike amount of 0.5 ng was chosen as it approximated the concentration in the middle of the calibration curve. Matrix effects and recoveries were not directly determined for brain samples due to lack of available brain tissue for this purpose. However, we monitored internal standard recoveries during analysis of brain samples and observed internal standard recoveries were similar to those found in hair and plasma samples, indicating similar recovery and matrix effects.
We applied this method to analysis of mouse plasma and brain samples, and human hair samples taken from subjects ranging in age from newborn to adult. For hair assays from active smokers, determination could be made in 2–3 strands of 2 inches or less, and for second-hand exposures 5 strands of hair were generally sufficient. The actual range of hair weights was 0.44 – 13.34 mg. High sensitivity analysis is particularly important for determining nicotine levels in passively-exposed newborns or toddlers from whom only small hair samples are generally available, and where hair nicotine levels can be relatively low. The use of 96-well SLE extraction plates speeds throughput for clinical samples.
Although minor sample type-specific alterations to the sample preparation procedure were required for hair and brain samples, we successfully developed a method to extract and analyze nicotine from multiple sample types. To our knowledge, this is only the second report of nicotine analysis in tissue samples using SLE for sample preparation [10]. This method can be used for monitoring acute, chronic, direct, and environmental exposures to cigarette smoke. The high sensitivity of the method allows for small plasma, hair, and brain tissue sample sizes which can help reduce the invasiveness of sample acquisition. The SLE extraction method is compatible with multiple sample types, offers faster throughput, and is less labor-intensive than liquid-liquid extraction (LLE). SLE also provides the possibility that certain steps of the sample preparation procedure can be automated. The washing procedure also reduces incidence of environmental contamination of samples, such as from smokers contaminating their hair samples.
Acknowledgements:
This work was supported by NIH grants P51 OD011092, R01 HL105447, and UH3 OD023288.
References:
- 1.Xu AS, et al. , Determination of nicotine and cotinine in human plasma by liquid chromatography-tandem mass spectrometry with atmospheric-pressure chemical ionization interface. J Chromatogr B Biomed Appl, 1996. 682(2): p. 249–57. [DOI] [PubMed] [Google Scholar]
- 2.Chetiyanukornkul T, et al. , Hair analysis of nicotine and cotinine for evaluating tobacco smoke exposure by liquid chromatography-mass spectrometry. Biomed Chromatogr, 2004. 18(9): p. 655–61. [DOI] [PubMed] [Google Scholar]
- 3.Seong MW, et al. , Neonatal hair nicotine levels and fetal exposure to paternal smoking at home. Am J Epidemiol, 2008. 168(10): p. 1140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer L, et al. , Simultaneous quantification of tobacco alkaloids and major phase I metabolites by LC-MS/MS in human tissue. Int J Legal Med, 2015. 129(2): p. 279–87. [DOI] [PubMed] [Google Scholar]
- 5.Vieira-Brock PL, et al. , Simultaneous quantification of nicotine and metabolites in rat brain by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci, 2011. 879(30): p. 3465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Delaimy WK, Crane J, and Woodward A, Is the hair nicotine level a more accurate biomarker of environmental tobacco smoke exposure than urine cotinine? J Epidemiol Community Health, 2002. 56(1): p. 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uematsu T, et al. , The axial distribution of nicotine content along hair shaft as an indicator of changes in smoking behaviour: evaluation in a smoking-cessation programme with or without the aid of nicotine chewing gum. Br J Clin Pharmacol, 1995. 39(6): p. 665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryu HJ, et al. , Simultaneous and sensitive measurement of nicotine and cotinine in small amounts of human hair using liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom, 2006. 20(18): p. 2781–2. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration guidance for industry: Bioanalytical method validation. 2013.
- 10.Aoki Y, et al. , Evaluation of the distribution of nicotine intravenous injection: an adult autopsy case report with a review of literature. Int J Legal Med, 2020. 134(1): p. 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]


