Abstract
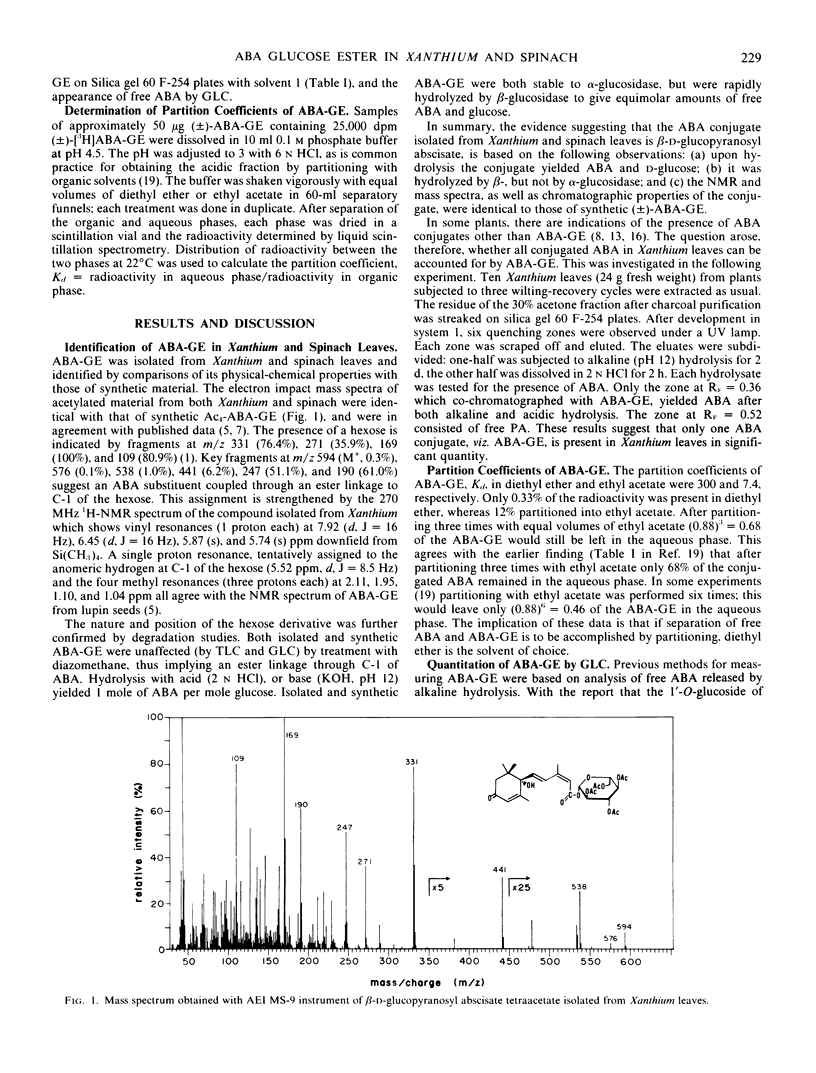
From previous work (Zeevaart 1980 Plant Physiol 66: 672-678) Xanthium leaves are known to contain a high level of alkali-hydrolyzable conjugated abscisic acid. This abscisic acid conjugate has been isolated and identified by mass spectrometry, nuclear magnetic resonance, and chemical and enzymic degradation techniques, as the glucosyl ester of abscisic acid, β-d-glucopyranosyl abscisate. The glucosyl ester of abscisic acid was the only abscisic acid conjugate found in Xanthium leaves. It was also isolated from spinach leaves.
An insignificant amount of the glucosyl ester of abscisic acid partitioned into diethyl ether, whereas 12% partitioned into ethyl acetate. Consequently, removal of abscisic acid by partitioning with ethyl acetate will result in considerable losses of the glucosyl ester of abscisic acid from the aqueous phase. Diethyl ether is, therefore, recommended for separation of abscisic acid and the glucosyl ester of abscisic acid by solvent partitioning.
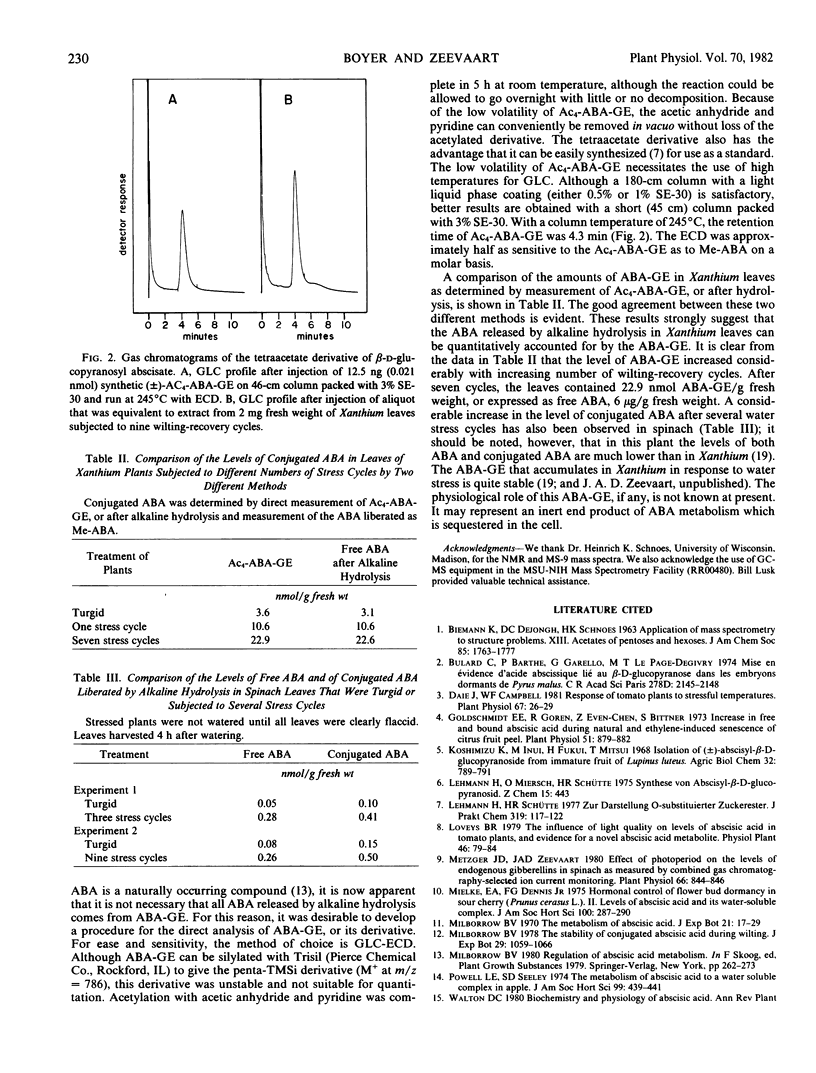
A method for quantitation of the glucosyl ester of abscisic acid as the tetraacetate derivative by gas-liquid chromatography with an electron capture detector was developed. The level of β-d-glycopyranosyl abscisate in Xanthium leaves increased from 3.6 nanomoles per gram fresh weight in turgid leaves to 22.9 nanomoles in leaves from plants subjected to seven wilting-recovery cycles. β-d-glycopyranosyl abscisate in Xanthium leaves may be a stable end product of abscisic acid metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daie J., Campbell W. F. Response of Tomato Plants to Stressful Temperatures : INCREASE IN ABSCISIC ACID CONCENTRATIONS. Plant Physiol. 1981 Jan;67(1):26–29. doi: 10.1104/pp.67.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt E. E., Goren R., Even-Chen Z., Bittner S. Increase in Free and Bound Abscisic Acid during Natural and Ethylene-induced Senescence of Citrus Fruit Peel. Plant Physiol. 1973 May;51(5):879–882. doi: 10.1104/pp.51.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger J. D., Zeevaart J. A. Effect of Photoperiod on the Levels of Endogenous Gibberellins in Spinach as Measured by Combined Gas Chromatography-selected Ion Current Monitoring. Plant Physiol. 1980 Nov;66(5):844–846. doi: 10.1104/pp.66.5.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Changes in the Levels of Abscisic Acid and Its Metabolites in Excised Leaf Blades of Xanthium strumarium during and after Water Stress. Plant Physiol. 1980 Oct;66(4):672–678. doi: 10.1104/pp.66.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeevaart J. A. Sites of Abscisic Acid Synthesis and Metabolism in Ricinus communis L. Plant Physiol. 1977 May;59(5):788–791. doi: 10.1104/pp.59.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]


