Abstract
A small-colony variant (Vsm) of the primary form (Vp) of Photorhabdus luminescens MD from in vitro and in vivo cultures is described. Unlike the primary form, Vp, the Vsm variant is not the preferred diet of its nematode symbiont, a Heterorhabditis sp., does not support development and reproduction of the nematode, and is less pathogenic than Vp to Galleria mellonella larvae. Vsm cells were carried by 25% of infective juveniles, but they comprised a very low percentage (∼0.4%) of the total cells carried by the juvenile. In vitro subculture and in vivo injection into the larvae with either Vp or Vsm always produced a mixture of both Vp and Vsm. In nematode-bacterium-infected G. mellonella larvae, the Vp population in the hemocoel was high (4 × 109 to 5 × 109 CFU/g of wet insect tissue) at 24 h after infection, decreased about 10-fold by 48 h, and then regained a high level at day 5 before decreasing at day 7 and then remaining relatively constant through day 15 postinfection. The Vsm population, under the same conditions as those of Vp, increased gradually to a high level (9 × 108 CFU/g of wet insect tissue) at day 5 postinfection and then declined gradually through day 15.
Infective juveniles (IJs) of the entomopathogenic nematode Heterorhabditis spp. carry symbiotic bacteria, Photorhabdus luminescens (Enterobacteriaceae), in their gut (22, 23), and after the IJs have penetrated an insect host, they release their bacteria into the insect hemocoel (21–23). The bacteria multiply rapidly, produce an environment in which it is suitable for the nematodes to develop, complete their life cycle (2, 19, 20), and, in combination with the nematode, kill the insect. This nematode-bacterium combination is marketed as an insect biocontrol agent and applied to the soil environment.
Strains of P. luminescens (previously known as Xenorhabdus luminescens) typically occur in two forms, designated the primary form and the secondary form (3, 6). The primary form is the one that is usually isolated from IJs and from the nematode-infected host insect, and it can be cultured in vitro. If not subcultured frequently (e.g., every 2 to 4 weeks), the primary form may convert to the secondary form under in vitro conditions. The primary form absorbs dye (e.g., bromothymol blue) from agar media, is highly luminescent, produces antimicrobial substances (2, 14, 18, 19) and pigments (18), and produces nutrients required to support the optimal growth of the nematode symbionts. The secondary form is deficient in most of these characteristics. Reversion from the secondary to the primary form has not been detected in plate cultures (11, 16) but has been reported to occur under certain conditions in liquid media (17). Despite significant differences in their morphology and biology, the primary and secondary forms are equally pathogenic to insects (1, 3).
Other polymorphisms in P. luminescens, named form variants or colony variants, have been found in an in vitro culture (11, 16, 25). These variants of P. luminescens show some differences and similarities to the primary or secondary form in cell and colony morphology, luminosity, dye absorption, pathogenicity, antibiotic production, and pigmentation (11, 16). In addition, the variants can revert from one to the other and to either the parental primary or the secondary form at a high frequency in plate cultures (11, 16). The significance of the colony variants is unknown; but, in addition to the secondary form, they have been assumed to have a survival advantage for these bacteria under changing environmental conditions (1, 3, 11, 16). The nature of the interaction between the variants and their nematode symbionts under different conditions and their occurrence in IJs and in infected insect larvae are not known. To build on the current knowledge base of these polymorphisms (11, 16), an investigation to characterize a small-colony variant (Vsm) of the primary form (Vp) of P. luminescens MD was performed under in vitro and in vivo conditions.
MATERIALS AND METHODS
Bacterium and nematode.
The nematode Heterorhabditis sp. strain HMD from which the symbiotic bacterium P. luminescens MD was isolated (originally supplied by Z. Mracek, Czech Academy of Sciences, Ceske Budejovice, Czech Republic) was used. The nematode was maintained in our laboratory by passage through last-instar Galleria mellonella larvae (supplied by the Insectary of the Department of Biological Sciences, Simon Fraser University). The IJs of the nematode were collected on a water trap within 4 days of their initial emergence from the infected insect cadaver and allowed to pass through two layers of wet-strength paper tissue to ensure that only active IJs were collected (13). The IJs were surface sterilized by immersion in 0.2% thimerosal for 2 h and washed four times with phosphate-buffered saline (PBS) (9). They were then homogenized, and the suspension was streaked onto tryptic soy agar (TSA; Difco) containing 25 mg of the dye bromothymol blue per liter (TSAD) and incubated at 25°C in the dark. After 48 h, typical, isolated primary-form Vp colonies were selected and clonally purified on TSAD plates. A stock culture of discrete, 48-h-old Vp colonies was prepared by suspending the colonies in a 12% sucrose solution, which was then freeze-dried and stored at −20°C. Bacterial cultures used in this study were subcultures of the stock culture, and these cultures were subcultured weekly on TSAD or TSA plates but not more than eight times before being discarded. The culture medium for all experiments was either TSA, TSAD, or tryptic soy broth (TSB; Difco), and the culture plates were incubated at 25°C unless otherwise stated. Sterilized polystyrene petri dishes (diameter, 9 cm) were used in all experiments, and all subcultures or transfers of the bacterium were done under standard sterile conditions.
Bacterial cells from distinct, isolated Vp and Vsm colonies from 48 h of culture on TSA plates were measured for cell size (20 of each). The luminosity of the 2- to 3-day-old bacterial cultures was checked by eye in the darkroom for up to 5 min. Catalase activity was tested by immersing the bacterial mass of 48-h-old Vp and Vsm cultures from TSA plates into 10% hydrogen peroxide and observing the release of oxygen. Antibiotic production was determined by observing the clear, inhibitory zone around the agar discs (diameter, 6 mm) on Bacillus subtilis plates after incubation (36°C for 24 h in the dark) (13). The agar discs were taken separately from 3- to 12-day-old Vp and Vsm cultures on TSAD plates.
Derivation of Vp and Vsm during in vitro culture.
To determine quantitatively the proportion of Vsm cells in a typical Vp colony and the proportion of Vp cells in a typical Vsm colony, a discrete 48-h-old Vp colony from a TSAD plate was subcultured onto a new TSAD plate. After 48 h, four discrete Vp colonies and four Vsm colonies were selected randomly, under a microscope, and suspended separately in a 0.8% NaCl solution. From each of these eight suspensions, an aliquot of about 200 to 300 cells was transferred separately and spread over the surface of TSAD plates. The plates were left open in the sterile, laminar-flow hood to allow surface water to dry. Then they were sealed and incubated for 48 h, and the numbers of Vp and Vsm cells in each respective suspension were determined by counting the numbers of Vp and Vsm colonies on each plate.
Growth of Vsm on different media.
Suspensions (∼200 cells) of a single, 48-h-old Vsm colony were plated on separate plates, each containing TSAD, TSAR (TSA plus neutral red and crystal violet at the ratio described for MacConkey agar [8]), or MacConkey agar. The numbers of Vp and Vsm colonies per plate and their morphologies were observed under a microscope (magnification, ×25) at 2 and 10 days after incubation.
Pathogenicity of Vp and Vsm to larval G. mellonella.
Suspensions of 40 cells of Vp and Vsm per μl were prepared separately by suspending in a 0.8% NaCl solution a single, 48-h-old colony of either Vp or Vsm from a TSAD plate. Last-instar larvae of G. mellonella of a similar weight (∼0.17 g each) were selected, and each larva was injected with 5 μl of either the Vp or Vsm suspension (∼200 cells/larva). Twenty-five larvae each were used for injection with Vp, Vsm, and the control (0.8% NaCl only). The injected larvae were incubated at 25°C in the dark, and their mortality and color change were recorded every 24 h after injection until 100% larval mortality was achieved by each variant. The relative proportion of Vp and Vsm cells inside the larvae was determined by homogenizing five randomly selected larval cadavers from each of the treatments 48 h after injection and plating the suspension on TSAD plates. The ratio of Vp to Vsm cells was determined 48 h after incubation of the plates. The experiment was repeated once as described above except that 30 larvae (weight, ∼0.2 g each) were used for each treatment.
Relative preference for Vp and Vsm by the nematode symbiont.
An experiment was done to determine whether the nematode had a preference for Vp or Vsm. Each of four single colonies of Vp and Vsm from a 48-h-old subculture on TSAD was inoculated separately onto new TSA plates. One colony each of Vp and Vsm was spread 1-cm apart on the surface of the same TSA plate. The four replicate plates were sealed and incubated for 48 h. Then, 50 μl of PBS containing about 1,400 IJs, sterilized as described above, were inoculated into the center of the plate in the middle of the central band of clear, uninoculated agar between the two bacterial lawns. The plates were left open in the sterile, laminar-flow hood to allow surface water to dry. As soon as the IJs began to move freely from the point of inoculation, the plates were sealed with Parafilm and incubated at 25°C in the dark. The numbers of IJs and female nematodes on each of the bacterial lawns were counted under a stereomicroscope at 1 h, 24 h, and 8 days after the plates were sealed. The experiment was repeated once as described above except that about 700 IJs were inoculated onto each plate.
Development and reproduction of the nematode symbiont on either Vp or Vsm.
An experiment was done to determine whether the nematodes were able to develop when either Vp or Vsm was available as the food source. Each of five discrete colonies of Vp and Vsm from a 48-h-old culture on TSAD was spread separately over the whole surface of 10 TSA plates (five plates of Vp and five plates of Vsm). The plates were sealed and incubated for 48 h at 25°C in the dark, and about 900 IJs in 50 μl of PBS, sterilized as described above, were inoculated onto the center of each plate. The plates were left open, as described above, to allow surplus water to dry and then sealed and incubated at 25°C in the dark. The number of nematodes and their developmental stage were determined for each plate at 2 and 6 days after inoculation.
Isolation of Vp and Vsm from IJs of the nematode symbiont.
Twenty active IJs, collected and surface sterilized as described above, were randomly selected, and each one was homogenized separately with a fine needle in a drop of TSB while being monitored under a stereomicroscope in a sterile laminar-flow hood. The suspension of each homogenized IJ was spread onto one of each of 20 TSAD plates. After incubation for 48 h, the numbers of Vp and Vsm colonies on each plate were counted under a stereomicroscope.
Growth of Vp and Vsm in nematode-bacterium-infected G. mellonella larvae: Experiment 1.
An experiment was done to determine whether Vp and Vsm coexist inside G. mellonella larvae following nematode infection and to monitor their relative population levels over time in the insect cadavers. Larval G. mellonella, all of a similar weight (∼0.22 g each), were each injected with 4 μl of PBS containing about 25 IJs that had been collected and sterilized as described above and then incubated at 25°C in the dark. At 0, 3, 6, 12, 24, 48, and 72 h after injection, and then on alternate days until day 15 after injection, five larvae were randomly collected, their body surfaces were washed three times with TSB for cleaning, and then the larvae were homogenized with 2 ml of TSB in a small mortar. The macerated material was transferred to a measuring bottle and adjusted to 10 ml with TSB. Standard dilution-plating methods were followed, and then the TSAD plates with bacterial cells were incubated. After 48 h of incubation, the numbers of Vp and Vsm bacterial colonies per plate were recorded. This process was repeated for samples collected at each sampling time. Five larvae injected with PBS alone served as a control.
Experiment 2.
To determine the time needed for the nematode symbiont to complete its life cycle inside the infected G. mellonella larvae and to correlate nematode development with the growth of Vp and Vsm in the experiment described above, three larvae were selected randomly every day from the infected larvae described above until the emergence of a new generation of IJs from the cadaver was observed. The collected insect cadavers were dissected under a stereomicroscope, and the nematode developmental stages were recorded. Both experiments 1 and 2 were repeated once.
RESULTS
General observations.
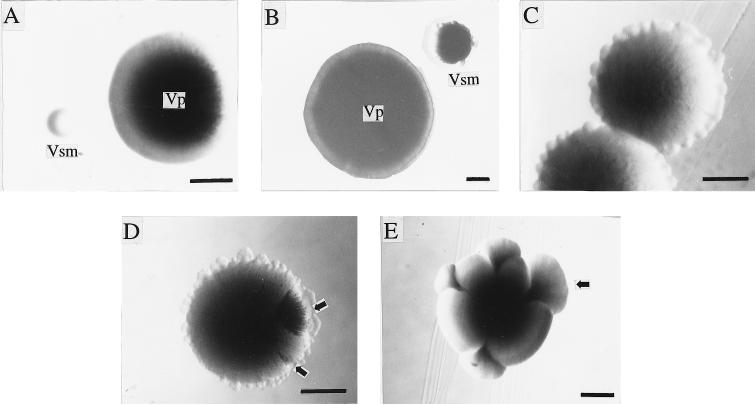
Both the primary form, Vp, and the colony variant, Vsm, were readily isolated from Vp or Vsm subcultures, with no exception. Some characteristics were held in common by Vp and Vsm, but others were not (Table 1). Although both Vp and Vsm colonies were initially smooth and round (Fig. 1A), they both became peripherally serrated in a crowded area on TSAD plates after about 7 days of incubation (Fig. 1C). The change of colony form was more obvious in Vsm colonies. They developed radial stripes and fan-like sectors that were similar in appearance to part of a Vp colony and that were common in aged Vsm colonies (Fig. 1D and E). Vsm colonies grew much more slowly than Vp colonies after the same period of incubation on TSAD plates (Fig. 1A and B).
TABLE 1.
Characteristics of Vp (primary form) and Vsm (small-colony variant) of P. luminescens MD
| Characteristic | Vp | Vsm |
|---|---|---|
| Gram stain | Negative | Negative |
| Mean cell size (μm) (range) | 5.0 by 1.3 (3.0 by 1.0 to 8.0 by 1.8) | 2.1 by 0.9 (1.5 by 0.8 to 3.0 by 1.0) |
| Proteinaceous granules | Yes | No |
| Colony color on TSA | Yellowish | Light grey |
| Colony size and form on TSAD | Large; dark-green center with radial stripes | Small; light green and homogeneous |
| Colony adhesion | Strong | None |
| Dye absorption | ||
| Bromothymol blue on TSA | Strong | Weak |
| Neutral red on TSA | Strong | Weak |
| Neutral red on MacConkey agar | Strong | Weak |
| Pigment diffusible on TSA | Brown | None |
| Catalase | Yes | Yes (weak) |
| Antibiotic production | Yes | No |
| Luminescence | Strong | Weak |
FIG. 1.
Colony morphology of the primary form (Vp) and small-colony variant (Vsm) of P. luminescens MD. (A) Colonies of 48-h-old Vp and Vsm; (B) colonies of aged Vp and Vsm; (C) colonies of aged Vsm with serrated edge in crowded area on TSAD plate; (D) colony of aged Vsm with serrated edge and stripes and fan-like sectors (arrows) in crowded area on TSAD plate; (E) colony of aged Vsm with fan-like sectors (arrow) in uncrowded area on TSAD plate. It is important to note that the aged colony was 17 days old. Bars, 0.5 mm.
The cell morphologies and physiologies of Vp and Vsm differed significantly. A 48-h-old Vp colony was composed mainly of large, rod-shaped cells often with one or two proteinaceous granules. A few long cells (3 to 5 times longer than average) and a few small, rod-shaped or nearly ovoid cells were also observed. The 48-h-old Vsm colony was composed mainly of small, rod-shaped cells which varied little in cell size, but a few large, rod-shaped cells, typical of Vp cells, as well as a few small or nearly ovoid cells occurred. Except for a few large, Vp-like cells, most cells from a 48-h-old Vsm colony did not contain proteinaceous granules (Table 1). Aged (17-day-old) Vp and Vsm colonies contained a mixture of cell types with the shapes described above, but all of the cells were smaller than those in 48-h-old colonies and the granules were evident within the cells of both Vp and Vsm colonies. The cells in the fan-like sectors of aged Vsm colonies (17 days old) looked similar to those in the aged Vp colony. When cells from either the fan-like sector or the non-fan-like sector of a Vsm colony were transferred separately to TSAD plates, about 95% formed Vp colonies and 5% formed Vsm colonies. The same proportions of colony types were observed for cells from aged Vp colonies (17 days old) when plated on TSAD plates. This indicated that although the aged Vsm colony comprised different sectors, such as stripes, fan-like sectors, and non-fan-like sectors, the cell compositions were the same in the different sectors, and most of them were virtually Vp cells. As well, the results indicated that prolonged incubation greatly enhanced the change of Vsm to Vp cells but not of Vp to Vsm cells. Despite the variety of cell shapes and sizes within the colonies of Vp and Vsm, no other colony types were observed in any of the subcultures examined.
Derivation of variants during in vitro culture.
In the 48-h-old Vp colonies, 2.6% of the cells were typical Vsm cells, and in the 48-h-old Vsm colonies, 1.3% of the cells were typical Vp cells under the prevailing experimental conditions.
Growth of Vsm on different solid media.
For Vsm colonies that grew on MacConkey agar, weak dye absorption was demonstrated, the growth rate of the Vsm cells was extremely low, and the size of each colony was tiny. Only a few, larger, red Vp colonies were observed in addition to many tiny Vsm colonies at 2 days after incubation on MacConkey agar. The total numbers of Vp and Vsm and Vp/Vsm ratios were the same on each of the 12 plates containing different media (4 plates for each medium) at 2 days, suggesting that there was no influence of medium selection on Vp and Vsm colony numbers. However, by 10 days, almost all Vsm colonies on MacConkey agar developed into red, Vp-like colonies whereas on the TSAD and TSAR plates the number of Vsm colonies and the few Vp colonies remained unchanged. Further observation revealed that Vp-like colonies growing on MacConkey agar developed from the original Vsm colonies, with the tiny Vsm colonies embedded at the center of the Vp-like colonies.
Pathogenicity of the two variants to larval G. mellonella.
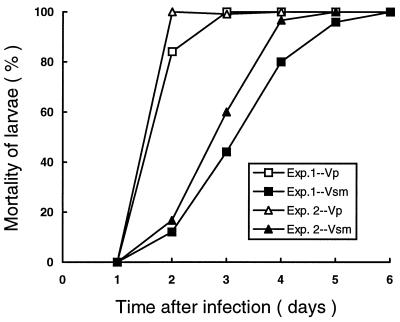
Vp and Vsm cells that had been injected into the larvae killed them at different rates (Fig. 2). After 48 h of infection, 84 to 100% of the G. mellonella larvae infected with Vp had died but only 12 to 16.7% infected with Vsm had died. Two to 3 and 5 to 6 days were needed to achieve 100% mortality with Vp and Vsm, respectively. No mortality was observed for the first 24 h of infection with either Vp or Vsm. Infected larvae also showed differences in color change. After 72 h, about 30 to 45% of the larvae that had been killed at 24 to 72 h after infection with Vsm remained the same color as that of the living controls, but after a further 24 h, the dead larvae turned the orange-brown color typical of those killed by Vp. A similar low rate of color change by some larvae that had been killed within the previous 24 h was shown at each sampling time of 4, 5, and 6 days postinfection with Vsm. The results of a repeated experiment were similar. No mortality or larval color change was observed among the control larvae of either experiment.
FIG. 2.
Percentage mortality of G. mellonella larvae infected with cells from either the primary form (Vp) or the small-colony variant (Vsm) of P. luminescens MD at 200 cells/larva (25°C, in the dark). Data are from two experiments.
Both Vp and Vsm were present in dead larvae 48 h after the larvae were infected by either Vp or Vsm, and Vp was the dominant form regardless of whether Vp or Vsm had been injected into the larvae. However, the percentage of Vsm cells was relatively high (∼33%) in the cadavers 48 h postinfection with Vsm in comparison with the percentage in those infected with Vp (∼20%). The larvae that died 3 to 4 days after infection with Vsm and had not changed color until day 4 contained both Vp and Vsm, but Vp cells predominated (93%) over Vsm cells (∼7%).
Relative preference for Vp and Vsm by their nematode symbiont.
In the first experiment concerned with this preference, about 60% of the IJs moved to the Vp lawn within 1 h of inoculation, and by 24 h this percentage had increased to 76%. By 8 days after inoculation, many dead and living female nematodes were observed in the Vp half of the plate (Table 2). Also, females on the Vp side were larger and more active, and they contained more eggs or IJs than did those on the Vsm side of the plates. The relative preference was confirmed in a repeat experiment (Table 2).
TABLE 2.
Preference of Heterorhabditis sp. strain HMD for bacterial lawns of the primary form (Vp) and small-colony variant (Vsm) of P. luminescens MDa
| Inoculum size (IJs/plate) and time after inoculation | Nematode stage | No. of nematodes/area of petri plate (mean ± SE) (n = 4)b
|
||
|---|---|---|---|---|
| Vp lawn | Middle area | Vsm lawn | ||
| 1,400 | ||||
| 1 h | IJs | 822.3 ± 32.0a | 20.25 ± 2.5c | 535.75 ± 48.0b |
| 24 h | Juveniles | 1,039.5 ± 74.2a | 3.5 ± 1.3c | 319.75 ± 24.0b |
| 8 days | Females | 522 ± 52.2a | 0.25 ± 0.3b | 39.0 ± 6.9b |
| 700 | ||||
| 1 h | IJs | 360.8 ± 46.3a | 5.75 ± 0.9b | 279.75 ± 26.4a |
| 24 h | Juveniles | 505.3 ± 59.9a | 6.75 ± 0.3b | 117.5 ± 16.5b |
| 8 days | Females | 122.25 ± 16.3a | 0.5 ± 0.5b | 39.75 ± 18.1b |
Preference was determined on TSA plates over time at two inoculation levels of the nematode.
Values in the same horizontal row followed by different letters are significantly different (P = 0.05, Student-Newman-Keuls test).
Development and reproduction of the nematode symbiont on either Vp or Vsm.
Nematodes did not develop and reproduce on a Vsm bacterial lawn. At 48 h after inoculation, no female nematodes could be found on the Vsm-inoculated plates, but about a hundred female nematodes were feeding actively on each of the Vp-inoculated plates. Three days after inoculation, 11 to 40 female nematodes developed on the Vsm plates, but most of them were found associated with a Vp-like lawn that had developed on the Vsm-inoculated plates. In contrast, hundreds of female nematodes developed on the Vp plates. By 4 days, no eggs had been laid by female nematodes on the Vsm plates but thousands of eggs had been laid over the surface of the Vp plates. Some juveniles hatched, and the female nematodes on the Vp plates were much larger than those on the Vsm plates. These differences between Vp and Vsm were even more pronounced at 6 days after inoculation, when there were 1,519 (n = 5) females on the Vp plates but only 103 females on the Vsm plates.
Isolation of colony variants from the IJs of the nematode symbiont.
Cells of both Vp and Vsm were present inside the IJs, but the number of Vsm cells was extremely low. Of a total of 20 IJs studied, 5 IJs (25%) carried Vsm cells, and the ratios of Vp to Vsm cells were 181:1, 250:1, 231:1, 240:1, and 228:1 based on colony numbers developed on the plates (∼0.4% of the total cells in these 5 IJs were Vsm).
In vivo growth of Vp and Vsm: experiment 1.
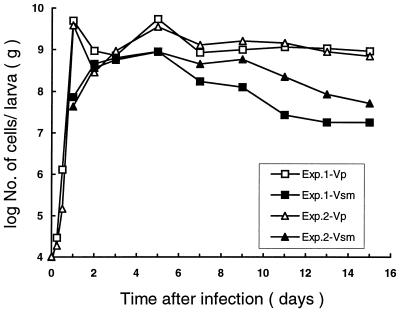
After 48 h of infection, all insect larvae except the controls died and turned orange-brown. Both Vp and Vsm cells were isolated from infected insect larvae, but they showed very different growth patterns from each other (Fig. 3). Within 12 h of infection, Vp cells and bacterial contaminants were readily isolated from the infected insect larvae. At 24 h, Vsm cells were also isolated from larvae but in smaller numbers than those of Vp cells. By 48 h, the numbers of Vp and Vsm cells were nearly equal. The number of Vsm cells increased dramatically from 4 × 107 to 7 × 107 CFU/g of wet insect tissue (WIT) at 24 h to 4 × 108 to 5 × 108 CFU/g of WIT at 2 days, but the numbers of Vp cells dropped sharply from 4 × 109 to 5 × 109 CFU/g of WIT to 3 × 108 to 9 × 108 CFU/g of WIT in the same period of time. The Vsm count increased gradually and reached a high level (9 × 108 CFU/g of WIT) at day 5, while the Vp count remained at a lower level from 2 to 3 days after infection before regaining a high population (4 × 109 to 5 × 109 CFU/g of WIT) at 5 days after infection. The population of Vsm in the larvae declined gradually after day 5, but the Vp population remained relatively constant through day 15 postinfection. Bacterial contaminants, probably from the alimentary system or body surface of the insect, were readily detected during the first 12 h, but their number decreased rapidly during the first 24 h after infection and few of them could be detected thereafter. No Vp or Vsm was detected in the control insect larvae, only the bacterial contaminants were. The results were confirmed by a repeat experiment.
FIG. 3.
Population of the primary form (Vp) and the small-colony variant (Vsm) of P. luminescens MD inside G. mellonella larvae over time. Data are from two experiments.
Experiment 2.
The symbiotic nematode completed its life cycle about 9 days after infection of G. mellonella larvae and produced new IJs. Population peaks of hermaphroditic female, amphimictic female, and new IJs occurred at 2, 5, and 9 days, respectively.
DISCUSSION
The occurrence of primary and secondary forms of P. luminescens is well documented (1, 3–7, 10). In the present study, Vp cells had all the characteristics of a primary form of Photorhabdus, but Vsm cells were without most of these primary-form characteristics. Vsm cells were less preferable and less suitable as a food source for their nematode symbiont and in this sense were similar to the secondary form (12). In addition, Vsm cells were less pathogenic to G. mellonella than were Vp cells. Vsm colonies had characteristically a larger proportion of small cells and grew more slowly than Vp cells on TSA, TSAD, and MacConkey agar. Vsm colonies were weakly luminescent in comparison with Vp colonies and absorbed dyes slowly, especially neutral red and bromothymol blue, the differential absorption of which characterizes the primary and secondary forms of Xenorhabdus and Photorhabdus (1, 3, 6). Although a secondary form was not detected in this study, small-colony variants similar to Vsm have been reported for strains of P. luminescens where the secondary form was present (16). Consequently, Vsm appears to be a variant of the primary form, Vp, rather than a distinct secondary form. It should be recognized that due to the inseparability of Vp and Vsm, some characteristics of Vp and Vsm in this study may be partially attributed to the presence of one cell type from a Vsm colony being in a Vp colony or vice versa.
The change of colony form of Vsm on MacConkey agar might explain the formation of observed, fan-like sectors of Vsm in this study and in earlier reports (11, 16). Each Vsm colony contained Vp cells, as shown in this study. Vp cells in a Vsm colony grew faster than Vsm cells on MacConkey agar, formed fan-like sectors around the edges of the tiny Vsm colonies, and, finally, completely surrounded or even covered the Vsm colonies. As a result, almost all Vsm colonies by day 10 of incubation became Vp-like, red colonies with visible or invisible parts of a Vsm colony embedded within them. Our findings for P. luminescens suggest that the fan-like sectors of aged Vsm colonies on TSAD observed in this study (Fig. 1C to E) and those observed by others (11, 16) might have been formed in a way similar to that just described. However, the sectors could not completely surround or cover the original Vsm colony as those did on MacConkey agar, probably because of the lower differential growth rates of the Vp and Vsm cells in the Vsm colony on TSAD, as influenced by limiting nutrients and/or space on the plate. This was further supported by the fact that fan-like sectors of aged Vsm colonies were much more evident and encompassed more of the edges of the Vsm colonies in an uncrowded area on the plate, where the space and nutrients were less limiting (Fig. 1E) and the sectors could finally surround the edges of the Vsm colonies.
Polymorphism appears to be a common property of Xenorhabdus spp. (11, 25) and Photorhabdus spp. at both colonial and cellular levels (11, 16). But, its significance is unknown, although there is speculation that the small-colony variants may have a survival advantage (11, 16). Such cells do not produce secondary metabolites, and so more energy could be diverted to cell division and growth (11, 24). This study has demonstrated that the Vsm variant is virtually inseparable from the primary form, Vp, in both agar culture and in insect cadavers, is less pathogenic to G. mellonella larvae than Vp, and does not produce some of the secondary metabolites, such as pigments and antibiotics, that Vp does (Table 1). This study shows for the first time that Vsm is carried by its nematode symbiont, is less preferred than Vp by the nematode symbiont, and does not support the development and reproduction of the nematode symbiont. Vsm and Vp coexist in infected insects and show quite different growth patterns under in vivo conditions. The question arises as to why the Vsm variant should occur so early in the development of the nematode and at such a high level in newly infected insect larvae when presumably the nutrient level is high. What is the possible role of Vsm in this natural, nematode-bacterium-insect association? Gerritsen (11) proposed that the nematode might prefer the primary form over the small-colony variant and so the presence of the small-colony variant might prevent the nematode from removing all the bacterial cells in the cadaver when feeding. The present study shows that the nematode does, in fact, prefer Vp over Vsm. However, this hypothesis for survival of the bacterial symbionts may not apply to all strains of P. luminescens because most strains are not known to have colony variants under in vivo conditions.
It is not clear whether the population dynamics of Vp and Vsm inside the insect were simply the result of differential growth of Vsm and Vp cells or whether it was the result of the change of cell type from one to the other. Vsm was undetectable, although it might have been present at very low levels compared with those of Vp, within 12 h of infection. Even at 24 h, significantly less than 1% of the total number of cells were Vsm. This is coincident with the fact that Vsm was proved to be carried at very low numbers by the IJs and grew slowly in vitro. The sharp decrease of the Vp cell level at 48 h of infection may perhaps be due to the increasing number of Vsm cells that were competing for nutrients or the effect of Vsm metabolites. The decreased number of the Vp cells at this stage of the infection might be beneficial to the nematodes because there were only a few hermaphroditic females at this stage and more food or food reserves could be used in the subsequent development and reproduction of large numbers of amphimictic females. In fact, the Vp cells regained high population levels within the insect cadaver when there were hundreds of amphimictic females and males 5 days after infection. This bacterial growth pattern contrasts with the pattern that has been observed (15) for the in vivo growth of strain C9 of P. luminescens, where the primary-form population of this strain increased gradually to a peak level at about day 9, at which time large numbers of amphimictic females had developed. In both cases, however, the high population levels of the primary-form bacteria appeared to be associated with the appearance of large numbers of amphimictic female nematodes. It would be interesting to know whether other strains of P. luminescens that have colony variants in in vitro cultures have similar growth patterns as strain MD and to compare them with strains which do not show any variant or secondary form under in vivo conditions.
ACKNOWLEDGMENTS
The study was supported by a research grant from the Natural Sciences and Engineering Research Council of Canada.
We thank Zdenek Mracek for supplying the nematode strain used in this study. Thanks are also due to Bruce Leighton, Jianxiong Li, and Victor Bourne for assistance.
REFERENCES
- 1.Akhurst R J. Morphological and functional dimorphism in Xenorhabdus spp., a bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. J Gen Microbiol. 1980;121:303–309. [Google Scholar]
- 2.Akhurst R J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982;128:3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- 3.Akhurst R J, Boemare N E. Biology and taxonomy of Xenorhabdus. In: Gaugler R, Kaya H K, editors. Entomopathogenic nematodes in biological control—1990. Boca Raton, Fla: CRC Press; 1990. pp. 75–90. [Google Scholar]
- 4.Akhurst R J, Smigielski A J, Mari J, Boemare N E, Mourant R G. Restriction analysis of phase variation in Xenorhabdus spp. (Enterobacteriaceae), entomopathogenic bacteria associated with nematodes. Syst Appl Microbiol. 1992;15:469–473. [Google Scholar]
- 5.Bleakley B, Nealson K H. Characterization of primary and secondary forms of Xenorhabdus luminescens strain HM. FEMS Microbiol Ecol. 1988;53:241–250. [Google Scholar]
- 6.Boemare N E, Akhurst R J. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriaceae) J Gen Microbiol. 1988;134:751–761. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- 7.Boemare N E, Akhurst R J, Mourant R G. DNA relatedness between Xenorhabdus spp. (Enterobacteriaceae), symbiotic bacteria of entomopathogenic nematodes, and a proposal to transfer Xenorhabdus luminescens to a new genus, Photorhabdus gen. nov. Int J Syst Bacterial. 1993;43:249–255. [Google Scholar]
- 8.Difco Laboratories. Difco manual. 10th ed. Detroit, Mich: Difco Laboratories; 1984. [Google Scholar]
- 9.Dunphy G B, Webster J M. Influence of the Mexican strain of Steinernema feltiae and its associated bacterium Xenorhabdus nematophilus on Galleria mellonella. J Parasitol. 1986;72:130–135. [Google Scholar]
- 10.Forst S, Nealson K H. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol Rev. 1996;60:21–43. doi: 10.1128/mr.60.1.21-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerritsen L J M, de Raay G, Smits P H. Characterization of form variants of Xenorhabdus luminescens. Appl Environ Microbiol. 1992;58:1975–1979. doi: 10.1128/aem.58.6.1975-1979.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerritsen L J M, Smits P. The influence of Photorhabdus luminescens strains and form variants on the reproduction and bacterial retention of Heterorhabditis megidis. Fundam Appl Nematol. 1997;20:317–322. [Google Scholar]
- 13.Hickey K D. Methods for evaluating pesticides for control of plant pathogens. St. Paul, Minn: The American Phytopathological Society; 1986. [Google Scholar]
- 14.Hu K, Li J, Webster J M. Quantitative analysis of a bacteria-derived antibiotic in nematode-infected insects using HPLC-UV and TLC-UV methods. J Chromatogr B. 1997;703:177–183. doi: 10.1016/s0378-4347(97)00398-8. [DOI] [PubMed] [Google Scholar]
- 15.Hu, K. Unpublished data.
- 16.Hurlbert R E, Xu J, Small C L. Colonial and cellular polymorphism in Xenorhabdus luminescens. Appl Environ Microbiol. 1989;55:1136–1143. doi: 10.1128/aem.55.5.1136-1143.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasomil-Osterfeld K C. Influence of osmolarity on phase shift in Photorhabdus luminescens. Appl Environ Microbiol. 1995;61:3748–3749. doi: 10.1128/aem.61.10.3748-3749.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li J, Chen G, Wu H, Webster J M. Identification of two pigments and a hydroxystilbene antibiotic from Photorhabdus luminescens. Appl Environ Microbiol. 1995;61:4329–4333. doi: 10.1128/aem.61.12.4329-4333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Chen G, Webster J M, Czyzewska E. Antimicrobial metabolites from a bacterial symbiont. J Nat Prod. 1995;58:1081–1086. doi: 10.1021/np50121a016. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Berry R, Poinar G O, Moldenke A. Phylogeny of Photorhabdus and Xenorhabdus species and strains as determined by comparison of partial 16S rRNA gene sequences. Int J Syst Bacteriol. 1997;47:948–951. doi: 10.1099/00207713-47-4-948. [DOI] [PubMed] [Google Scholar]
- 21.Poinar G O, Jr, Thomas G M. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136. (Neoaplectana sp. Steinernematidae) Parasitology. 1966;56:385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- 22.Poinar G O., Jr Description and biology of a new insect parasite rhabditol, Heterorhabditis bacteriophora n. gen., n. sp. (Rhabditida: Heterorhabditidae n. fam.) Nematologica. 1975;21:463–470. [Google Scholar]
- 23.Poinar G O, Jr, Thomas G, Haygood M, Nealson K H. Growth and luminescence of the symbiotic bacteria associated with terrestrial nematodes, Heterorhabditis bacteriophora. Soil Biol Biochem. 1980;12:5–10. [Google Scholar]
- 24.Smigielski A J, Akhurst R J, Boemare N E. Phase variation in Xenorhabdus nematophilus and Photorhabdus luminescens: difference in respiratory activity and membrane energization. Appl Environ Microbiol. 1994;60:120–125. doi: 10.1128/aem.60.1.120-125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wouts W M. The primary form of Xenorhabdus species (Enterobacteriaceae: Eubacteriales) may consist of more than one bacterial species. Nematologica. 1990;36:313–318. [Google Scholar]