Abstract
The control of night-break timing was studied in dark-grown seedlings of Pharbitis nil (Choisy cv. Violet) following a single continuous or skeleton photoperiod. There was a rhythmic response to a red (R) interruption of an inductive dark period, and the phasing of the rhythm was influenced by the preceding light treatment.
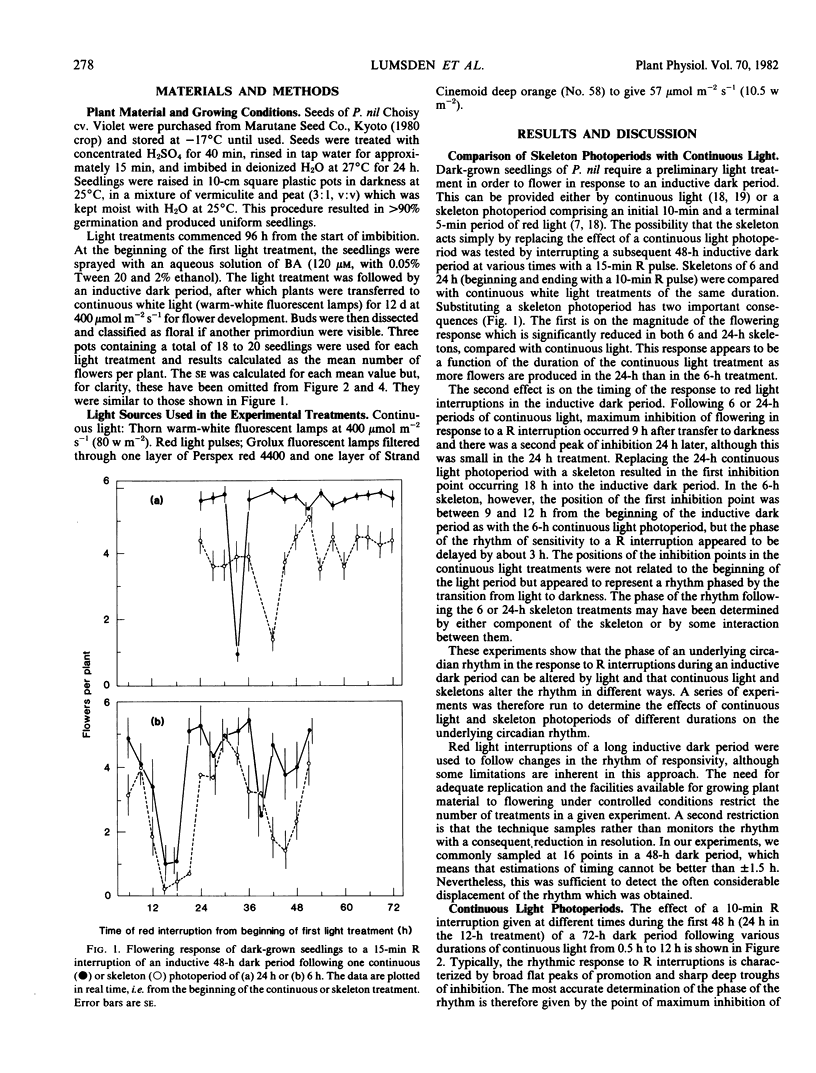
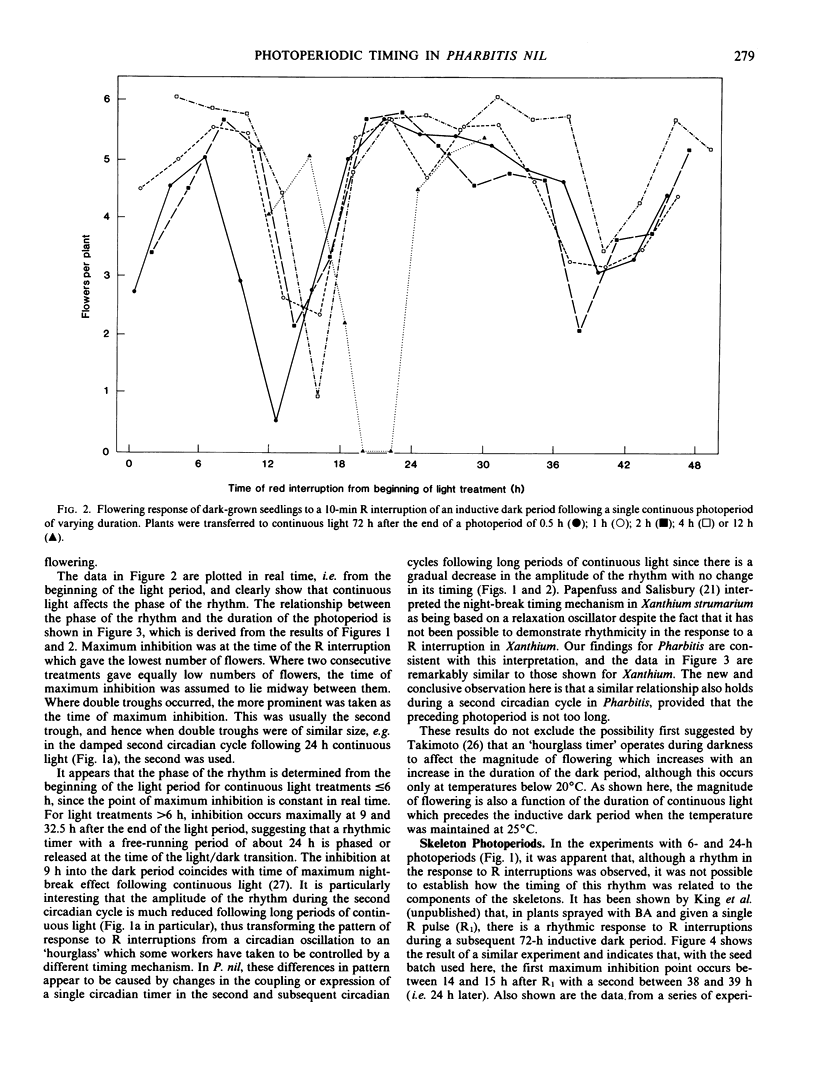
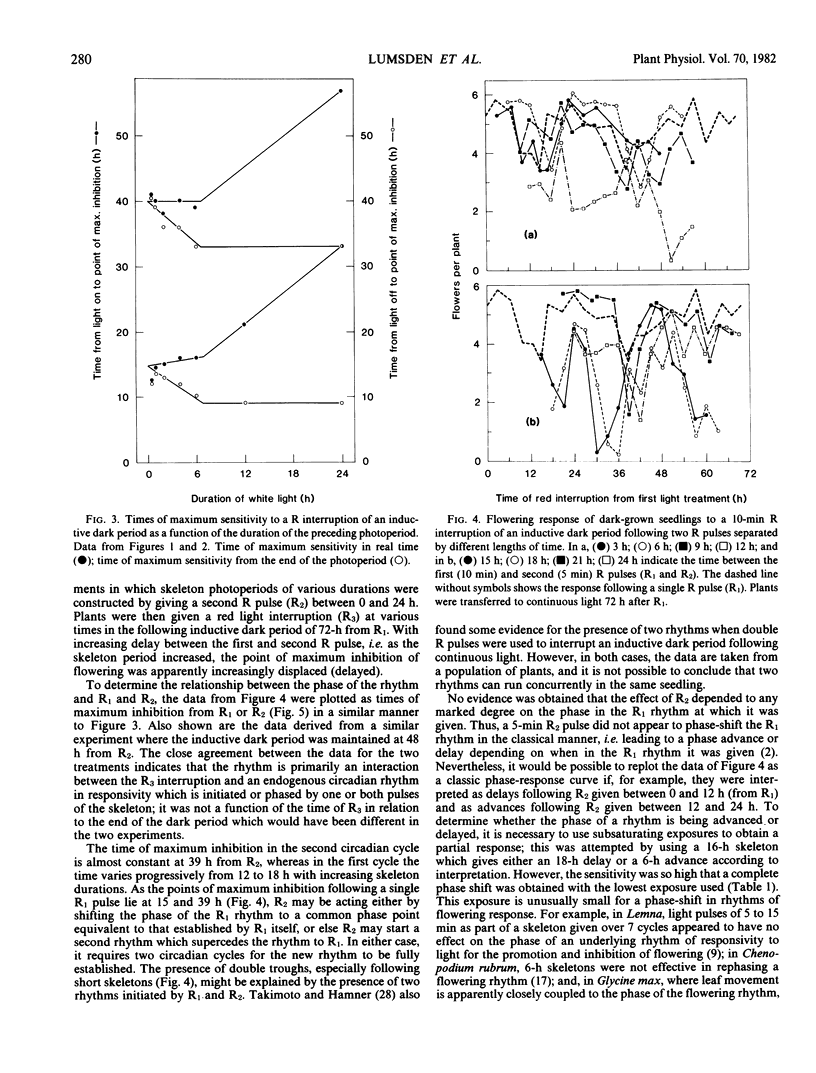
Following a continuous white light photoperiod of 6 hours or less, the points of maximum inhibition of flowering were constant in real time. Following a continuous photoperiod of more than 6 hours, maximum inhibition occurred at 9 and 32.5 hours after the end of the light period. The amplitude of the rhythm during the second circadian cycle was much reduced following prolonged photoperiods.
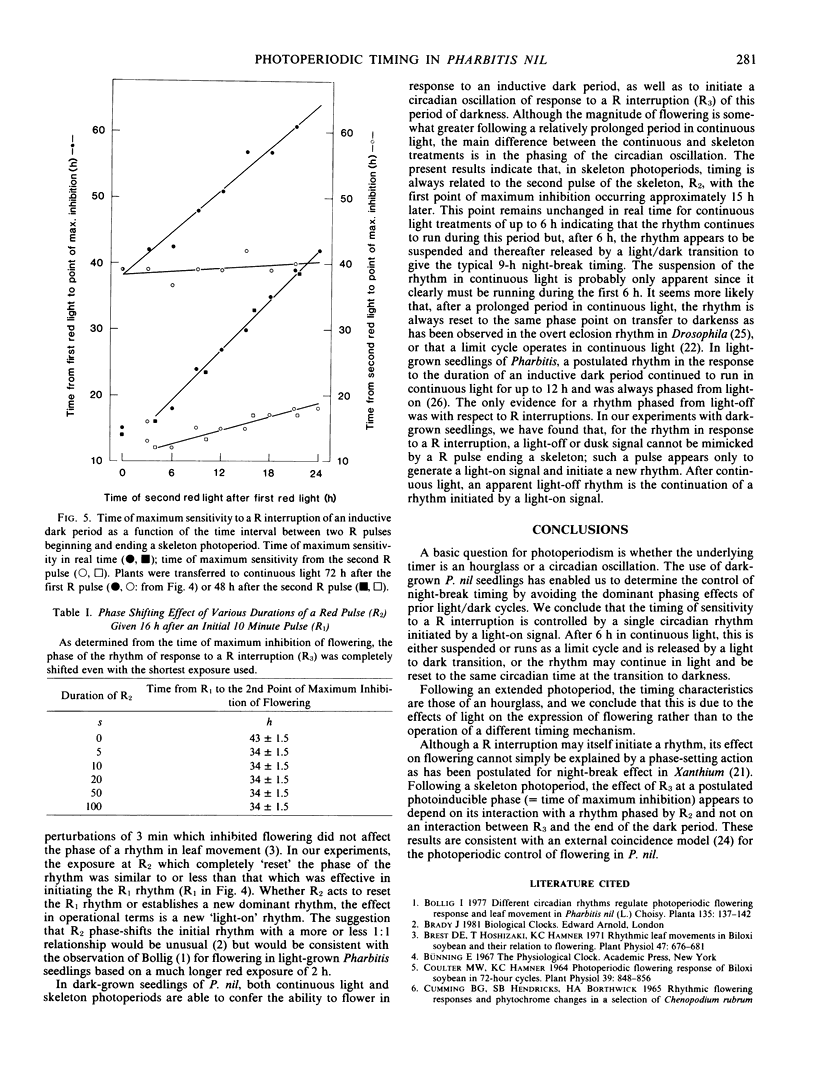
Following a skeleton photoperiod, the time of maximum sensitivity to a R interruption was always related to the second pulse of the skeleton, R2, with the first point of maximum inhibition of flowering occurring after 12 to 18 hours and the second after 39 hours. Without a second R pulse, the time of maximum sensitivity to a R interruption was related to the initial R1 pulse. A `light-off' or dusk signal was not mimicked by a R pulse ending a skeleton photoperiod; such a pulse only generated a `light-on' signal and initiated a new rhythm.
It is concluded that the timing of sensitivity to a R interruption of an inductive dark period in Pharbitis nil is controlled by a single circadian rhythm initiated by a light-on signal. After 6 hours in continuous white light, the phase of this rhythm is determined by the transition to darkness. Following an extended photoperiod, the timing characteristics were those of an hourglass; this seemed to be due to an effect on the coupling or expression of a single circadian timer during the second and subsequent cycles, rather than to the operation of a different timing mechanism.
In addition to the effects on timing, the photoperiod affected the magnitude of the flowering response.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brest D. E., Hoshizaki T., Hamner K. C. Rhythmic leaf movements in biloxi soybean and their relation to flowering. Plant Physiol. 1971 May;47(5):676–681. doi: 10.1104/pp.47.5.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter M. W., Hamner K. C. Photoperiodic Flowering Response of Biloxi Soybean in 72-Hour Cycles. Plant Physiol. 1964 Sep;39(5):848–856. doi: 10.1104/pp.39.5.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman W. S. A metabolic indicator of photoperiodic timing. Proc Natl Acad Sci U S A. 1976 Feb;73(2):501–504. doi: 10.1073/pnas.73.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman W. S. Calibrating duckweeds: light, clocks, metabolism, flowering. Science. 1976 Aug 6;193(4252):453–458. doi: 10.1126/science.193.4252.453. [DOI] [PubMed] [Google Scholar]
- Hillman W. S. Effects of inorganic nitrogen on the response of Lemna carbon dioxide output to light quality and timing. Photochem Photobiol. 1975 Jan;21(1):30–47. doi: 10.1111/j.1751-1097.1975.tb06627.x. [DOI] [PubMed] [Google Scholar]
- Hsu J. C., Hamner K. C. Studies of the Involvement of an Endogenous Rhythm in the Photoperiodic Response of Hyoscyamus niger. Plant Physiol. 1967 May;42(5):725–730. doi: 10.1104/pp.42.5.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfuss H. D., Salisbury F. B. Aspects of clock resetting in flowering of xanthium. Plant Physiol. 1967 Nov;42(11):1562–1568. doi: 10.1104/pp.42.11.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C. S. Circadian surfaces and the diversity of possible roles of circadian organization in photoperiodic induction. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2734–2737. doi: 10.1073/pnas.69.9.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto A., Hamner K. C. Effect of Double Red Light Interruptions on the Photoperiodic Response of Pharbitis nil. Plant Physiol. 1965 Sep;40(5):855–858. doi: 10.1104/pp.40.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]


