Abstract
Periodontitis has significant public health implications, affecting individuals’ overall health, well-being, and quality of life. This study aimed to assess the risk factors associated with the extent of clinical attachment loss (CAL) in a population diagnosed with periodontitis. Six hundred and sixty-seven patients with different degrees of CAL (mild, n = 223; moderate, n = 256; and advanced, n = 188) were enrolled. Socio-demographics, lifestyle, microbiological profiles, specific immune response, obesity, and single-nucleotide polymorphism of the IL1 gene were determined. Unconditional logistic regression models were conducted to determine the factors associated with the extent of CAL. Aging, smoking, microbial factors, plaque index, and IgG2 antibodies against Aggregatibacter actinomycetemcomitans were associated with advanced CAL. IgG2 antibodies against A. actinomycetemcomitans (OR 1.50; CI 95% 1.23–1.81), plaque accumulation (OR 2.69; CI 95% 2.20–3.29), Porphyromonas gingivalis (OR 1.93; CI 95% 1.35–2.76), Tanerella forsythia (OR 1.88; CI 95%1.30–2.70), and current smoking (OR 1.94; CI 95% 1.31–2.87) were associated with advanced CAL. Gene IL polymorphisms, obesity, and stress were not associated with the extent of CAL. Aging, plaque accumulation, smoking, and having antibodies against A. actinomycetemcomitans were the most critical factors associated with advanced CAL. In contrast, obesity, stress, and gene polymorphisms were not associated with the extent of CAL.
Keywords: periodontitis, IgG antibodies, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitan, smoking, plaque index
1. Introduction
Periodontitis is a chronic inflammatory oral condition characterized by the dysbiotic alteration of the oral microbiota, resulting in an imbalance in the composition of the microbial community within the periodontal biofilm. Dysbiosis in periodontitis involves a shift from a commensal microbiota to a pathogenic one, typically marked by an overabundance of periodontal pathogens. This microbial dysbiosis triggers a host immune response, leading to sustained inflammation and the progressive destruction of the periodontal tissues, ultimately resulting in tooth-supporting structure loss and potential tooth mobility [1,2]. Periodontitis is one of the most frequent causes of tooth loss and affects large populations worldwide. This condition can have significant public health implications due to its prevalence and potential impact on overall health. By addressing risk factors and promoting preventive measures, public health initiatives can help reduce the prevalence and impact of periodontitis, leading to improved oral health and overall well-being for the population [1]. Evidence has shown an increase in the prevalence and severity of periodontitis [2]; however, the results are influenced by the level of socio-economic development [2,3,4]. Both the severity and extent of periodontal disease increase with age [5] and are influenced by tobacco smoking, specific subgingival microorganisms, and uncontrolled diabetes [6,7,8]. However, local factors such as calculus and dental plaque are also recognized in periodontitis progression [9]. Genetic aspects, in particular, the increase in the allelic frequency of single-nucleotide polymorphisms (SNPs) for IL-1 beta (IL-1b) (+3954), SNP IL alpha −889 (IL-1a), and a positive genotype of IL gene increased risk of developing periodontitis within the European Caucasian [9,10]. However, it is still controversial in Latin American populations [11,12,13]. Likewise, an association between periodontitis and putative risk factors such as high levels of stress [13,14,15] and obesity [16,17,18] has been reported.
Clinical attachment loss (CAL) is the best marker of periodontal breakdown, and this has been used to study the risk indicators for the severity of periodontal disease among the adult population [19,20]. However, a few studies evaluate the association of classic and putative factors with the extent of CAL in multivariate analyses. The hypothesis of this study posits that advanced CAL is correlated with certain established and potential risk factors.
The aim of this study was to assess the correlation between geographic, genetic, microbiological, and immunological factors, lifestyle, and obesity, and their influence on the extent of clinical attachment loss within a population through multifactorial modeling.
2. Materials and Methods
2.1. Population
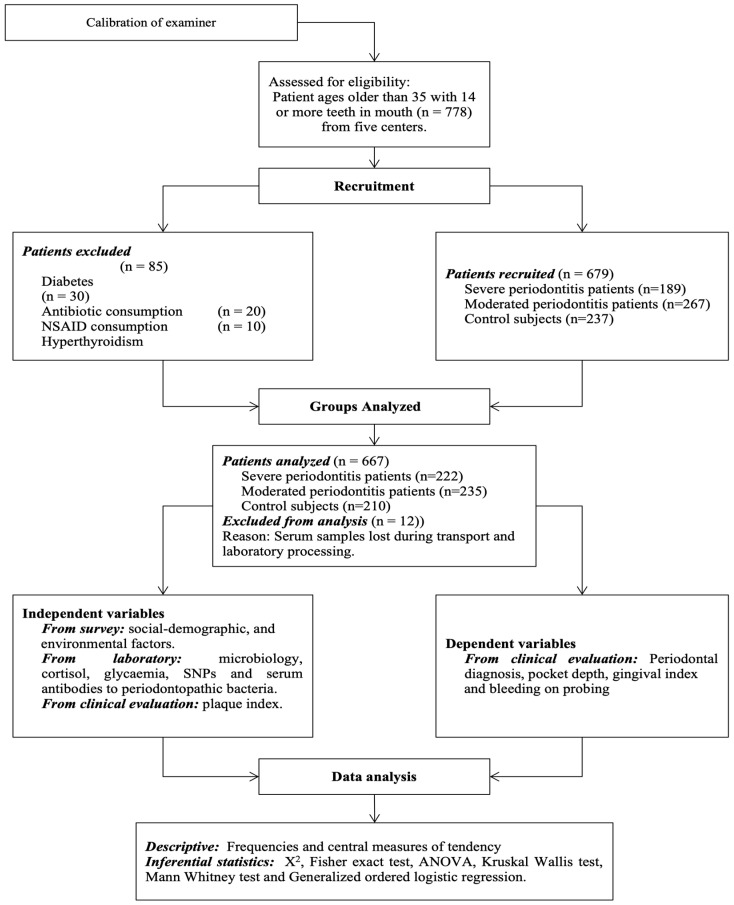
Six hundred and sixty-seven untreated adult patients over 35 years old with at least 14 teeth were studied. The patients belonged to 3 cities in Colombia: Bogotá, Medellin, and Cali, and they were attending advanced periodontal programs at 5 universities. The Institutional Review Board of each participating university approved this study, and the patients signed their informed consent. Previous periodontal treatment, antibiotic intake, immunosuppressive medication, non-steroidal anti-inflammatory drugs (NSAIDs), diagnosed diabetes, and autoimmune diseases were exclusion criteria (Figure 1).
Figure 1.
Flowchart of study protocol.
2.2. Clinical Evaluation
A complete periodontal examination was performed by using the CP15 probe to measure clinical attachment loss (CAL). The included 667 individuals were categorized into three groups based on the mean of CAL of the whole dentition. (1) Mild CAL included individuals with ≤2 mm CAL on average; (2) moderate CAL included those having >2/<4 mm; and (3) advanced CAL included those having ≥4 mm [20]. The exam was carried out by three calibrated periodontists with an Intra-examiner (IE) Intra-Class Correlation Coefficient (IE- ICC) of 0.90–0.98 in each city. Other data obtained during the clinical examination were bleeding on probing (BOP) (IE-kappa index 0.75–0.95), plaque index (PI) (IE-kappa index 0.85–0.96), gingival index (GI) (IE-kappa index 0.75–0.90), and pocket depth (IE-kappa index 0.95–0.98). A middle plaque index refers to a score of 2, while a high plaque index refers to a score of 3. These scores indicate a substantial accumulation of plaque on the teeth [21].
2.3. Sample Size
The sample size was calculated with a power of 80%, and an error α of 5% for detecting an odds ratio (OR) ≥ 2 for risk indicators with a prevalence ≥ 15% in the control population (mild CAL). A minimum of 200 patients were calculated in each group. However, although 223 patients with mild CAL and 256 with moderate CAL were included, only 188 with advanced CAL could be enrolled.
2.4. Socio-Demographic Features and Lifestyle
A questionnaire was used for establishing the socio-demographics and habits including current smoking, smoking history, and alcohol intake. In addition, a blood sample was taken from every patient for carrying out a test of serum cotinine, according to the manufacturer’s guidelines to verify the tobacco habit in which a level ≥ 10 ng/mL was taken as indicative of current smoking, and serum cortisol for determining the levels of stress. Finally, the body mass index (BMI), the complete blood count (CBC), and levels of post-prandial glycemia were also assessed.
2.5. Single-Nucleotide Polymorphism (SNP) Analysis of IL 1 Gene by Polymerase Chain Reaction (PCR)
To determine the polymorphism of SNPs from interleukin IL-1a (-889) and interleukin IL-1b (+3954), cells were collected from the oral mucosa by mouthwash and swab sampling. To obtain DNA, the epithelial cells were subjected to extraction with the Chelex-100 resin [22]. After the sample was cooled down and the sediment allowed to settle, 40 µL supernatant was reconstituted in 10 µL of buffer Tris- Borato EDTA 5X (TBE). Samples were frozen at −20 °C until the process. Analysis of the SNP of IL-1b and IL-1a used the primers reported by Kornman et al. [23]. The reaction was amplified by initial denaturation at 94 °C for 3 min followed by 30 cycles with a temperature of 94 °C, 53 °C, and 72 °C for 1 min to IL-1b, and 94 °C, 53 °C, and 72 °C for 45 sec to IL-1a. Following amplification, the digestion of the amplification products was carried out with 4U of Taq I for 2 h at 65 °C for IL-1b and with 5U of NcoI for 16 h at 37 °C for IL-1a††. The fragments of enzymatic restriction were displayed in agarose gels after staining with ethidium bromide.
The following electrophoretic profile set the genotypic assay: for IL-1b, fragments of 97, 85, and 12 pb (genotype CC, absence of polymorphism), fragments of 12 and 182 pb (genotype TT, presence of polymorphism), fragments of 182, 97, 85, and 12 pb (genotype CT, presence of polymorphism on one of its alleles); for IL-1a, fragments of 83 + 16 pb (genotype CC absence of polymorphism), fragments of 99 pb (genotype TT, presence of polymorphism), fragments of 99, 83, and 16 pb (genotype CT, presence of polymorphism on one of its alleles). Analysis of Hardy–Weinberg balance on genotypic frequencies was performed by Chi-square (X2) test.
2.6. Microbiological Aspects
Supragingival plaque was eliminated using curettes, and subsequently, sterile paper points were inserted for a duration of 20 s into the six deepest periodontal sites within each subject for PCR detection of Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Aggregatibacter actinomycetemcomitans. P. gingivalis yields a band corresponding to 404 bp; T. forsythia yields a band of 641 bp; T. denticola yields a band of 316 bp; and A. actinomycetemcomitans yields a band of 557 bp after electrophoresis in agarose of 1.5% with 0.5 µg/mL of ethidium bromide. Reference strains of each bacterium were used as positive DNA controls. The negative control was sterile water added to the master mix [24].
2.7. Indirect Immunoassay (ELISA) for the Determination of Serum Antibodies IgG1 and IgG2 against Periodontal Pathogens
A blood sample determined serum antibodies IgG1 and IgG2 against 3 selective periodontal pathogens. ELISA was performed as follows. A 96-well plate was covered with 50 μL at a concentration 10 µg/mL of sonicated P. gingivalis (ATCC 33277), T. forsythia (ATCC 43037), or A. actinomycetemcomitans (ATCC 29523) in carbonate buffer (pH 9.6) and incubated overnight. The plates were blocked for an hour at 37 °C with 150 μL of a solution of phosphate buffer (PBS, pH: 7.2) with 1% bovine serum albumin, 1% milk, and avidin. The sera were diluted 1/100 in PBS-Tween-BSA (1%)-biotin solution and incubated at 37 °C per 1 h. Anti-IgG1- dilution 1:5000ǁǁ or anti-IgG2 dilution 1:10,000 in PBS-Tween-BSA (1%) solution were incubated for 1 h at 37 °C. Streptavidin peroxidase in a dilution 1/1500 dilution was incubated for 1 h at room temperature. Three washes were carried out between each incubation period with 250 µL PBS-tween (0.05%) solution for 2 min under stirring. Finally, 50 µL phosphate/citrate buffer solution (0.5 M, pH = 5) was added with O-Phenylenediamine and a concentration of 1 mg/mL was added and activated with H2O2 for 5 min at room temperature.
The reaction was stopped with a solution of sulfuric acid (2.5 M). Absorbance values were read at 490 nm. The concentration of IgG1 and IgG2 in the samples for each of the microorganisms was measured in triplicate and calculated by linear regression analysis with a known concentration curve (5 µg/mL–0.152 µg/mL, dilution 1:2) of human immunoglobulin IgG1 or IgG2. The reliability of the test was 98% (R2 > 98%) with a variation coefficient of <20%.
2.8. Statistical Analyses
Descriptive analyses of frequency distribution were carried out for the categorical variables: region (Central—Medellin, Bogotá, and Pacific—Cali), sex (female, male), age (</>55 years), socio-economic status (low, medium, high), according to the classification used by Department of National Planning of Colombia [25], smoking history (never, current, former), current smoking (serum cotinine ≥ 10 ng/mL/<10 ng/mL), alcohol consumption (yes, no), class of drinks (none, soft, strong), blood sugar levels (≥110 mg/dL, <110 mg/dL), and obesity (BMI > 30). Mean, median, and standard deviation were obtained for continuous variables. Antibody levels were categorized into terciles: low, medium, and high. Bivariate analysis was carried out with chi-squared and Fisher’s exact tests. All variables which showed a significance level < 0.10 in the bivariate analysis were included in a logistic regression analysis to establish the odds ratio (OR) and confidence intervals of 95%. Subgroup analyses were conducted by multivariate unconditional logistic regression using a stepwise function by primary outcomes such as CAL (mild vs. moderate and mild vs. advanced CAL), lifestyle (smoking and type of consumed alcohol), genetic factors (SNPs of IL-1a), microbial factors (presence of P. gingivalis, A. actimomycetemcomitans, T denticola, T. forsythia, and plaque index), and IgG antibodies (IgG1 and IgG2 subclass against P. gingivalis, T. forsythia, and A. actimomycetemcomitans). The factors associated with subgroup analysis were included in a final model, and obesity as a category was included.
Potential confounders were defined a priori (region and age). For all logistic models, the goodness-of-fit tests for logistic regression (Hosmer–Lemeshow test) were performed. The adjusted and unadjusted models were compared using the likelihood ratio chi-square (G2), Akaike’s Information Criterion (AIC), and the Bayesian Information Criterion (BIC).
A final model was performed with the factors associated with the logistic regression using a generalized ordered logistical regression analysis to determine the factors that kept the proportionality of the association in two levels according to the extent of CAL [26]. All data analysis was undertaken using STATA version 11.
3. Results
Table 1 shows the characteristics of the studied population. Medellin presented the highest number of patients with advanced CAL, compared to the other cities (p < 0.05). There was a difference in gender among the geographic region, although most of the patients were women. The age was similar for moderate and advanced CAL groups, but the mild CAL group was younger (p < 0.05). The periodontal status for the study population is also described in Table 1. The number of remaining teeth between the study groups decreases with the advance of the severity of CAL (p < 0.05), and periodontal clinical parameters increase with CAL severity (p < 0.05).
Table 1.
Demographic and periodontal clinical characteristics of population.
| Parameter | Mild CAL | Moderate CAL | Advanced CAL | |||
|---|---|---|---|---|---|---|
| Total | Percentage | Total | Percentage | Total | Percentage | |
| Subjects | 223/667 | 33.4% | 256/667 | 38.3% | 188/667 | 28.1% |
| Cities * | ||||||
| Medellín | 51/226 | 22.6% | 88/256 | 38.9% | 87/188 | 38.5% |
| Bogotá | 126/297 | 42.2% | 100/256 | 33.6% | 71/188 | 23.9% |
| Cali | 46/144 | 31.9% | 68/256 | 47.2% | 30/188 | 20.8% |
| Gender * | F: 163/223 M: 60/223 |
73.0% 27.0% |
F: 166/256 M: 90/256 |
64.8% 35.2% |
F: 120/188 M: 68/188 |
63.8% 36.2% |
| Socio-economic Status * |
L: 140/223 ME: 83/223 |
62.7% 37.3% |
L: 183/256 ME: 73/256 |
71.4% 28.6% |
L: 152/188 ME: 36/188 |
80.8% 19.2% |
| Age (Mean ± SD) * | 45.0 ± 7.7 b,c | 48.4 ± 9.19 a | 48.3 ± 9.8 a | |||
| No. of teeth * | 25.8 ± 3.3 b,c | 24.1 ± 4.1 a,c | 23.1 ± 3.8 a,b | |||
| PD (Mean ± SD) * | 2.2 ± 0.4 b,c | 2.7 ± 0.5 a,c | 3.8 ± 0.9 a,b | |||
| CAL (Mean ± SD) * | 1.3 ± 0.6 b,c | 2.8 ± 0.6 a,c | 4.8 ± 1.1 a,b | |||
| Bleeding (%) * | 36 ± 25 b,c | 52 ± 28 a,c | 67 ± 28 a,b | |||
| Gingival Index (%) * |
42 ± 30 b,c | 55 ± 31 a | 67 ± 33 a,b | |||
F = female; M = male; L = low; ME = medium; PD: probing depth full-mouth; CAL: clinical attachment loss in full-mouth; IG: gingival index. * ANOVA, Kruskal–Wallis, Mann–Whitney U, or Chi-square test. p < 0.05; a differences with mild CAL; b differences with moderate CAL; c differences with advanced CAL.
Table 2 shows the descriptive and bivariate analysis of lifestyle, systemic, immunogenic, and microbiologic factors, and serum antibodies by CAL. Smoking history, current smoking, and microbial factors such as elevated plaque index, P. gingivalis, and T. forsythia showed a high association with advanced CAL (p < 0.0001). BMI, SNP IL1a, and SNP IL1b, A. actinomycetemcomitans, IgG1, and IgG2 antibodies against P. gingivalis and A. actinomycetemcomitans also were associated with advanced CAL (p < 0.05). IgG1 and IgG2 antibodies against T. forsythia, alcohol consumption, stress, and glycemia > 120 mg/dL showed no associations in bivariate analysis.
Table 2.
Descriptive and bivariate analyses of lifestyle, systemic, immunogenetic, and microbial factors, and serum antibodies against periodontophatogens by CAL.
| Independent Variables | Clinical Attachment Loss | ||||||||
|
Mild CAL
n = 223 |
Moderate CAL
n = 253 |
Advanced CAL
n = 188 |
Missing Data | p-Value | |||||
| F | % | F | % | F | % | F | % | ||
| LIFESTYLE | |||||||||
| Smoking History | |||||||||
| Negative | 168 | 76 | 179 | 70.7 | 105 | 55.8 | 5 | 0.7 | <0.0001 |
| Current | 31 | 14 | 44 | 17.4 | 55 | 29.3 | |||
| Former | 22 | 10 | 39 | 11.9 | 28 | 14.9 | |||
| Current Smoking | |||||||||
| Negative | 190 | 85.2 | 209 | 82.6 | 133 | 70.7 | 5 | 0.9 | <0.0001 |
| Positive | 31 | 14.8 | 44 | 17.4 | 55 | 29.3 | |||
| Alcohol consumption | |||||||||
| Not | 195 | 88.2 | 220 | 88.3 | 168 | 89.1 | 9 | 1.3 | NS |
| Occasional | 20 | 9 | 21 | 8.4 | 15 | 8.1 | |||
| Frequent | 6 | 2.7 | 8 | 3.2 | 5 | 2.7 | |||
| Stress (Cortisol) mean ± SD | 13.31 ± 1.20 | 13.04 ± 0.75 | 13.49 ± 0.72 | 12 | 1.8 | NS | |||
| SYSTEMIC FACTORS | |||||||||
| BMI | |||||||||
| Normal | 132 | 59.2 | 131 | 51.6 | 104 | 55.6 | 3 | 0.4 | <0.05 |
| Overweight | 81 | 36.3 | 85 | 33.5 | 64 | 34.2 | |||
| Obesity | 10 | 4.5 | 38 | 14.9 | 19 | 10.2 | |||
| Glycemia | |||||||||
| <120 mg/dL | 191 | 85.6 | 204 | 83.9 | 157 | 83.5 | 10 | 1.4 | NS |
| >120 mg/dL | 32 | 14.3 | 39 | 16 | 31 | 16.4 | |||
| IMMUNOGENETIC FACTORS | |||||||||
| SNP IL-1b | |||||||||
| C-C | 147 | 66.8 | 162 | 66.2 | 135 | 73.4 | 16 | 2.3 | <0.05 |
| T-T | 18 | 8.2 | 29 | 11.8 | 22 | 12.1 | |||
| CT/TC | 55 | 25 | 44 | 18 | 23 | 12.6 | |||
| SNP IL-1a | |||||||||
| CC | 127 | 60.5 | 125 | 53.2 | 124 | 55.9 | 16 | 2.4 | <0.05 |
| T-T | 21 | 10 | 45 | 19.1 | 51 | 23 | |||
| CT/TC | 58 | 27.6 | 57 | 24.2 | 43 | 19.3 | |||
| MICROBIAL FACTORS | |||||||||
| Plaque index | |||||||||
| Low | 124 | 56.4 | 70 | 28.6 | 25 | 13.5 | 0 | 0.0 | <0.0001 |
| Middle | 65 | 29.6 | 95 | 38.7 | 51 | 27.6 | |||
| High | 31 | 14 | 80 | 32.7 | 109 | 58.9 | |||
| P. gingivalis | |||||||||
| Not | 115 | 51.8 | 80 | 31.3 | 45 | 23.9 | 2 | 0.3 | <0.0001 |
| Yes | 107 | 48.2 | 145 | 68.7 | 143 | 76.1 | |||
| T. forsythia | |||||||||
| Not | 119 | 53.6 | 68 | 26.6 | 52 | 22.3 | 2 | 0.3 | <0.0001 |
| Yes | 103 | 46.4 | 187 | 73.4 | 143 | 77.6 | |||
| T. denticola | |||||||||
| Not | 160 | 71.3 | 158 | 62.9 | 106 | 56.3 | 2 | 0.3 | <0.05 |
| Yes | 63 | 29.7 | 93 | 37.1 | 82 | 43.7 | |||
| A. actinomycetemcomitans | |||||||||
| Not | 215 | 96.8 | 232 | 90.9 | 169 | 89.9 | 2 | 0.3 | <0.05 |
| Yes | 7 | 3.3 | 22 | 9.1 | 22 | 10.1 | |||
| SERUM ANTIBODIES | |||||||||
| IgG1 P. gingivalis | |||||||||
| Low | 76 | 34.1 | 99 | 38.7 | 47 | 25 | 0 | 0.0 | <0.10 |
| Middle | 61 | 27.3 | 92 | 35.9 | 69 | 36.7 | |||
| High | 86 | 38.6 | 75 | 25.4 | 62 | 38.3 | |||
| IgG1 T. forsythia | |||||||||
| Low | 75 | 33.6 | 82 | 32.4 | 63 | 33.5 | 0 | 0.0 | NS |
| Middle | 65 | 29.1 | 80 | 31.6 | 73 | 38.8 | |||
| High | 83 | 37.3 | 91 | 36.0 | 52 | 27.7 | |||
| IgG1 A. actinomycetemcomitans | |||||||||
| Low | 99 | 39.1 | 80 | 31.6 | 56 | 29.7 | 0 | 0.0 | <0.10 |
| Middle | 84 | 33.2 | 81 | 32.1 | 64 | 34 | |||
| High | 70 | 27.6 | 92 | 36.3 | 68 | 36.3 | |||
| IgG2 P. gingivalis | |||||||||
| Low | 91 | 41 | 81 | 31.7 | 50 | 26.6 | 0 | 0.0 | <0.05 |
| Middle | 67 | 30.2 | 83 | 32.4 | 73 | 38.8 | |||
| High | 64 | 28.8 | 92 | 35.9 | 65 | 34.6 | |||
| IgG2 T. forsythia | |||||||||
| Low | 88 | 39.4 | 84 | 33.2 | 51 | 27.1 | 0 | 0.0 | NS |
| Middle | 66 | 29.6 | 87 | 34.4 | 68 | 36.2 | |||
| High | 69 | 31.1 | 82 | 32.4 | 69 | 36.7 | |||
| IgG2 A. actinomycetemcomitans | |||||||||
| Low | 92 | 41.4 | 84 | 32.8 | 46 | 24.5 | 0 | 0.0 | <0.05 |
| Middle | 65 | 29.3 | 92 | 35.9 | 75 | 34.6 | |||
| High | 65 | 29.3 | 80 | 31.3 | 67 | 40.9 | |||
The multivariate logistic analysis for the risk factors associated with CAL severity is shown in Table 3. In both models, the unadjusted models were compared with adjusted models, and the likelihood ratio and BIC provided support for selecting the unadjusted model. In model 1, an association between moderate CAL compared with mild CAL was performed. The socio-demographic factors were not similar among the groups. The region and age (>55 years) were associated with moderate CAL. Microbial factors such as the presence of P. gingivalis, T. forsythia, and elevated plaque index were associated with moderate CAL. However, IgG1 against P. gingivalis antibodies proved to be a protective factor for moderate CAL (OR 0.71, CI 95% 0.55–0.91). Although SNP IL1a and SNP ILb were not associated in the regression model with moderate CAL, obesity was associated (OR 1.40, CI 95% 1.01–1.94). In model 2, an association between advanced CAL and mild CAL was also found. In model 2, advanced CAL was also associated with high levels of IgG2 antibodies against A. actinomycetemcomitans (OR 1.63, CI 95% 1.19–2.21). Other factors, such as current smoking (OR 1.97, CI 95% 1.07–3.75) and P. gingivalis (OR 3.22, CI 95% 1.89–6.46), were associated with advanced lesions. Nevertheless, T. forsythia and IgG1 P. gingivalis were associated with the most severe CAL. In this model, plaque index was the most important factor associated with severe CAL (OR 4.59, CI 95% 3.28–6.43). Region and age were also associated with advanced CAL. Obesity was associated with moderate CAL but was not associated with advanced CAL.
Table 3.
Logistic regression models. Risk factors associated with clinical attachment loss.
| MODEL 1 | |||
| BASE OUTCOME Mild CAL | Unadjusted OR (IC 95%) | Adjusted OR (IC 95%) | |
| Compared with moderate CAL | |||
| Base comparison | |||
| CITIES | 1 | 1 | |
| Bogotá | 1.51 ** (1.14–1.99) | 1.85 (0.90–3.80) | |
| Other | |||
| AGE | 1 | 1 | |
| <55 years | 1.50 ** (1.15–1.95) | 2.36 † (1.22–4.55) | |
| >45 years | |||
| MICROORGANISMS | 1 | 1 | |
| P. gingivalis positive | 1.95 ** (1.23–3.08) | 2.78 ** (1.07–7.19) | |
| T. forsythia positive | 1 2.10 ** (1.33–3.32) |
1 5.02 ** (1.95–12.9) |
|
| ANTIBODIES | low | 1 | 1 |
| IgG1 P. gingivalis Middle-High |
0.71 ** (0.55–0.91) |
0.70 (0.42–1.17) |
|
| PLAQUE INDEX | low | 1 2.25 † (1.72–2.95) |
1 1.91 (1.32–3.25) |
| Obesity | 1 | 1 | |
| Positive | 1.40 ** (1.01–1.94) | 1.41 (0.69–2.84) | |
| MODEL 2 | |||
| BASE OUTCOME Middle CAL | Unadjusted OR (IC 95%) | Adjusted OR (IC 95%) | |
| Compared with advanced CAL | |||
| Base comparison | |||
| CITIES | 1 | 1 | |
| Bogotá | 1.85 † (1.31–2.61) | 1.32 (0.50–3.48) | |
| Other | |||
| AGE | |||
| <45 years | 1 | 1 | |
| >55 years | 1.63 ** (1.19–2.23) | 2.70 ** (1.12–6.54) | |
| MICROORGANISMS | 1 | 1 | |
| P. gingivalis positive | 3.22 † (1.89–6.46) | 2.54 (0.88–7.31) | |
| ANTIBODIES | low level | 1 | 1 |
| IgG2 A. actinomycetemcomitans Middle-High Level |
1.63 ** (1.19–2.21) | 1.98 ** (1.06–3.69) | |
| PLAQUE INDEX Middle-High level |
low | 1 4.59 † (3.28–6.43) |
1 5.65 † (2.97–10.7) |
| SMOKING | never | 1 | 1 |
| Current | 1.97 ** (1.07–3.65) | 1.76 (0.49–6.27) | |
Middle CAL (clinical attachment loss) < 2 mm, MP = moderate CAL > 2 < 3.5 mm, advanced CAL ≥ 3.5 mm. ** p < 0.05. † p < 0.000. Model 1: comparison of unadjusted OR and adjusted OR (adjusted to age and region). Likelihood ratio p = 0.36. Difference in BIC = unadjusted 597.7816 − adjusted 658.5311 = −60.7495 provides positive support for the unadjusted OR. Model 2: comparison of unadjusted OR and adjusted OR (adjusted to age and region). Likelihood ratio p = 0.10. Difference in BIC = unadjusted 433.6645 − adjusted 489.7469 = −446.0824 provides positive support for the unadjusted OR.
A generalized ordered regression model was carried out to determine the factors associated with the extent of CAL. This analysis has been considered in particular due to the outcome variable CAL, which is truly ordered and is composed of three categories; it satisfies the assumption of proportional odds, namely, different models are needed to describe the relationship between, on the one hand, mild versus moderate and severe categories, and on the other hand, mild and moderate versus severe categories. In consequence, this will cause the model to be more parsimonious (Table 4).
Table 4.
Generalized ordered regression model. Risk factors associated with CAL.
| Level 1 | MODEL 1 | MODEL 2 |
| BASE OUTCOME Mild CAL | OR (IC 95%) | OR (IC 95%) |
| Compared with Moderate + Advanced CAL | ||
| CITIES Bogotá | 1 | 1 |
| Other | 1.34 ** (1.09–1.64) | 1.58 † (1.25–2.01) |
| AGE < 45 years | 1 | 1 |
| >55 years | 1.46 ** (1.21–1.77) | 1.45 † (1.20–1.75) |
| MICROORGANISMS | ||
| P. gingivalis Negative | 1 | 1 |
| Positive | 1.93 ** (1.35–2.76) | 2.44 † (1.77–3.38) |
| T. forsythia Negative | 1 | |
| Positive | 1.88 ** (1.30–2.70) | |
| ANTIBODIES | ||
| Low levels | 1 | |
| IgG1 P. gingivalis | 0.85 (0.67–1.06) | |
| Middle-High | ||
| Low levels | 1 | |
| IgG2. A. actinomycetemcomitans | 1.50 † (1.23–1.81) | 1.48 † (1.22–1.78) |
| Middle-High | ||
| PLAQUE INDEX Low | 1 | 1 |
| Middle-High | 2.69 † (2.20–3.29) | 2.76 † (2.27–3.36) |
| OBESITY Negative | 1 | |
| Positive | 2.99 ** (1.40–6.36) | |
| SMOKING Negative | 1 | 1 |
| Positive | 1.94 ** (1.31–2.87) | 1.88 ** (1.28–2.76) |
| Level 2 | ||
| BASE OUTCOME | Mild + Moderate CAL | |
| Comparison with advanced CAL | ||
| REGION | 1 | 1 |
| Bogota | 1.34 ** (1.09–1.64) | 1.54 † (1.21–1.96) |
| Other regions | ||
| AGE < 45 years | 1 | 1 |
| >55 years | 1.46 † (1.21–1.77) | 1.46 † (1.21–1.76) |
| MICROORGANISMS | ||
| P. gingivalis Negative | 1 | 1 |
| Positive | 1.93 † (1.35–2.76) | 1.89 † (1.33–2.69) |
| T. forsythia Negative | 1 | |
| Positive | 1.88 ** (1.30–2.70) | 2.33 † (1.56–3.49 |
| ANTIBODIES Low level | 1 | |
| IgG1 P. gingivalis | 1.42 ** (1.12–1.80) | |
| Middle-High | ||
| Low levels | 1 | 1 |
| IgG2 A. actinomycetemcomitans Middle-High Level |
1.50 † (1.23–1.81) | 1.50 † (1.24–1.81) |
| PLAQUE INDEX Low level | 1 | 1 |
| Middle-High level | 2.69 † (2.20–3.29) | 2.67 † (2.19–3.26) |
| OBESITY Negative | 1 | |
| Positive | 0.94 (0.50–1.74) | |
| SMOKING Never | 1 | 1 |
| Current | 1.94 ** (1.31–2.87) | 1.86 ** (1.26–2.74) |
Middle CAL (clinical attachment loss) < 2 mm, MP= moderate CAL > 2 < 3.5 mm, advanced CAL ≥ 3.5 mm. ** p < 0.05. † p < 0.000. Level 1: comparison of mild vs. moderate and advanced CAL. Level 2: comparison mild and moderate vs. advanced CAL. Difference in BIC: model 1 = 1276.918, model 2 = 1287.819 = −10.901; this provides positive support for model 1.
For logistic regression, model 1 included all variables associated with unconditional logistic regression models, and model 2 included only factors that showed proportionally in OR in the two levels. Model 1 proved to be the most adjusted model. The factors that followed the assumption of proportionality for both levels that showed the same value of OR were region, age, smoking, plaque index level, P. gingivalis, T. forsythia, and IgG2 antibodies against A. actinomycetemcomitans; therefore, the severity of CAL can be attributed to these factors. Obesity and IgG1 antibodies against P. gingivalis were only associated with one level and did not follow the course of proportionality; hence, these cannot be associated with the extent of severity of CAL.
4. Discussion
Periodontal disease has been considered a multifactorial disease associated with immunogenic, microbial, and unhealthy lifestyle habits like irregular dental plaque control, excessive alcohol consumption, and smoking. In this study, bacterial factors were linked to the severity of CAL. High supragingival plaque level was relevant for moderate and advanced CAL and an important factor for the severity of CAL. The best available evidence indicates that supragingival prophylaxis and subgingival debridement are comparable concerning the probing depth and clinical attachment level outcomes after 12 months of non-surgical treatment [27]. However, in long-term studies, only supragingival plaque control fails to prevent further periodontal tissue destruction in subjects with advanced periodontal disease [28,29]. In patients with periodontitis, subgingival debridement in conjunction with supragingival plaque control is more effective for reducing probing pocket depth and improving the clinical attachment level than supragingival plaque control alone [30]. Likewise, strict plaque control performed during and after periodontal treatment improves probing depth and clinical attachment level and reduction in the proportions of red and orange complexes in periodontitis [31]. In fact, the supragingival plaque composition seems to be like the subgingival plaque but with a lower proportion of periodontal pathogens, and the supragingival biofilm appears to be a reservoir of periodontopathic microorganisms for the colonization of subgingival plaque [32]; hence, high levels of supragingival plaque can continuously increase the risk of clinical attachment loss. It is possible that high levels of supragingival plaque for long periods might increase the risk of clinical attachment loss by plaque maturation. It augments the inflammatory response in gingival crevicular fluid as the bacterial biomass of the human microbiome escalates alongside increasing periodontal inflammation [33]. Nevertheless, these hypotheses require validation in a clinical context.
In this study, P. gingivalis and T. forsythia were associated with advanced CAL. The evidence supports the association of these microorganisms with the progression of periodontal disease [34,35,36,37]. Nevertheless, individuals harboring microorganisms in subgingival plaque generally respond with a humoral immune response against them [38]. Plasma IgG levels against these pathogens are highly stable for 15 years in subjects with and without periodontitis and can be a good marker of infection history [39]. In this study, it was only possible to establish an association between advanced CAL and serum markers for A. actinomicetemcomitans. High antibodies against A. actinomycetemcomitans reflect periods of active disease and recurrence in periodontitis [40,41]. Its detection is a significant risk factor for the onset of attachment loss and disease progression [42]. However, the presence of A. actinomycetemcomitans was not associated with the severity of CAL. It is possible that this is due to the observed low frequency of this microorganism in the subgingival plaque. Nevertheless, its levels were lower in mild CAL compared with moderate and advanced CAL. The presence of IgG2 antibodies against A. actinomycetemcomitans supports the hypothesis that the increase in severity of CAL is associated with the history of A. actinomycetemcomitans infection.
There is strong evidence of the association between smoking and the increase in the severity of CAL in this study. Current smoking significantly predicts clinical attachment loss in longitudinal studies [42,43]. A negative effect of smoking on bone regeneration and the response to periodontal treatment is known from a systematic review and meta-analysis, confirming the importance of this factor [6]. Otherwise, alcohol consumption frequency was not associated with this population’s prevalence or severity of periodontitis. However, although the relationship between periodontitis and alcohol use is still controversial [44], other populations have found an association between periodontitis and a high frequency of alcohol consumption [45,46].
Here, several putative factors for periodontitis were studied. A meta-analysis that evaluated associations of genetic polymorphisms and periodontal disease revealed a greater frequency of IL-1a and IL-b polymorphisms for developing periodontitis; however, this association is variable between regions [47]. In this study, SNPs of the IL gene were not associated with periodontal disease severity. Other types of polymorphism need to be studied to determine their influence on periodontitis severity among Latin Americans [48,49].
Obesity was a risk for the presence of periodontitis but not for advanced CAL. A systematic review of longitudinal studies showed evidence of a stronger association of periodontal disease with BMI [15,16]. However, the proof of a poor response to non-surgical periodontal therapy in the obese is still not consistent [49]. This study shows an association between obesity and moderate CAL in a multivariate model. However, due to the lack of association with advanced CAL, a direct relationship between obesity and the severity of CAL in periodontal patients cannot be demonstrated.
In this study, the selection of participants was not at random, and a sequential allocation of patients attending clinics of universities was used. This was not a representative sample of the general population of the regions studied but individuals who attend university service. There was a low prevalence of obesity, which could influence the lack of association with severe forms of periodontitis. IL-1a and IL-1b SNPs were the factors with the most data missing. However, these missing data did not appear to impact the analysis. Moreover, Hardy–Weinberg balance on genotypic frequency analysis did not show differences between cases and controls in this population. Hence, an examination of a randomized, representative sample from the study population could address these limitations. Additional investigations featuring these attributes and larger sample sizes are imperative to elucidate the present findings.
To summarize, in this study, two different analyses were carried out. The logistic regression can estimate the association of the independent variables with CAL compared with the control (mild CAL); nevertheless, this analysis cannot estimate the effect of these variables in the extent of CAL. Finally, CAL is an ordinal variable, and the best analysis for establishing the impact of the factors in its increase is the generalized ordered logistic model.
5. Conclusions
This study reaffirms the significance of previously identified risk factors linked to the severity of periodontitis. Specifically, aging, plaque accumulation, and IgG2 to A. actinomycetemcomitans antibodies emerged as the most influential factors associated with advanced clinical attachment loss. Conversely, genetic factors, obesity, and stress were found to be inadequate in explaining variations in the extent of CAL.
Author Contributions
Conceptualization, G.I.L., A.C., A.D., C.M.A. and D.M.C.; methodology, G.I.L., S.J.G., M.A.S., J.D.A. and T.G.T.; validation, G.I.L., M.A.S., D.F.G., J.D.A., T.G.T., S.D. and S.J.G.; formal analysis, G.I.L., A.C., C.M.A., A.D. and A.G.; investigation, G.I.L., M.A.S., A.C., D.M.C., D.F.G., J.D.A., T.G.T., A.D., A.G., S.D., S.J.G. and C.M.A.; resources, G.I.L., A.D., A.C., D.M.C. and C.M.A.; data curation, G.I.L., C.M.A. and A.C.; writing—original draft preparation, G.I.L., A.C. and C.M.A.; writing—review and editing, G.I.L., A.C. and C.M.A.; project administration, G.I.L.; funding acquisition, G.I.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The Ethical Review Board Committee approved the study for human research at the Health Faculty of the participating universities. All procedures performed were under the ethical standards of the institutional research committee and in accord with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent Statement
All participants of the study signed their informed consent and their voluntary participation.
Data Availability Statement
Data are unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The Institute of Science and Technology, Francisco José de Caldas-COLCIENCIAS Grant number 1308-459-21661 and the participant Universities funded the study.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Janakiram C., Dye B.A. A public health approach for prevention of periodontal disease. Periodontol. 2000. 2020;84:202–214. doi: 10.1111/prd.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu L., Zhang S.Q., Zhao L., Ren Z.H., Hu C.Y. Global, regional, and national burden of periodontitis from 1990 to 2019: Results from the Global Burden of Disease study 2019. J. Periodontol. 2022;93:1445–1454. doi: 10.1002/JPER.21-0469. [DOI] [PubMed] [Google Scholar]
- 3.Weijdijk L.P.M., Ziukaite L., Van der Weijden G.A.F., Bakker E.W.P., Slot D.E. The risk of tooth loss in patients with diabetes: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2022;20:145–166. doi: 10.1111/idh.12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gjermo P., Rösing C.K., Susin C., Oppermann R. Periodontal diseases in Central and South America. Periodontol. 2000. 2002;29:70–78. doi: 10.1034/j.1600-0757.2002.290104.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Xu S., Cai Q., Chen Y., Zhu P., Du M., Visser A., Li A. Does Periodontitis Affect the Association of Biological Aging with Mortality? J. Dent. Res. 2023;102:909–918. doi: 10.1177/00220345231179117. [DOI] [PubMed] [Google Scholar]
- 6.Leite F.R.M., Nascimento G.G., Scheutz F., López R. Effect of Smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018;54:831–841. doi: 10.1016/j.amepre.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Lafaurie G.I., Castillo D.M., Iniesta M., Sanz M., Gómez L.A., Castillo Y., Pianeta R., Delgadillo N.A., Neuta Y., Diaz-Báez D., et al. Differential analysis of culturable and unculturable subgingival target microorganisms according to the stages of periodontitis. Clin. Oral Investig. 2023;27:3029–3043. doi: 10.1007/s00784-023-04907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nascimento G.G., Leite F.R.M., Vestergaard P., Scheutz F., López R. Does diabetes increase the risk of periodontitis? A systematic review and meta-regression analysis of longitudinal prospective studies. Acta Diabetol. 2018;55:653–667. doi: 10.1007/s00592-018-1120-4. [DOI] [PubMed] [Google Scholar]
- 9.Ramseier C.A., Anerud A., Dulac M., Lulic M., Cullinan M.P., Seymour G.J., Faddy M.J., Bürgin W., Schätzle M., Lang N.P. Natural history of periodontitis: Disease progression and tooth loss over 40 years. J. Clin. Periodontol. 2017;44:1182–1191. doi: 10.1111/jcpe.12782. [DOI] [PubMed] [Google Scholar]
- 10.Liu X., Li H. A Systematic Review and Meta-Analysis on Multiple Cytokine Gene Polymorphisms in the Pathogenesis of Periodontitis. Front. Immunol. 2022;12:713198. doi: 10.3389/fimmu.2021.713198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebersole J.L., Kirakodu S., Novak M.J., Stromberg A.J., Shen S., Orraca L., Gonzalez-Martinez J., Burgos A., Gonzalez O.A. Cytokine gene expression profiles during initiation, progression and resolution of periodontitis. J. Clin. Periodontol. 2014;41:853–861. doi: 10.1111/jcpe.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira P.R., de Sá A.R., Xavier G.M., Costa J.E., Gomez R.S., Gollob K.J., Dutra W.O. A functional interleukin-1 beta gene polymorphism is associated with chronic periodontitis in a sample of Brazilian individuals. J. Periodontal Res. 2005;40:306–311. doi: 10.1111/j.1600-0765.2005.00801.x. [DOI] [PubMed] [Google Scholar]
- 13.Trevilatto P.C., de Souza Pardo A.P., Scarel-Caminaga R.M., de Brito R.B., Jr., Alvim-Pereira F., Alvim-Pereira C.C., Probst C.M., Garlet G.P., Sallum A.W., Line S.R.P. Association of IL1 gene polymorphisms with chronic periodontitis in Brazilians. Arch. Oral Biol. 2011;56:54–62. doi: 10.1016/j.archoralbio.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Marruganti C., Gaeta C., Romandini M., Ferrari Cagidiaco E., Parrini S., Discepoli N., Grandini S. Multiplicative effect of stress and poor sleep quality on periodontitis: A University-based cross-sectional study. J. Periodontol. 2023 doi: 10.1002/JPER.23-0209. [DOI] [PubMed] [Google Scholar]
- 15.Coelho J.M.F., Miranda S.S., da Cruz S.S., Trindade S.C., Passos-Soares J.S., Cerqueira E.M.M., Costa M.D.C.N., Figueiredo A.C.M.G., Hintz A.M., Barreto M.L., et al. Is there association between stress and periodontitis? Clin. Oral Investig. 2020;24:2285–2294. doi: 10.1007/s00784-019-03083-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim C.M., Lee S., Hwang W., Son E., Kim T.W., Kim K., Kim Y.H. Obesity and periodontitis: A systematic review and updated meta-analysis. Front. Endocrinol. 2022;13:999455. doi: 10.3389/fendo.2022.999455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tegelberg P., Tervonen T., Knuuttila M., Saxlin T., Ylöstalo P. Association of obesity and weight gain with alveolar bone loss: Results of the Northern Finland Birth Cohort 1966 study. J. Clin. Periodontol. 2023;50:1051–1063. doi: 10.1111/jcpe.13829. [DOI] [PubMed] [Google Scholar]
- 18.Colombo A.P., Wu B. Aging and Oral Health: Biological and Sociobehavioral Perspectives. J. Dent. Res. 2023;102:841–843. doi: 10.1177/00220345231181885. [DOI] [PubMed] [Google Scholar]
- 19.Yaragani A., Sushuma K., Guduri V., Thirumalasetty S.S.M.K., Vishnubhotla G., Kandikatla P., Chandu V.C. The influence of tobacco consumption on periodontal health: A stratified analysis based on type of tobacco use. J. Fam. Med. Prim. Care. 2020;9:2061–2066. doi: 10.4103/jfmpc.jfmpc_1071_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duque A., Martínez P.J., Giraldo A., Gualtero D.F., Ardila C.M., Contreras A., Duarte S., Lafaurie G.I. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med. Oral Patol. Oral Cir. Bucal. 2017;22:e425–e431. doi: 10.4317/medoral.21292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silness J., Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol. Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 22.Zhang X., Dai J., Long Y., Wu H., Li X.J., Ding Y. Correlation of estrogen receptor alpha gene polymorphisms and bone mineral density in Chinese women with chronic periodontitis. Chin. Med. J. 2010;123:3262–3267. [PubMed] [Google Scholar]
- 23.Kornman K.S., Crane A., Wang H.Y., di Giovine F.S., Newman M.G., Pirk F.W., Wilson T.G., Jr., Higginbottom F.L., Duff G.W. The interleukin-1 genotype as a severity factor in adult periodontal disease. J. Periodontol. 1997;24:72–77. doi: 10.1111/j.1600-051X.1997.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 24.Lafaurie G.I., Contreras A., Barón A., Botero J., Mayorga-Fayad I., Jaramillo A., Giraldo A., Gonzalez F., Mantilla S., Botero A., et al. Demographic, clinical, and microbial aspects of chronic and aggressive periodontitis in Colombia: A multicenter study. J. Periodontol. 2007;78:629–639. doi: 10.1902/jop.2007.060187. [DOI] [PubMed] [Google Scholar]
- 25.National Planning Department of Colombia [(accessed on 15 November 2013)]; Available online: http://www.dnp.gov.co/
- 26.Hosmer D., Lemeshow S. Applied Logistic Regression. 2nd ed. John Wiley & Sons, Inc.; New York, NY, USA: 2000. [Google Scholar]
- 27.Heasman P.A., McCracken G.I., Steen N. Supportive periodontal care: The effect of periodic subgingival debridement compared with supragingival prophylaxis with respect to clinical outcomes. J. Clin. Periodontol. 2002;29((Suppl. S3)):163–172. doi: 10.1034/j.1600-051X.29.s3.9.x. [DOI] [PubMed] [Google Scholar]
- 28.Westfelt E., Rylander H., Dahlén G., Lindhe J. The effect of supragingival plaque control on the progression of advanced periodontal disease. J. Clin. Periodontol. 1998;25:536–541. doi: 10.1111/j.1600-051X.1998.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 29.Albandar J.M., Buischi Y.A., Oliveira L.B., Axelsson P. Lack of effect of oral hygiene training on periodontal disease progression over 3 years in adolescents. J. Periodontol. 1995;66:255–260. doi: 10.1902/jop.1995.66.4.255. [DOI] [PubMed] [Google Scholar]
- 30.Van der Weijden G.A., Timmerman M.F. A systematic review on the clinical efficacy of subgingival debridement in the treatment of chronic periodontitis. J. Clin. Periodontol. 2002;29((Suppl. S3)):55–71; discussion 90–91. doi: 10.1034/j.1600-051X.29.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 31.Feres M., Gursky L.C., Faveri M., Tsuzuki C.O., Figueiredo L.C. Clinical and microbiological benefits of strict supragingival plaque control as part of the active phase of periodontal therapy. J. Clin. Periodontol. 2009;36:857–867. doi: 10.1111/j.1600-051X.2009.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ximénez-Fyvie L.A., Haffajee A.D., Socransky S.S. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J. Clin. Periodontol. 2000;27:648–657. doi: 10.1034/j.1600-051x.2000.027009648.x. [DOI] [PubMed] [Google Scholar]
- 33.Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol. Oral Microbiol. 2014;29:248–257. doi: 10.1111/omi.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayanagi G., Sato T., Shimauchi H., Takahashi N. Detection frequency of periodontitis-associated bacteria by polymerase chain reaction in subgingival and supragingival plaque of periodontitis and healthy subjects. Oral Microbiol. Immunol. 2004;19:379–385. doi: 10.1111/j.1399-302x.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 35.Ardila C.M., Olarte-Sossa M., Guzmán I.C. Association between immunoglobulin G1 against Tannerella forsythia and reduction in the loss of attachment tissue. J. Periodontal Implant Sci. 2014;44:274–279. doi: 10.5051/jpis.2014.44.6.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Winkelhoff A.J., Loos B.G., van der Reijden W.A., van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J. Clin. Periodontol. 2002;29:1023–1028. doi: 10.1034/j.1600-051X.2002.291107.x. [DOI] [PubMed] [Google Scholar]
- 37.Byrne S.J., Dashper S.G., Darby I.B., Adams G.G., Hoffmann B., Reynolds E.C. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol. Immunol. 2009;24:469477. doi: 10.1111/j.1399-302X.2009.00544.x. [DOI] [PubMed] [Google Scholar]
- 38.Kinane D.F., Mooney J., Ebersole J.L. Humoral immune response to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in periodontal disease. Periodontol. 2000. 1999;20:289–340. doi: 10.1111/j.1600-0757.1999.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 39.Lakio L., Antinheimo J., Paju S., Buhlin K., Pussinen P.J., Alfthan G. Tracking of plasma antibodies against Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis during 15 years. J. Oral Microbiol. 2009;1:1979. doi: 10.3402/jom.v1i0.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebersole J.L., Cappelli D., Sandoval M.N. Subgingival distribution of A. actinomycetemcomitans in periodontitis. J. Clin. Periodontol. 1994;21:65–75. doi: 10.1111/j.1600-051X.1994.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 41.Ebersole J.L., Cappelli D., Steffen M.J., Willmann D.E., O’Dell D.S. Host response assessment in recurring periodontitis. J. Clin. Periodontol. 1996;23 Pt 2:258–262. doi: 10.1111/j.1600-051X.1996.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 42.Van der Velden U., Abbas F., Armand S., Loos B.G., Timmerman M.F., Van der Weijden G.A., Van Winkelhoff A.J., Winkel E.G. Java project on periodontal diseases. The natural development of periodontitis: Risk factors, risk predictors and risk determinants. J. Clin. Periodontol. 2006;33:540–548. doi: 10.1111/j.1600-051X.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Canut P. Predictors of tooth loss due to periodontal disease in patients following long-term periodontal maintenance. J. Clin. Periodontol. 2015;42:1115–1125. doi: 10.1111/jcpe.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dye B.A. The relationship between periodontitis and alcohol use is not clear. J. Evid. Based Dent. Pract. 2010;10:225–227. doi: 10.1016/j.jebdp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Lages E.J., Costa F.O., Lages E.M., Cota L.O., Cortelli S.C., Nobre-Franco G.C., Gilson C., Cyrino Renata M., Cortelli J.R. Risk variables in the association between frequency of alcohol consumption and periodontitis. J. Clin. Periodontol. 2012;39:115–122. doi: 10.1111/j.1600-051X.2011.01809.x. [DOI] [PubMed] [Google Scholar]
- 46.Ryder M.I., Couch E.T., Chaffee B.W. Personalized periodontal treatment for the tobacco- and alcohol-using patient. Periodontol. 2000. 2018;78:30–46. doi: 10.1111/prd.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu X., Offenbacher S., Lόpez N.J., Chen D., Wang H.Y., Rogus J., Zhou J., Beck J., Jiang S., Bao X., et al. Association of interleukin-1 gene variations with moderate to severe chronic periodontitis in multiple ethnicities. J. Periodontal Res. 2005;50:52–61. doi: 10.1111/jre.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akram Z., Safii S.H., Vaithilingam R.D., Baharuddin N.A., Javed F., Vohra F. Efficacy of non-surgical periodontal therapy in the management of chronic periodontitis among obese and non-obese patients: A systematic review and meta-analysis. Clin. Oral Investig. 2016;20:903–914. doi: 10.1007/s00784-016-1793-4. [DOI] [PubMed] [Google Scholar]
- 49.Gerber F.A., Sahrmann P., Schmidlin O.A., Heumann C., Beer J.H., Schmidlin P.R. Influence of obesity on the outcome of non-surgical periodontal therapy—A systematic review. BMC Oral Health. 2016;16:90. doi: 10.1186/s12903-016-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are unavailable due to privacy or ethical restrictions.