Abstract
We have studied pressure-induced germination of Bacillus subtilis spores at moderate (100 MPa) and high (500 to 600 MPa) pressures. Although we found comparable germination efficiencies under both conditions by using heat sensitivity as a criterion for germination, the sensitivity of pressure-germinated spores to some other agents was found to depend on the pressure used. Spores germinated at 100 MPa were more sensitive to pressure (>200 MPa), UV light, and hydrogen peroxide than were those germinated at 600 MPa. Since small, acid-soluble proteins (SASPs) and dipicolinic acid (DPA) are known to be involved in spore resistance to UV light and hydrogen peroxide, we studied the fate of these compounds during pressure germination. DPA was released upon both low- and high-pressure germination, but SASP degradation, which normally accompanies nutrient-induced germination, occurred upon low-pressure germination but not upon high-pressure germination. These results adequately explain the UV and hydrogen peroxide resistance of spores germinated at 600 MPa. The resistance to pressure inactivation of 600-MPa-germinated spores could also, at least partly, be attributed to α/β-type SASPs, since mutants deficient in α/β-type SASPs were more sensitive to inactivation at 600 MPa. Further, germination at 100 MPa resulted in rapid ATP generation, as is the case in nutrient-induced germination, but no ATP was formed during germination at 600 MPa. These results suggest that spore germination can be initiated by low- and high-pressure treatments but is arrested at an early stage in the latter case. The implications for the use of high pressure as a preservation treatment are discussed.
A major obstacle to the application of high hydrostatic pressure as a technology for the preservation of foods and pharmaceuticals is the inefficient inactivation of bacterial spores. Ungerminated bacterial spores are believed to be extremely pressure resistant (1, 13). Spores of B. subtilis were shown to survive pressure treatments at 1,000 MPa for 40 min at temperatures below 10°C (17). However, it has been observed for the spores of various Bacillus spp. that inactivation was more efficient at moderate (200 to 500 MPa) than at higher (>500 MPa) pressure. This was explained by the finding that pressure can induce spores to germinate and lose their resistance to high pressure and heat and by the assumption that this germination is less efficient at high pressure (5, 7, 20, 26). This pressure-induced germination was strongly temperature dependent, being virtually absent at <10°C and most prominent at 40 to 50°C.
In line with these observations, it has been demonstrated that significant reductions in spore survival can be obtained by application of a cyclic process alternating between low and high pressures at moderate temperatures (40 to 70°C) (4, 10, 26). A problem that remains unsolved is the existence of a relatively large fraction of superdormant spores that remain ungerminated and thus viable after prolonged pressurization (20). There is only limited knowledge about the factors affecting pressure-induced germination and about its mechanism.
Gould and Sale (7) assumed that pressure-induced germination is caused by activation of enzymes involved in spore germination. For instance, they showed that a pressure of 25 MPa increased the activity of alanine racemase in B. cereus T spores. It is possible that pressure results in a changed environment in the spores by affecting the permeability of the spore envelope. The changed environment, in turn, could lead to activation of germination enzymes. Alternatively, pressure could directly induce conformational changes in enzymes, which could lead to activation of the enzymes (16). The observation that inhibitors of nutrient-induced germination also inhibited pressure-induced germination suggests that common enzymatic reactions are involved in both germination processes (7).
The reduced germination assumed to occur at higher pressures could be explained by conformational changes leading to inactivation of a critical germination enzyme. This inactivation must be reversible, since spores exposed to 800 MPa at 25°C germinate normally at 0.1 MPa in the presence of nutrients (8).
That spore inactivation exhibits a maximum as a function of pressure has been attributed by most investigators to the existence of a pressure maximum for spore germination. However, the data reported by Gould and Sale (7) shows that at 45°C, the degree of pressure-induced germination of B. coagulans spores increases between 100 and 600 MPa, while the inactivation is maximal at 300 MPa and decreases at higher pressures. In this case, the pattern of spore germination cannot explain the existence of a pressure maximum for spore inactivation. In the present paper, we report a similar discrepancy between B. subtilis spore germination and inactivation by high pressure. In addition, we provide an explanation by demonstrating that spores germinated at high pressure pass through an incomplete germination process and therefore retain some of their resistance properties.
MATERIALS AND METHODS
Preparation of B. subtilis spore suspensions.
B. subtilis ATCC 6051, obtained from the LMG culture collection (Ghent, Belgium), and B. subtilis PS832 (wild-type), PS356 (small, acid-soluble protein [SASP] α−β−), PS483 (SASP γ−), and PS482 (SASP α−β−γ−), obtained from P. Setlow (University of Connecticut Health Center), were used throughout this study. To induce sporulation, cells from a −80°C glycerol stock culture were grown at 37°C in a humid atmosphere on the surface of nutrient agar CM3 (Oxoid, Basingstoke, United Kingdom) supplemented with 0.03-g/ml MgSO4 and 0.25-g/ml KH2PO4. After 7 days, spores were harvested, washed two times by centrifugation at 4,000 × g for 15 min each time, and finally resuspended in sterile, deionized water. The spore suspension was adjusted to 107 to 108 spores ml−1 and kept at 4°C for up to 1 month. Plating of these spore suspensions before and after heat treatment (60°C for 15 min) did not result in significantly different counts. This indicated that the spore suspensions consisted exclusively of spores. The eventual presence of low numbers of vegetative cells did not influence the results of the experiments, since these are more sensitive than spores to pressure and to the other lethal agents used in this work.
Pressure treatment and measurement of germination.
Aqueous spore suspensions, diluted fivefold with 50 mM potassium phosphate buffer (pH 7), were pressurized at 40°C in heat-sealed, sterile polyethylene bags. The pressurization equipment (Resato, Roden, The Netherlands) consisted of a manually operated spindle pump, two parallel thermostatted vessels with a circulating-water jacket, and the necessary high-pressure valves to allow independent use of both vessels.
To count the ungerminated spores after pressurization, the treated spore suspensions were immersed in water at 60°C for 15 min to inactivate germinated spores. The percentage of ungerminated spores was calculated as the ratio of the number of CFU after germination and heat treatment to the number of CFU before germination but with heat treatment multiplied by 100.
UV treatment.
A UV transilluminator (254 nm; 60 W/m2) was positioned upside down at approximately 10 cm above an open flat-bottom vial (diameter, 2 cm) containing 1 ml of a spore suspension diluted fivefold in 50 mM potassium phosphate buffer, agitated by a small magnetic stirrer. Samples were withdrawn at different UV exposure times and plated to count survivors.
Measurement of DPA release.
The dipicolinic acid (DPA) content of the filtered supernatant of the pressure-germinated spore suspensions was measured by a method based on the characteristic change in the absorbance spectrum of DPA upon addition of Ca2+ (21). The total DPA content of the spores was determined after autoclaving the pressure-germinated spore suspensions to completely release DPA (11).
Extraction and immunoblot analysis of SASPs.
Spores (or pressure-germinated spores) were suspended in 3 ml of ice-cold 2 N HCl and 6 g of 106-μm-diameter glass beads (Sigma, St. Louis, Mo.) were added. The samples were vortexed five times for 1 min and kept on ice for 3 min between mixing periods. After 10 min on ice, the suspensions were centrifuged for 10 min at 10,000 × g. The pellets were extracted a second time with 3 ml of 2 N HCl for 30 min on ice and finally extracted twice with 2 ml of 3% acetic acid for 20 min at room temperature. The combined HCl and acetic acid extracts were dialyzed in Spectrapor no. 3 tubing (molecular weight cutoff, 3,500) against 1% acetic acid for 62 h at 8°C and lyophilized. The dry residues were dissolved in a small volume of sample buffer containing 10 mM Tris-HCl (pH 7.5), 1 mM EDTA, 2.5% sodium dodecyl sulfate, 0.01% bromophenol blue, and 1% dithiothreitol. The samples were run on a sodium dodecyl sulfate-polyacrylamide gel (PhastGel Homogeneous 20; Pharmacia, Uppsala, Sweden). The proteins on the gel were electroblotted onto a polyvinylidene difluoride microporous membrane (Immobilon-P Transfer Membrane; Millipore, Bedford, Mass.). SASPs on the polyvinylidene difluoride membrane were then detected by using rabbit antisera against B. subtilis SASP α plus β or B. subtilis SASP γ obtained from P. Setlow (University of Connecticut Health Center) and peroxidase-coupled swine anti-rabbit immunoglobulins (Dako a/s, Glostrup, Denmark).
Measurement of ATP.
The generation of ATP in pressure-germinated spore suspensions was analyzed by using a firefly luciferase assay (HY-LiTE; Merck, Darmstadt, Germany). The ATP content of germinated spores was expressed as the ratio of the number of relative light units after germination to the number of relative light units before germination.
Reproducibility of results.
All experiments were done at least in triplicate. The data presented are either means of three replicate experiments or from a single representative experiment.
RESULTS
Germination and inactivation of B. subtilis spores by high pressure.
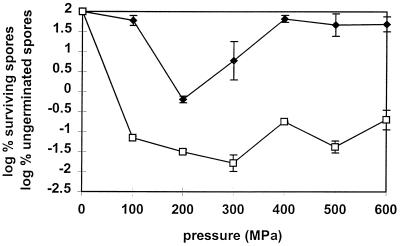
To study the pressure dependence of spore germination in B. subtilis spores, spore suspensions were subjected to different constant pressures (100 to 600 MPa) at 40°C for 30 min. Germination and inactivation were determined by plating heat-treated and unheated spore suspensions. The fraction of ungerminated spores was almost independent (±0.5 log unit) of pressure in the pressure range studied (Fig. 1). However, the fraction of survivors showed a minimum at around 200 MPa, while at >400 MPa there was almost no inactivation in spite of considerable germination. This suggested that spores germinated at high pressure would be less sensitive to pressure than those germinated at low pressure. At 100 MPa, we also observed considerable germination but negligible inactivation. However, in this case, the most likely explanation is that a pressure of 100 MPa is too low to have a lethal effect on the germinated spores, since this level of pressure is also generally insufficient to kill vegetative bacteria (4, 20).
FIG. 1.
Surviving spores (⧫) and ungerminated spores (□) of B. subtilis after treatment at different pressures for 30 min at 40°C.
In the next set of experiments, we tried to confirm the different pressure sensitivities of spores germinated at low and at high pressures, and we compared the sensitivities of these germinated spores to some other lethal agents.
Sensitivity of spores germinated at low versus high pressure. (i) Pressure sensitivity.
The sensitivity of spores germinated at 100 and 500 MPa for 30 min to an increase in pressure to 600 MPa for 10 min was investigated. Pressure was not released to atmospheric pressure before the upshift. Approximately the same level of germination was reached at 100 and 500 MPa, and the upshift to 600 MPa caused little or no additional germination in either case. However, spores germinated at 100 MPa were very sensitive to inactivation at 600 MPa, while spores germinated at 500 MPa remained almost unaffected (Table 1). This result confirmed that while the same level of germination was obtained at low (100 MPa) and high (500 to 600 MPa) pressures, the germinated spores obtained under both conditions differed in sensitivity to pressure killing.
TABLE 1.
Sensitivity of B. subtilis spores to a pressure of 600 MPa after pregermination at 100 or 500 MPa
| Pressure treatment (MPa) (time [min]) | Log percentage of:
|
|
|---|---|---|
| Surviving spores | Ungerminated spores | |
| 100 (30) | 0.92 | −2.16 |
| 100 (30) + 600 (10) | −1.83 | −2.63 |
| 500 (30) | 1.47 | −1.48 |
| 500 (30) + 600 (10) | 1.38 | −1.19 |
(ii) Heat sensitivity.
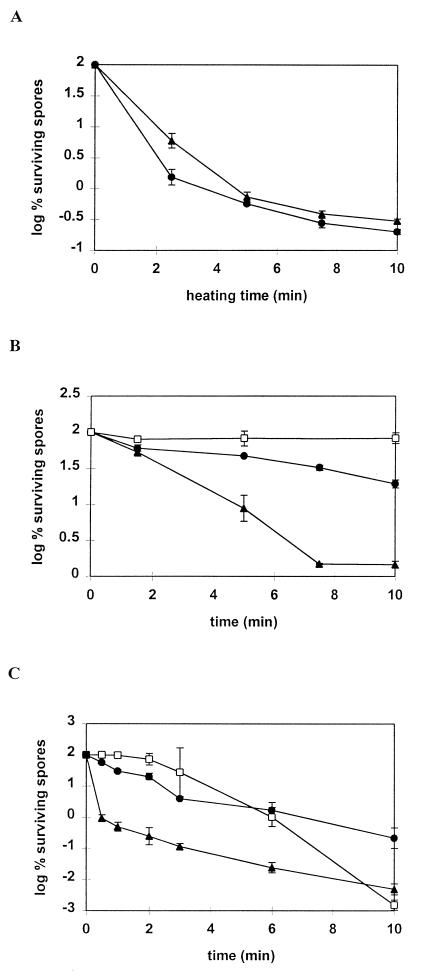
Germination renders spores sensitive to heat. While ungerminated spores survived treatment at 80°C for 15 min (results not shown), pressure-germinated spores were rapidly inactivated at 55°C (Fig. 2A). However, there was no significant difference in the inactivation rate at 55°C for spores germinated at 100 or 600 MPa.
FIG. 2.
Inactivation by heat (55°C) (A), hydrogen peroxide (0.4 M) (B), or UV light (C) of ungerminated B. subtilis spores (□) and spores germinated at 100 (▴) and 600 (•) MPa.
Decimal reduction times could not be calculated because inactivation did not proceed according to first-order kinetics.
(iii) Hydrogen peroxide sensitivity.
Inactivation of ungerminated spores and spores germinated at 100 and 600 MPa in 0.4 M H2O2 was monitored for 10 min (Fig. 2B). The viability of the ungerminated spores was not reduced by this treatment. The 100-MPa-germinated spores were inactivated significantly faster (decimal reduction time D = 4.1 ± 0.4 min) than the 600-MPa-germinated spores (D = 14.7 ± 2.1 min), at least during the first 8 min. After that, a fraction of more resistant cells (log % CFU = 0.16) remained in the 100-MPa-treated suspension, that was still 10 times larger than the fraction of ungerminated spores (log % CFU = −0.83). Thus, spores germinated at 100 MPa are more sensitive to H2O2 than are those germinated at 600 MPa, except for a small fraction that remains rather resistant in spite of being germinated.
(iv) UV sensitivity.
Ungerminated spores were inactivated by UV treatment after a lag phase of 2 min (Fig. 2C). Spores germinated at 600 MPa did not show a lag phase but were more resistant to UV than were the ungerminated spores after their lag phase. The inactivation of spores germinated at 100 MPa occurred at a very high rate during the first 30 s (2-log CFU decrease) and then continued at approximately the same rate as for the 600-MPa-germinated spores. As was the case with the H2O2 inactivation, the more UV-resistant fraction of spore populations treated at 100 MPa (log % CFU = −0.03) was larger than the fraction of ungerminated spores (log % CFU = −0.56).
Physiological processes during pressure-induced germination at low versus high pressure. (i) Release of DPA.
DPA release from the spores was measured after pressure-induced germination at 40°C for 60 min at 100 and 600 MPa. Considerable DPA release occurred with both treatments, but a slightly higher value was noted for the 100-MPa treatment (97.5% of the total spore DPA content) than for the 600-MPa treatment (87.0% of the total spore DPA content). Since the percentage of ungerminated spores was less than 0.1% in both cases, this difference was not due to a difference in germination efficiency.
(ii) Degradation of SASPs.
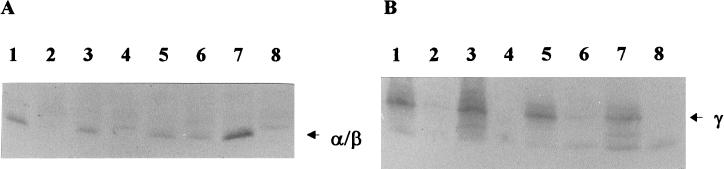
The degradation of SASPs during pressure-induced germination was studied by immunoblot analysis of spore suspensions subjected to pressure treatment at 100 or 600 MPa and 40°C for 60 min (Fig. 3).
FIG. 3.
Immunoblot detection of α/β-type (A) and γ-type (B) SASPs in extracts of B. subtilis spores. Lanes: 1 to 4, HCl extracts; 5 to 8, acetic acid extracts. (A) Lanes: 1 and 5, ungerminated PS832 (wild type); 2 and 6, 100-MPa-germinated PS832; 3 and 7, 600-MPa-germinated PS832; 4 and 8, ungerminated PS356 (SASP α−β−). (B) Lanes: 1 and 5, ungerminated PS832; 2 and 6, 100-MPa-germinated PS832; 3 and 7, 600-MPa-germinated PS832; 4 and 8, ungerminated PS483 (SASP γ−).
After pressure treatment at 100 MPa, no α/β-type SASPs were found in the HCl extracts, while a low level was found in the acetic acid extracts. However, after pressure treatment at 600 MPa, α/β-type SASPs were found in the HCl extracts and in the acetic acid extracts. The amount of α/β-type SASPs in the acetic acid extracts was much greater after the 600-MPa treatment than after the 100-MPa treatment.
No γ-type SASPs were found in the HCl extracts or in the acetic acid extracts after pressure treatment at 100 MPa, while after treatment at 600 MPa, γ-type SASPs were found in both extracts.
Since both pressure treatments resulted in a high level of germination (>95%), we can conclude that γ-type SASPs and most of the α/β-type SASPs are degraded in spores germinated at 100 MPa, while SASP degradation is inhibited or delayed in spores germinated at 600 MPa.
A noteworthy observation that was made in these experiments concerns the extraction of the SASPs. As can be seen in Fig. 3, the major amount of SASPs from ungerminated spores was extracted in the first extraction with HCl and only a minor amount was extracted subsequently with acetic acid. For germinated spores in contrast, the acetic acid extract contained the greatest amount of SASPs. Therefore, the SASP contents of spores before and after germination should not be quantitatively compared by these methods.
(iii) Generation and subsequent consumption of ATP.
Immediately after pressure treatment, i.e., 10 min after pressure release, ATP levels in spore suspensions treated at 100 MPa were considerably increased (35-fold-increased luminescence signal) compared to those of untreated suspensions, whereas no change in ATP levels was found after treatment at 600 MPa. During further storage in potassium phosphate buffer, the ATP content in spores germinated at 100 MPa decreased rapidly during the first hour after pressurization and stabilized at a value about sixfold the value for ungerminated spores. In spores germinated at 600 MPa, the ATP content increased very slowly after pressurization, being about doubled after 200 min.
The role of SASPs in the pressure resistance of spores.
The finding that SASPs are not degraded upon germination at 600 MPa can explain the higher UV and H2O2 resistance of 600-MPa-germinated spores compared to spores germinated at 100 MPa (see Discussion) but may also explain the higher pressure resistance of the former. To investigate this hypothesis, B. subtilis PS832 (wild type), PS356 (SASP α−β−), PS483 (SASP γ−), and PS482 (SASP α−β−γ−) spore suspensions were subjected to pressure treatment at 600 MPa and 40°C for 120 min. Germination and inactivation were determined by plating heat-treated and unheated spore suspensions (Table 2). All of the strains showed a high level of germination (log % CFU = −3), while high-pressure inactivation of the SASP α−β−-mutant and the SASP α−β−γ−-mutant was significantly (P < 0.05) higher than inactivation of the wild type and the SASP γ−-mutant. Thus, α/β-type SASPs seem to provide some protection from high-pressure inactivation of germinated spores.
TABLE 2.
Inactivation and germination of spores of B. subtilis SASP mutants after pressure treatment for 120 min at 600 MPa and 40°C
| Strain | Log percentage
|
|
|---|---|---|
| Surviving spores | Ungerminated spores | |
| PS832 (wild type) | 0.60 ± 0.3 | −2.9 ± 0.3 |
| PS356 (SASP α−β−) | −0.36 ± 0.3 | −3.3 ± 1.0 |
| PS483 (SASP γ−) | 0.55 ± 0.3 | −3.1 ± 0.2 |
| PS482 (SASP α−β−γ−) | −0.80 ± 0.6 | −3.8 ± 0.5 |
DISCUSSION
It has been reported by several groups in the 1960’s and 1970’s that under particular conditions, the inactivation of bacterial spores by high pressure occurs faster at moderate pressure (200 to 300 MPa) than at high pressure (>500 MPa) (5, 7, 19). Since a similar pressure response was also, in many cases (particularly at temperatures below 35°C), observed for pressure-induced germination, a model was proposed in which pressure would cause spores to germinate and lose their pressure resistance, and the germinated spores would subsequently be inactivated by pressure. However, some of the reported data did not support this simple model. For instance, at 45 to 55°C, germination of B. coagulans increased with pressure without showing a maximum, whereas inactivation decreased at pressures above 200 to 300 MPa (8, 20). Apparently, high pressure did not inactivate all of the spores it caused to germinate.
In this work, we have observed a similar discrepancy for B. subtilis. At 40°C, the degree of pressure-induced germination was roughly constant between 100 and 600 MPa, while inactivation was maximal at 200 MPa (Fig. 1) and almost nonexistent at 600 MPa. Sensitivity of pressure-germinated spores to inactivation by high pressure (600 MPa) was subsequently shown to depend on the pressure used to induce germination. Spores germinated at low pressure (100 MPa) were much more sensitive to pressure inactivation at 600 MPa than were spores germinated at a higher pressure (500 MPa) (Table 1). This can only be explained by postulating a difference in the germination process occurring at 100 versus 500 or 600 MPa, leading to qualitatively different germinated spores.
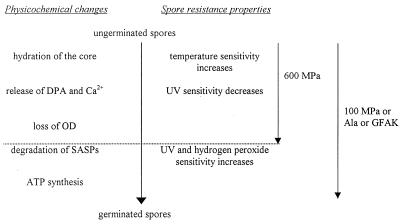
Nutrient-induced germination has been well described and entails a complex chain of events, leading gradually to more-sensitive spores (Fig. 4) (15, 19, 22, 23, 25). To identify which events occur during pressure-induced germination, the sensitivity of the 100- and 600-MPa-germinated spores to a number of lethal agents was further investigated, as well as the physiological processes occurring during pressure-induced germination at 100 and 600 MPa. For heat sensitivity, no significant differences were observed (Fig. 2A). Since the most important factor believed to determine spore heat resistance is the hydration of the core (25), this result suggests that spores germinated at different pressures have been hydrated to similar degrees.
FIG. 4.
Scheme of events occurring during nutrient-induced germination (compiled from references 15, 19, 22, 23, and 25 and our own results). As indicated by the dotted lines, our results suggest that 600-MPa treatment causes an incomplete germination process. OD, optical density; GFAK, glucose, fructose, asparagine, and potassium.
On the other hand, spores germinated at 100 MPa were much more sensitive to both hydrogen peroxide and UV light than were spores germinated at 600 MPa (Fig. 2B and C). Since the α/β-type SASPs protect spore DNA from various types of damage caused by UV light and hydrogen peroxide (15, 23–25), these results are in agreement with the finding that SASPs are degraded in 100-MPa-germinated spores but not in 600-MPa-germinated spores (Fig. 3). A possible explanation for the absence of SASP degradation during pressure-induced germination at 600 MPa is that the germination protease that is responsible for SASP degradation (24) may be inactivated at high pressure.
UV resistance of spores is known to be also affected by DPA. DPA was shown to sensitize spore DNA to UV light-induced mutations (22). During nutrient-induced germination, spores lose DPA before SASPs are degraded, which explains why spores in the intermediate germination stage are more resistant to UV light than are dormant spores (24). The release of DPA together with the inhibition of SASP degradation upon pressure-induced germination at 600 MPa then explains why 600-MPa-germinated spores are more resistant to UV light than are ungerminated spores (Fig. 2C).
Finally, the finding that 600-MPa-germinated spores are more sensitive to hydrogen peroxide than are ungerminated spores (Fig. 2B) may be related to the hydration of the core of 600-MPa-germinated spores (18).
An interesting issue is the high-pressure inactivation resistance of the 600-MPa-germinated spores compared to the 100-MPa-germinated spores. One of the prime reasons for the resistance of dormant spores to high-pressure inactivation (at low temperature when no germination takes place) is believed to be the dehydrated state of the core (6, 27). Spore mineralization has also been demonstrated to influence spore heat resistance (3) and pressure-induced germination (2), but its role in pressure resistance remains unclear. Another possibility that was investigated here is that pressure resistance in the 600-MPa-treated spores would be caused by SASPs, which are not degraded under this condition. The results from a comparison of the pressure resistances of spores from various SASP-deficient mutants (Table 2) suggest a slight but significant contribution of α/β-type SASPs to pressure resistance in 600-MPa-germinated spores. γ-type SASPs appear to play no role. Other factors than the α/β-type SASPs are likely to be involved because the mutants lacking α/β-type SASPs were still more resistant to 600-MPa treatment than were wild-type spores pregerminated at 100 MPa.
Whatever the reasons for their high-pressure inactivation resistance, the observation that the 600-MPa-germinated spores are at the same time very heat sensitive but pressure resistant is remarkable in its own right, because vegetative bacterial cells have been generally assumed to be necessarily heat and pressure sensitive (6, 12, 14). Our results reported here, together with results reported earlier for Escherichia coli (9), strongly suggest that there is no necessary link between heat and pressure resistance, and this may have important consequences for the use of high pressure as a nonthermal preservation process.
The lack of SASP degradation may indicate that the germination process that is initiated at 600 MPa is arrested at some early stage. Further evidence supporting this view was found by studying the generation of ATP during germination. During the first minutes of nutrient-induced germination, ATP is synthesized from 3-phosphoglycerate that is present in the dormant spore (25). Pressure-induced germination at 100 MPa was also accompanied by rapid ATP generation, while no ATP formation was detected during pressure-induced germination at 600 MPa. Taken together with the results from SASP degradation, this indicates that pressure-induced germination at 100 MPa initiates a number of enzymatic reactions in a similar way as during nutrient-induced germination. During 600-MPa germination, at least two of these enzymatic reactions do not occur, and a possible explanation is that some key enzymes are inactivated at high pressure.
ACKNOWLEDGMENTS
This work was supported by grants from the Belgian Fund for Scientific Research (N.F.W.O.G.0189.95) and the K. U. Leuven Onderzoeksfonds (OT/94/19). E.W. received a grant from the Flemish Institute for the Promotion of Scientific-Technological Research (I. W. T.)
We thank P. Setlow for generously providing antibodies and strains.
REFERENCES
- 1.Basset J, Macheboeuf MA. Etude sur les effets biologiques des ultrapressions: résistance des bactéries, des diastases et des toxines aux pressions très élevées. C R Hebd Seances Acad Sci Ser D. 1932;196:1431–1438. [Google Scholar]
- 2.Bender G R, Marquis R E. Sensitivity of various salt forms of Bacillus megaterium spores to the germination action of hydrostatic pressure. Can J Microbiol. 1982;28:643–649. [Google Scholar]
- 3.Bender G R, Marquis R E. Spore heat resistance and specific mineralization. Appl Environ Microbiol. 1985;50:1414–1421. doi: 10.1128/aem.50.6.1414-1421.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butz P, Ries J, Traugott U, Weber H, Ludwig H. Hochdruckinaktivierung von Bakterien und Bakteriensporen. Pharm Ind. 1990;52:487–491. [Google Scholar]
- 5.Clouston J G, Wills P A. Initiation of germination and inactivation of Bacillus pumilus spores by hydrostatic pressure. J Bacteriol. 1969;97:684–690. doi: 10.1128/jb.97.2.684-690.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould G W. The microbe as a high pressure target. In: Ledward D A, Johnston D E, Earnshaw R G, Hasting A P M, editors. High pressure processing of foods. Nottingham, England: Nottingham University Press; 1995. pp. 27–35. [Google Scholar]
- 7.Gould G W, Sale A J H. Initiation of germination of bacterial spores by hydrostatic pressure. J Gen Microbiol. 1970;60:335–346. doi: 10.1099/00221287-60-3-335. [DOI] [PubMed] [Google Scholar]
- 8.Gould G W, Sale A J H. Role of pressure in the stabilization and destabilization of bacterial spores. Symp Soc Exp Biol. 1972;26:147–157. [PubMed] [Google Scholar]
- 9.Hauben K J A, Bartlett D H, Soontjens C C F, Cornelis K, Wuytack E Y, Michiels C W. Escherichia coli mutants resistant to inactivation by high hydrostatic pressure. Appl Environ Microbiol. 1997;63:945–950. doi: 10.1128/aem.63.3.945-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa I, Kanno T, Yoshiyama K, Fujio Y. Oscillatory compared with continuous high pressure sterilization on Bacillus stearothermophilus spores. J Food Sci. 1994;59:164–167. [Google Scholar]
- 11.Janssen F W, Lund A J, Anderson L E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958;127:26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- 12.Knorr D. Hydrostatic pressure treatment of food: microbiology. In: Gould G W, editor. New methods of food preservation. Glasgow, Scotland: Blackie Academic and Professional; 1995. pp. 159–175. [Google Scholar]
- 13.Larson W P, Hartzell T B, Diehl H S. The effect of high pressures on bacteria. J Infect Dis. 1918;22:271–279. [Google Scholar]
- 14.Marquis R E. High-pressure microbial physiology. Adv Microb Physiol. 1976;14:159–241. doi: 10.1016/s0065-2911(08)60228-3. [DOI] [PubMed] [Google Scholar]
- 15.Mason J M, Setlow P. Essential role of small, acid-soluble spore proteins in the resistance of Bacillus subtilis spores to UV light. J Bacteriol. 1986;167:174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mozhaev V V, Lange R, Kudryashova E V, Balny C. Application of high hydrostatic pressure for increasing activity and stability of enzymes. Biotechnol Bioeng. 1996;52:320–331. doi: 10.1002/(SICI)1097-0290(19961020)52:2<320::AID-BIT12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama A, Yano Y, Kobayashi S, Ishikawa M, Sakai K. Comparison of pressure resistance of spores of six Bacillus strains with their heat resistances. Appl Environ Microbiol. 1996;62:3897–3900. doi: 10.1128/aem.62.10.3897-3900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popham D L, Sengupta S, Setlow P. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA-binding proteins. Appl Environ Microbiol. 1995;61:3633–3638. doi: 10.1128/aem.61.10.3633-3638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russell AD, Chopra I. Understanding antibacterial action and resistance. Chichester, England: Ellis Horwood; 1990. [Google Scholar]
- 20.Sale A J H, Gould G W, Hamilton W A. Inactivation of bacterial spores by hydrostatic pressure. J Gen Microbiol. 1970;60:323–334. doi: 10.1099/00221287-60-3-323. [DOI] [PubMed] [Google Scholar]
- 21.Scott I R, Ellar D J. Study of calcium dipicolinate release during bacterial spore germination by using a new, sensitive assay for dipicolinate. J Bacteriol. 1978;135:133–137. doi: 10.1128/jb.135.1.133-137.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setlow B, Setlow P. Dipicolinic acid greatly enhances production of spore photoproduct in bacterial spores upon UV irradiation. Appl Environ Microbiol. 1993;59:640–643. doi: 10.1128/aem.59.2.640-643.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setlow B, Setlow P. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl Environ Microbiol. 1993;59:3418–3423. doi: 10.1128/aem.59.10.3418-3423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- 25.Setlow P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. J Appl Bacteriol Symp Suppl. 1994;76:49S–60S. doi: 10.1111/j.1365-2672.1994.tb04357.x. [DOI] [PubMed] [Google Scholar]
- 26.Sojka B, Ludwig H. Pressure-induced germination and inactivation of Bacillus subtilis spores. Pharm Ind. 1994;56:660–663. [Google Scholar]
- 27.Timson W J, Short A J. Resistance of microorganisms to hydrostatic pressure. Biotechnol Bioeng. 1965;7:139–159. [Google Scholar]