Abstract
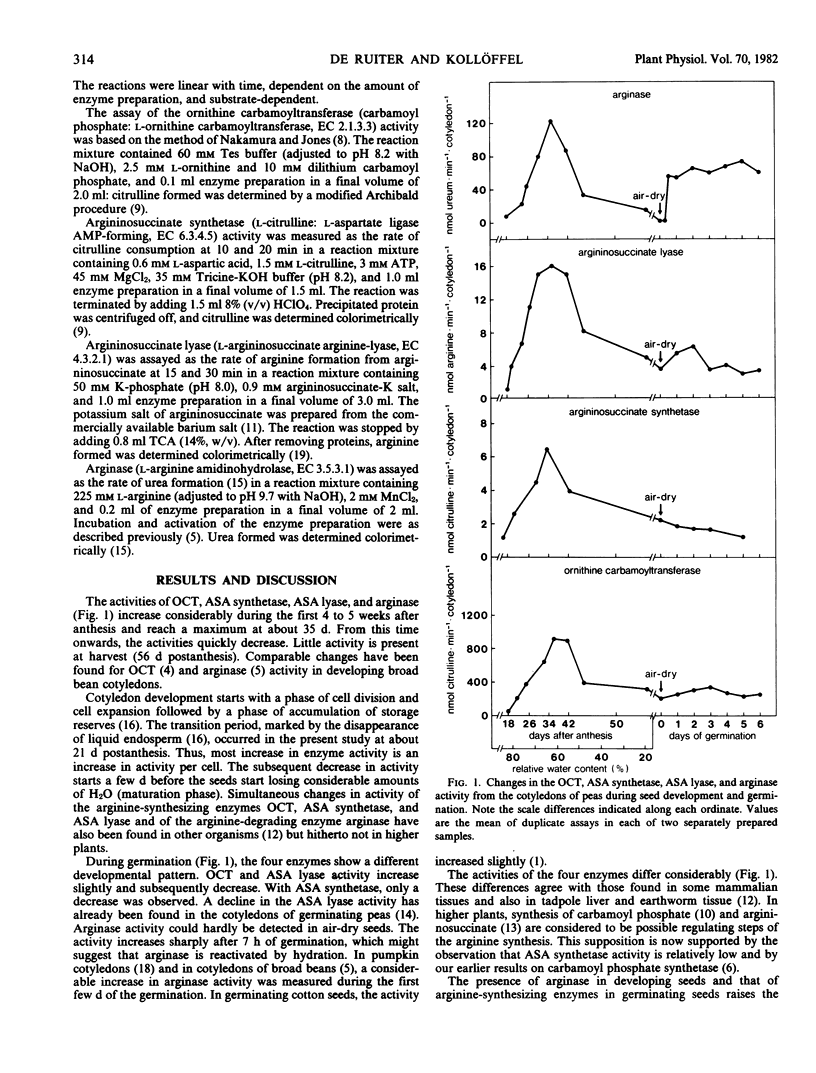
Ornithine carbamoyltransferase, argininosuccinate synthetase, argininosuccinate lyase, and arginase activity were measured in extracts from cotyledons of developing and germinating seeds of Pisum sativum L. The course of activity of these four urea cycle enzymes showed a similar pattern during seed development. The activity per cotyledon increased sharply initially and reached a maximum about 5 weeks after anthesis, when the relative water content of the seeds was about 60%. About 8 weeks after anthesis, the seeds were mature (air-dry) and had enzyme activities which were much lower. The activities of the enzymes differed considerably. Ornithine carbamoyltransferase showed the highest activity, followed in order of decreasing activity by arginase, argininosuccinate lyase, and finally argininosuccinate synthetase.
The course of the activity of the four enzymes was different during germination. Arginase activity increased sharply 7 hours after the onset of germination and remained at a constant level during the following days. Argininosuccinate synthetase activity decreased; the other enzymes showed a small increase in activity and a subsequent decrease. Results are discussed in relation to the regulation of the arginine metabolism during pea seed development and germination.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dilworth M. F., Dure L. Developmental biochemistry of cotton seed embryogenesis and germination: x. Nitrogen flow from arginine to asparagine in germination. Plant Physiol. 1978 Apr;61(4):698–702. doi: 10.1104/pp.61.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollöffel C., Verkerk B. C. Carbamoyl phosphate synthetase activity from the cotyledons of developing and germinating pea seeds. Plant Physiol. 1982 Jan;69(1):143–145. doi: 10.1104/pp.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollöffel C., van Dijke H. D. Mitochondrial Arginase Activity from Cotyledons of Developing and Germinating Seeds of Vicia faba L. Plant Physiol. 1975 Mar;55(3):507–510. doi: 10.1104/pp.55.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal T. D., Naylor A. W. Some regulatory properties of pea leaf carbamoyl phosphate synthetase. Plant Physiol. 1976 Jan;57(1):23–28. doi: 10.1104/pp.57.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- Shargool P. D., Cossins E. A. Isolation and some properties of argininosuccinate lyase from a higher plant source. Can J Biochem. 1968 May;46(5):393–399. doi: 10.1139/o68-060. [DOI] [PubMed] [Google Scholar]
- Shargool P. D. The response of soy bean argininosuccinate synthetase to different energy charge values. FEBS Lett. 1973 Jul 15;33(3):348–350. doi: 10.1016/0014-5793(73)80227-3. [DOI] [PubMed] [Google Scholar]
- Splittstoesser W. E. Metabolism of arginine by aging and 7 day old pumpkin seedlings. Plant Physiol. 1969 Mar;44(3):361–366. doi: 10.1104/pp.44.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]


