Abstract
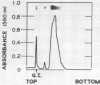
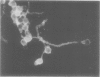
A hydroxyproline-rich glycoprotein was isolated from tobacco (Nicotiana tabacum L.) callus tissue cultures by an acidic-ethanol extraction procedure and purified to about 95% homogeneity by ion exchange chromatography on carboxymethyl cellulose. This glycoprotein agglutinated cells of an avirulent strain (B-1) of the bacterial pathogen Pseudomonas solanacearum but not its parental, virulent isolate (K-60). Bacterial lipopolysaccharide (from K-60 strain) inhibited this agglutination. The tobacco glycoprotein also agglutinated zoospores of both compatible and incompatible races of Phytophthora parasitica var. nicotianae. Although 34 potential haptens were tested, no low-molecular-weight carbohydrate that inhibited bacterial or fungal agglutination was found. The agglutination activity of the tobacco glycoprotein was sensitive to pronase and sodium periodate. The apparent molecular weight of the glycoprotein was 120,000. The protein moiety was basic (12% lysine and 5% histidine) and contained 38% hydroxyproline. The carbohydrate moiety comprised 26% (by weight) of the glycoprotein, and contained 87% arabinose, 8% galactose, and 5% glucose. The glycoprotein labeled with fluorescein isothiocyanate bound significantly better to the avirulent isolate (B-1) of P. solanacearum than to the virulent strain (K-60). Binding to the avirulent cells was inhibited by incubation in a higher ionic strength medium (e.g. 0.2 m NaCl). The labeled glycoprotein also bound to cystospores and mycelia of both races of P. parasitica var. nicotianae. This fungal-glycoprotein interaction was inhibited by the lipopolysaccharide from strain K-60 and by higher ionic strength conditions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and Secretion of Hydroxyproline-containing Macromolecules in Carrots: II. In vivo Conversion of Peptidyl Proline to Peptidyl Hydroxyproline. Plant Physiol. 1970 Feb;45(2):223–227. doi: 10.1104/pp.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland R. Distribution and metabolism of protein-bound hydroxyproline in an elongating tissue, the Avena coleoptile. Plant Physiol. 1968 Jun;43(6):865–870. doi: 10.1104/pp.43.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T. Cell Surfaces in Plant-Microorganism Interactions: I. A Structural Investigation of Cell Wall Hydroxyproline-rich Glycoproteins Which Accumulate in Fungus-infected Plants. Plant Physiol. 1979 Aug;64(2):314–319. doi: 10.1104/pp.64.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquerré-Tugayé M. T., Lafitte C., Mazau D., Toppan A., Touzé A. Cell Surfaces in Plant-Microorganism Interactions: II. Evidence for the Accumulation of Hydroxyproline-rich Glycoproteins in the Cell Wall of Diseased Plants as a Defense Mechanism. Plant Physiol. 1979 Aug;64(2):320–326. doi: 10.1104/pp.64.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. M., Albersheim P. A gas chromatographic method for the determination of aldose and uronic Acid constituents of plant cell wall polysaccharides. Plant Physiol. 1972 Jun;49(6):926–936. doi: 10.1104/pp.49.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klis F. M. Glycosylated seryl residues in wall protein of elongating pea stems. Plant Physiol. 1976 Feb;57(2):224–226. doi: 10.1104/pp.57.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARINKOVICH V. A. PURIFICATION AND CHARACTERIZATION OF THE HEMAGGLUTININ PRESENT IN POTATOES. J Immunol. 1964 Nov;93:732–741. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- RINDERKNECHT H. Ultra-rapid fluorescent labelling of proteins. Nature. 1962 Jan 13;193:167–168. doi: 10.1038/193167b0. [DOI] [PubMed] [Google Scholar]
- Stuart D. A., Varner J. E. Purification and Characterization of a Salt-extractable Hydroxyproline-rich Glycoprotein from Aerated Carrot Discs. Plant Physiol. 1980 Nov;66(5):787–792. doi: 10.1104/pp.66.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga J., Bartnicki-Garcia S. Structure and differentiation of the cell wall of Phytophthora palmivora: cysts, hyphae and sporangia. Arch Mikrobiol. 1971;79(4):293–310. doi: 10.1007/BF00424906. [DOI] [PubMed] [Google Scholar]
- Wong P. P. Interactions between Rhizobia and Lectins of Lentil, Pea, Broad Bean, and Jackbean. Plant Physiol. 1980 Jun;65(6):1049–1052. doi: 10.1104/pp.65.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]