Abstract
In this study, we compared three methods for extraction and quantification of RNA and DNA from marine sediments: (i) a spectrophotometric method using the diphenylamine assay; (ii) a fluorometric method utilizing selective fluorochromes (thiazole orange for total nucleic acids and Hoechst 33258 for DNA); and (iii) a high-pressure liquid chromatography (HPLC) method which uses a specific column to separate RNA and DNA and UV absorption of the nucleic acids for quantification. Sediment samples were collected in the oligotrophic Cretan Sea (eastern Mediterranean, from 40 to 1,540 m in depth) and compared to the distribution and composition of the benthic microbial assemblages (i.e., bacteria and microprotozoa). DNA concentrations measured spectrophotometrically and by HPLC were not significantly different, while fluorometric yields were significantly lower. Such differences appear mainly due to fact that the stain-DNA complex is strongly dependent on the DNA composition and structure. RNA concentrations determined by the three methods displayed some differences; fluorometric and spectrophotometric methods obtain RNA concentration by difference and therefore may be biased by DNA estimates. By contrast, the HPLC method provides independent assessments of RNA and DNA concentrations. We tentatively estimated the contribution of the detrital DNA to the total DNA pools in two ways. The two calculations provided quite similar results indicating that the majority of the DNA pool in the deep-sea sediments was detrital. Microbial RNA generally accounted for almost the entire sedimentary RNA pools below 100-m depth. RNA concentrations were found to decrease along the Cretan shelf and slope. The RNA/DNA ratio calculated by using fluorometric DNA concentrations was significantly correlated with values of sediment community oxygen consumption only below 100-m depth (dominated by the microbial biomass). These data suggest that the RNA/DNA ratio, based on fluorometric estimates of DNA, can be used as an indicator of benthic metabolic activity, but only when metazoan contribution to the microbial DNA is negligible.
Nucleic acid determination in marine habitats has attracted special attention because of the relationship between RNA/DNA ratios and the growth rates of a wide variety of marine organisms such as phytoplankton (3, 4, 12, 13, 44), bacteria (22, 24, 33), invertebrates (42), and fish (5, 6). Other studies of natural seawater and sediment samples have been undertaken to investigate the relationships between RNA/DNA ratio and the metabolic state of the microbial communities associated with suspended and sedimentary organic particles (9, 16). However, Jeffrey et al. (24) found no correlation between RNA/DNA ratios of natural bacterioplankton communities and other measures of metabolic activity or growth (thymidine and leucine incorporation). There are several problems which may compromise the interpretation of RNA/DNA ratios in intact sediments as a measure of benthic community metabolism. The most important of them is the presence of substantial amounts of DNA associated with dead cells and/or absorbed to particles (i.e., detrital DNA) in the DNA pools in the sediments and in the water column as well. Holm-Hansen et al. (21) first tried to use DNA concentrations as a measure for living biomass in the oceans; they found unreasonably high concentrations and postulated that most of the DNA was not associated with living cells and thus not immediately degraded. Also, Danovaro et al. (9) found a large fraction of the DNA in sediments from the deep Mediterranean to be unaccounted-for bacterial standing stocks. The significance and nature of this apparently detrital DNA is still uncertain (1).
For the determination of nucleic acids from sediments, two methods are generally used: spectrophotometric, based on the specific absorbance of nucleic acids (9, 18), and fluorometric, using specific fluorescent stains (32, 45). To date, comparative studies for nucleic acid determination have been performed with bacteria, algal cultures, and natural seawater samples (25, 37), but there are no examples of similar comparisons for nucleic acid determination in marine sediments.
In this study, for measuring RNA and DNA in marine sediments, we applied three methods which differ with respect to the nucleic acid extraction procedure. Moreover, two of the methods, referred to here for simplicity as the spectrophotometric and high-pressure liquid chromatography (HPLC) methods, use spectrophotometric quantification of nucleic acids, while the third (the fluorometric method) uses fluorescent dyes. The three procedures share the following advantages: (i) the extraction time is relatively short, allowing the processing of the large number of samples usually collected in field studies; and (ii) quantification of DNA and RNA in the same subsamples is possible, thus allowing for determination of the RNA/DNA ratio. The aims of this study were to (i) compare the results of nucleic acid determination using the above-named three methods; (ii) estimate the detrital fraction of the DNA and RNA pools in the Cretan sediments by calculating the DNA and RNA contribution by intact and stainable microbes (i.e., bacterial and protozoan densities by microscopy); and (iii) explore the relationships between the patterns of RNA/DNA ratios and sediment community oxygen consumption (SCOC).
MATERIALS AND METHODS
Study area and sampling.
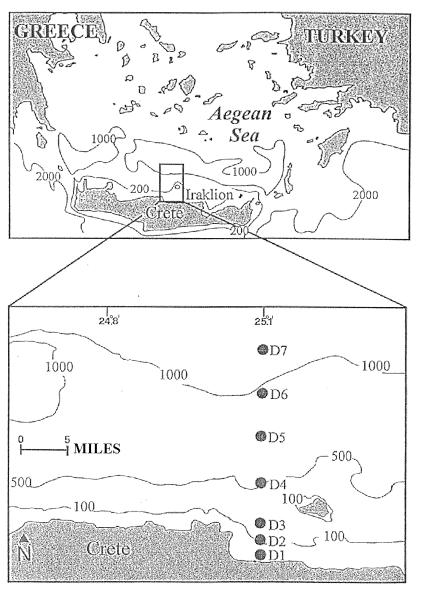
Sediment sampling was carried out on the continental shelf and slope of the north coast of Crete and in the adjacent deep basin of the Cretan Sea (south Aegean, eastern Mediterranean). This area is one of the world’s most oligotrophic seas, with a primary production as low as 20 to 30 g of C m−2 year−1 (14). The Cretan Sea is further characterized by high bottom-water temperatures (13 to 14.5°C) and strong water column stratification.
Undisturbed sediment samples were collected by using a multicorer in September 1995 at seven stations situated along a transect of depths from 40 to 1,540 m: 40, 100, 200, 500, 700, 900, and 1,540 m (Fig. 1). For nucleic acid determination, we used the top 1-cm slices of two cores, which were subsequently homogenized and deep frozen at −20°C. For bacterial and protozoan counting, three to five replicate subsamples (0.63 cm3) were collected with sterile cutoff syringes from the same cores used for nucleic acid analysis. The subsamples were fixed with filtered (0.2-μm-pore-size filter) seawater containing 2% buffered formalin and stored at 4°C for later analyses in the laboratory.
FIG. 1.
Sampling area and station locations.
Nucleic acid analysis.
Before analysis, larger macroscopic organisms were removed from the samples. All of the materials used for nucleic acid analysis were carefully cleaned by soaking in 1 N NaOH–10% HCl–MilliQ water to remove organic matter contamination and subsequently treated as described by Moran et al. (32) to avoid nuclease contamination. All of the solutions were prepared with MilliQ water and then autoclaved. Amounts of DNA and RNA were determined by the spectrophotometric, fluorometric, and HPLC methods.
For each method, internal standards of calf thymus DNA and baker’s yeast RNA (5 to 10 μg) were added to replicate subsamples before extraction. The final yields of the internal standards of DNA and RNA were on average 60 and 85% for the spectrophotometric method, 55 and 80% for the fluorometric method, and 95 and 90% for the HPLC method. DNA and RNA concentrations in the sediments were not corrected for percent recovery of the internal standards and were calculated from calibration curves of calf thymus DNA and baker’s yeast RNA prepared according to each analytical protocol. Data were normalized to sediment dry weight after desiccation (60°C, constant weight).
(i) Spectrophotometric method.
Nucleic acid extraction and measurement were done by the procedures of Zachleder (50) as applied by Danovaro et al. (9) and Danovaro (10), with a few modifications to enhance DNA extraction from the sediment. Briefly, 1 g of sediment (three replicates) was treated with 3.0 ml of 0.5 N perchloric acid, stirred for 3 min, and sonicated three times for 1 min (with intervals of 30 s). Nucleic acid extraction was carried out at 75°C for 30 min under continuous stirring. After centrifugation (3,000 × g, 10 min), the absorbance of the total nucleic acid content (TNA) in the supernatant was measured at 260 nm. DNA absorbance was determined with a diphenylamine (2% in acetic acid) light-activated reaction (40 W, 12 h) at 598 nm and converted to concentration, using standard solutions of calf thymus DNA. DNA concentration was then reported as equivalent of absorbance at 260 nm in order to calculate by difference the absorbance due to RNA: ABSRNA = ABSTNA − ABSDNA, where ABSRNA is the absorbance of RNA, ABSTNA is the absorbance of TNA, and ABSDNA is the absorbance of DNA. RNA absorbance (260 nm) was then converted to concentration, using standard solutions of baker’s yeast RNA. Since TNA absorbance at 260 nm might be affected by the interference due to inorganic compounds, we used sediment subsamples, previously treated in a muffle furnace (550°C, 4 h) as blanks. Sensitivity of the method has been tested on DNA and RNA standards (accuracy of ±1.0 μg) and appeared to be adequate for field investigations.
(ii) Fluorometric method.
Initially, nucleic acid extraction for the fluorometric determination was performed with sediments homogenized in Tris-Ca2+ buffer. This buffer provided the best recovery and the lowest variability in natural seawater samples (3). However, we found that this procedure was not effective in the extraction of both RNA and DNA from sediments. Therefore, to extract nucleic acids, we used sodium dodecyl sulfate (SDS), which is efficient in cellular lysis and is commonly used in protocols for extracting nucleic acids from marine sediments (35, 45). Nucleic acid extraction for fluorometric determination was carried out as follows: 1 g of sediment (three replicates) was added to 2 ml of 0.2 M sodium phosphate buffer containing 0.1 M EDTA (pH 8.0), stirred for 5 min, and then mixed with 2 ml of SDS (10%). The samples were incubated for 2 h at 65°C and subsequently subjected to repeated freeze-thaw steps (five times, from 65 to −80°C). Nucleic acids were extracted from the sediment by centrifuging the lysate at 12,000 × g at 10°C for 15 min. The supernatant was transferred to another tube; the pellet was resuspended in 2 ml of sodium phosphate buffer, incubated at 65°C for 15 min, and centrifuged as described above. This supernatant was added to the first and dialyzed against sterile water to remove low-molecular-weight contaminants such as SDS and salts. The volumes of the final dialysate were measured, added to absolute ethanol (ethanol volume = 3 × dialysate volume), and stored (−80°C, 2 h). After centrifugation (10,000 × g, 20 min) to precipitate nucleic acids, the pellet was washed with ethanol 70%, centrifuged again (10,000 × g for 20 min), dried under N2, and resuspended in 100 μl of MilliQ water for fluorometric analysis. Before fluorometric analysis, an aliquot of the resuspension was analyzed by gel electrophoresis to ensure the presence of nucleic acids in the extract (Fig. 2).
FIG. 2.
Agarose gel electrophoresis of sedimentary nucleic acids by extraction using the fluorometric method. Lanes: 1, lambda plasmid digested with HindIII (molecular weight marker); 4 and 5, calf thymus DNA standard (0.4 and 0.8 μg, respectively); 7, sediment sample from 1,540-m depth; 8, sediment sample from 100-m depth containing internal standard of calf thymus DNA, treated with RNase; 9, sediment sample from 100-m depth treated with RNase.
Subsamples (25 μl) were then analyzed with two fluorescent dyes, thiazole orange for TNA (26) and Hoechst 33258 (Hoechst) for DNA (37), using the procedure described by Berdalet and Dortch (3). Fluorescence was measured with a Perkin-Elmer LS50B spectrofluorometer (thiazole orange, 511-nm excitation and 533-nm emission; Hoechst, 360-nm excitation and 460-nm emission). DNA concentrations were calculated from calf thymus DNA standards stained with Hoechst. The same DNA standards were stained with thiazole orange to determine the DNA contribution to the total thiazole orange fluorescence. The RNA contribution in the thiazole orange fluorescence (FTORNA) was estimated as FTORNA = FTOTNA − FTODNA, where FTOTNA is the fluorescence of TNA after staining with thiazole orange and FTODNA is the calculated contribution of DNA in the thiazole orange fluorescence (as determined from Hoechst staining). RNA concentrations were calculated from baker’s yeast RNA standards after thiazole orange staining.
In addition, an aliquot of the samples was treated with RNase and stained with thiazole orange and Hoechst to compare the fluorescence yields obtained using the two stains on the same DNA matrix (see Discussion for comments on results).
(iii) HPLC method.
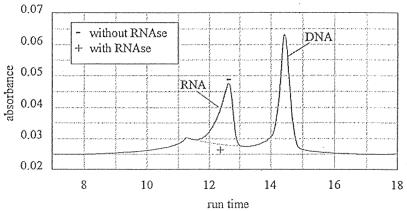
For HPLC determination of RNA and DNA, 0.2 g of sediment (three replicates) was added to 1 ml of Tris-HCl buffer (with 2% SDS and 10% EDTA) and sonicated (Soniprep 150 MSE) three times (10 s with 1-min interval). After sonication, the samples were centrifuged for 5 min (3,000 × g), and the supernatant was filtered over a 0.45-μm-pore-size cellulose acetate filter to remove particulate material. All manipulations were carried out at room temperature. The filtrate was directly injected into the HPLC system, which consisted of a Waters 600E pump and controller unit, connected via a Nucleogen 4000-7 DEAE anion-exchange column (Machery-Nagel) (8) to a Waters 994 photodiode array. In accordance with the method of Coppela et al. (8), we used a urea buffer with a KCl gradient. The elution gradient and the flow rate (Table 1) were adapted to overcome problems caused by precipitation of the Tris-HCl extraction buffer (with SDS and EDTA) when mixed with the urea buffer. Unretained material (proteins) produced a large peak at the start of the chromatogram. Figure 3 shows the chromatogram of sedimentary DNA and RNA. The identity of the peaks in the chromatogram was confirmed by inspection of the absorbance spectrum (DNA and RNA maximum absorbance at 260 nm) in combination with either coinjection of standards (calf thymus DNA and baker’s yeast RNA) or digestion of RNA with RNase. The areas of DNA and RNA peaks were integrated at 260-nm wavelength. Peak areas were converted to concentrations by using calibration curves obtained from standard solutions of calf thymus DNA and baker’s yeast RNA.
TABLE 1.
Gradient flow rate and relative composition of eluents used for HPLC determination of nucleic acids at a gradient flow rate of 1.6 ml/min
| Time (min) | Composition (%)a
|
||
|---|---|---|---|
| A | B | C | |
| 0 | 0 | 0 | 100 |
| 0.32 | 0 | 0 | 100 |
| 0.63 | 85 | 15 | 0 |
| 6.25 | 85 | 15 | 0 |
| 18.75 | 0 | 100 | 0 |
| 18.95 | 0 | 100 | 0 |
| 19.00 | 0 | 0 | 100 |
| 23.00 | 0 | 0 | 100 |
A, 300 g of urea, 1.361 g of KH2PO4, and 1.742 g of K2HPO4 in 1 liter of MilliQ water at pH 6.7 with H3PO4; B, 300 g of urea, 112 g of KCl, 1.361 g of KH2PO4, and 1.742 g of K2HPO4 in 1 liter of MilliQ water at pH 6.7 with H3PO4; C, 0.01% sodium azide in MilliQ water.
FIG. 3.
HPLC chromatogram of nucleic acids from sediment sample with and without RNase treatment.
Bacterial and protozoan analyses.
For bacterial analysis, subsamples were sonicated three times (Sonifier Branson 2200, 60 W for 1 min), diluted 100 times, stained with acridine orange (0.01%, final concentration) and filtered on black Nuclepore 0.2-μm-pore-size filters. This procedure appeared to be the most appropriate both for cell recovery and for normalization of data to sediment dry weight (31). The filters were analyzed under epifluorescence microscopy (Zeiss Universal microscope). Bacterial DNA and RNA were estimated by assuming conversion factors of 3.3 fg of DNA (41) and 4.2 fg of RNA (19) for cells of the same size encountered in this study. Data were normalized to sediment dry weight after desiccation (60°C, constant weight).
For protozoan (heterotrophic flagellates) analysis, the subsamples were diluted in prefiltered (0.2-μm-pore-size filter) seawater containing acridine orange (0.01%, final concentration) to stain the DNA and filtered onto 2.0-μm-pore-size black-stained Nuclepore filters. The filters were subsequently stained as described by Bak and Nieuwland (2) and scanned at a magnification of ×1,000. Protozoan DNA and RNA were estimated by assuming conversion factors of 3.2 pg of DNA cell−1 and 4.4 pg of RNA cell−1 (as average of the values reported for different protozoan species 30). Data were normalized to sediment dry weight after desiccation (60°C, constant weight).
RESULTS
Spectrophotometric method.
DNA and RNA concentrations and RNA/DNA ratios are reported in Table 2. DNA concentrations ranged from 39.7 ± 14.2 at 40-m depth down to 12.3 ± 8.2 μg g−1 at 1,540-m depth. The average DNA concentration (± the standard deviation [SD]) was 30.9 ± 10.6 μg g−1 (median, 37.4 μg g−1). The RNA concentration strongly decreased with water depth, from 29.9 ± 5.0 at 40 m to 0.4 ± 0.7 μg g−1 at 1,540 m, with an average of 11.1 ± 10.9 μg g−1 (median, 5.1 μg g−1). The RNA/DNA ratios showed a similarly decreasing pattern with increasing water depth and ranged from 0.96 to 0.004 (100 and 900 m, respectively).
TABLE 2.
DNA and RNA concentrations and RNA/DNA ratios at different depths
| Depth (m) | Comparison of methodsa
|
Fluorometric DNAb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a
|
b
|
c
|
|||||||||
| Mean concn ± SD (μg g−1)
|
Mean concn ± SD (μg g−1)
|
Mean concn ± SD (μg g−1)
|
|||||||||
| DNA | RNA | RNA/DNA | DNA | RNA | RNA/DNA | DNA | RNA | RNA/DNA | F/S | F/H | |
| 40 | 39.7 ± 14.2 | 29.9 ± 5 | 0.84 | 18.6 ± 0.3 | 5.7 ± 2.8 | 0.31 | 39.8 ± 4.4 | 24.1 ± 2.4 | 0.61 | 47 | 47 |
| 100 | 38.5 ± 11 | 19.1 ± 3.1 | 0.96 | 31.4 ± 1.9 | 25.0 ± 3.5 | 0.8 | 37.3 ± 0.5 | 9.7 ± 0.4 | 0.26 | 82 | 84 |
| 200 | 37.4 ± 19.3 | 20.5 ± 13.5 | 0.58 | 5.1 ± 0.4 | 5.3 ± 1 | 1.04 | 34.5 ± 3.1 | 6 ± 0.3 | 0.17 | 14 | 15 |
| 500 | 16.6 ± 4.7 | 5.1 ± 0.9 | 0.157 | 2.9 ± 0.1 | 3.6 ± 0.3 | 1.25 | 31.7 ± 3.3 | 2.9 ± 0.2 | 0.09 | 17 | 9 |
| 700 | 34 ± 5.8 | 2.2 ± 1.1 | 0.05 | 8.4 ± 0.7 | 7.5 ± 1.9 | 0.9 | 33.5 ± 2.2 | 2.8 ± 0.2 | 0.08 | 25 | 25 |
| 900 | 37.7 ± 10.4 | 0.8 ± 0.4 | 0.004 | 3.7 ± 0.4 | 6.7 ± 1.9 | 1.82 | 25.2 ± 4.3 | 2.7 ± 0.2 | 0.11 | 10 | 15 |
| 1,540 | 12.3 ± 8.2 | 0.4 ± 0.7 | 0.08 | 2.1 ± 0.5 | 4.7 ± 1.4 | 2.2 | 33.6 ± 1.2 | 1.3 ± 0.2 | 0.04 | 17 | 6 |
| Avg | 30.9 | 11.1 | 10.3 | 8.4 | 33.7 | 7.1 | 30 | 29 | |||
ANOVA results of DNA determinations by spectrophotometric (a), fluorometric (b), and HPLC (c) methods: a versus b, P < 0.01; a versus c, not significant; b versus c, P < 0.01. ANOVA results for RNA determinations: a versus b, not significant; a versus c, not significant at 40-m depth and below 200-m depth; b versus c, P < 0.05.
Contribution of fluorometric DNA concentrations (as percentage) to the spectrophotometric (F/S) and HPLC (F/H) DNA concentrations at different depths.
Fluorometric method.
DNA concentrations ranged from 18.6 ± 0.3 (40 m) to 31.4 ± 1.9 μg g−1 (100 m) in the shelf (<200-m depth) and from 8.4 ± 0.7 (700 m) and 2.1 ± 0.5 μg g−1 (1,540 m) in the slope and deep basin (Table 2). The average DNA concentration (±SD) was 10.3 ± 10.1 μg g−1 (median, 5.1 μg g−1). Also, the highest RNA concentrations (25 ± 3.5 μg g−1) were found at 100-m depth, whereas in the other stations, RNA concentrations varied in a narrow range (from 3.6 ± 0.3 to 7.5 ± 1.9 μg g−1 [Table 2]). The average RNA concentration was 8.4 ± 6.9 μg g−1 (median, 5.7 μg g−1). The RNA/DNA ratio showed an increase with depth, from 0.3 at 40-m depth to 2.15 at 1,540-m depth.
HPLC method.
DNA concentrations showed relatively little variation, ranging between 39.8 ± 4.4 μg g−1 at 40-m depth and 25.2 ± 4.3 μg g−1 at 900-m depth (Table 2). The average DNA concentration (±SD) was 33.6 ± 4.3 μg g−1 (median, 33.6 μg g−1). In contrast to DNA, concentrations of RNA showed a clear decrease with depth, from 24.1 ± 2.4 μg g−1 at 40-m depth to 1.3 ± 0.2 μg g−1 at 1,540-m depth (Table 2). The average RNA concentration was 7.0 ± 7.4 μg g−1 (median, 2.9 μg g−1). The RNA/DNA ratios showed a similar decreasing trend, from 0.6 to 0.04 (depths of 40 and 1,540 m, respectively).
Bacteria and protozoa.
The density distribution of bacteria in sediment samples is reported in Table 3. The highest bacterial density (3.48 × 108 g−1) was observed at 100-m depth. From 100 m downward, bacterial densities showed a general decline with increasing water depth, reaching the lowest value at 1,540 m (1.02 × 108 g−1). The average bacterial density was 2.66 × 108 g−1.
TABLE 3.
Bacterial and protozoan density in the top 1 cm of sediment at different water depths
| Depth (m) | Total density (Mean ± SDa)
|
Estimated concn (μg g−1)
|
Contribution of M-DNAb
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria (108) | Protozoa (105) | Bacterial
|
Protozoan
|
M-DNA/S-DNA | M-DNA/F-DNA | M-DNA/H-DNA | M-RNA/S-RNA | M-RNA/F-RNA | M-RNA/H-RNA | |||
| DNA | RNA | DNA | RNA | |||||||||
| 40 | 2.34 ± 0.24 | 8.53 ± 4.41 | 0.77 | 0.98 | 2.73 | 3.75 | 9 | 19 | 9 | 16 | 83 | 20 |
| 100 | 3.48 ± 0.43 | 11.91 ± 0.77 | 1.15 | 1.46 | 3.81 | 5.24 | 13 | 16 | 13 | 35 | 27 | 69 |
| 200 | 3.4 ± 1.08 | 14.35 ± 5.84 | 1.12 | 1.43 | 4.59 | 6.31 | 15 | 112 | 17 | 38 | 146 | 129 |
| 500 | 2.88 ± 0.63 | 10.99 ± 1.84 | 0.95 | 1.21 | 3.52 | 4.84 | 27 | 154 | 14 | 119 | 168 | 209 |
| 700 | 3.29 ± 0.33 | 11.96 ± 0.82 | 1.09 | 1.38 | 3.83 | 5.26 | 14 | 59 | 15 | 302 | 89 | 237 |
| 900 | 2.22 ± 0.49 | 11.24 ± 2.01 | 0.73 | 0.93 | 3.60 | 4.95 | 11 | 117 | 17 | 735 | 88 | 218 |
| 1,540 | 1.02 ± 0.11 | 8.86 ± 1.03 | 0.34 | 0.43 | 2.84 | 3.90 | 26 | 151 | 9 | 1,082 | 92 | 333 |
| Avg | 2.66 | 11.12 | 0.88 | 1.12 | 3.56 | 4.89 | 16 | 90 | 13 | 332 | 99 | 174 |
Calculated without log transformation.
Contribution of microbial DNA (M-DNA as sum of bacterial and protozoan DNAs) and microbial RNA (M-RNA as sum of bacterial and protozoan RNAs) to the total DNA and RNA pools determined by the three methods: S-DNA and S-RNA (spectrophotometric); F-DNA and F-RNA (fluorometric); and H-DNA and H-RNA (HPLC). Values are expressed as percentages.
As for bacterial density, protozoan density (Table 3) was low at the shallow 40-m station and increased toward the shelf edge (200 m), where highest densities (14.4 × 105 g−1) were found. At greater depths, protozoan densities dropped again, reaching a level of 8.9 × 105 g−1 at 1,540 m. The average protozoan density was 11.12 × 105 g−1.
The concentration of bacterial DNA estimated on the basis of conversion factors (see Materials and Methods) ranged from 1.15 to 0.34 μg of DNA g−1, and that of bacterial RNA ranged from 1.46 to 0.43 μg of RNA g−1 (Table 3). The average concentrations of bacterial DNA and RNA were 0.88 and 1.12 μg g−1, respectively. The estimated concentration of protozoan DNA varied between 2.73 and 4.59 μg of DNA g−1, and that of protozoan RNA ranged between 3.75 and 6.31 μg of RNA g−1 (Table 3). The average concentrations of protozoan DNA and RNA were 3.56 and 4.89 μg g−1, respectively.
Using the estimates of nucleic acid concentrations derived from microscopic counts, we calculated DNA and RNA microbial (bacterial plus protozoan) contribution to the total pools of the two nucleic acids determined by the different methods (Table 3). Microbial DNA of intact cells (as determined by epifluorescence microscopy) accounted for 16, 90, and 13% of the total DNA concentrations as measured by the spectrophotometric, fluorometric, and HPLC methods, respectively. Microbial RNA accounted on average for 332, 99, and 174% of the total RNA concentrations determined spectrophotometrically, fluorometrically, and by HPLC, respectively. In many samples, especially those from deeper stations, the estimated microbial RNA concentration greatly exceeded the total RNA concentrations measured spectrophotometrically and by HPLC. The contribution of microbial RNA to the RNA pools determined fluorometrically surpassed 100% only at depths of 200 and 500 m but was close to 90% in the other deep-sea stations.
DISCUSSION
Comparison of methods for DNA estimates.
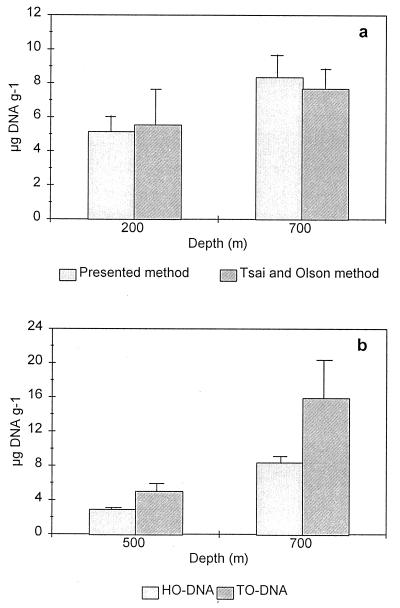
DNA concentrations measured spectrophotometrically and by HPLC in the seven stations of the Cretan Sea were not significantly different (analysis of variance [ANOVA] with replication; F = 0.89, P = 0.35 [i.e., all stations with all replicates]). By contrast, fluorometric DNA concentrations were significantly different, i.e. lower, than spectrophotometric and HPLC measurements (ANOVA; F = 49.3, P < 0.01 and F = 822.75, P < 0.01, respectively). Possible explanations for these discrepancies concern the extraction phase as well as the detection phase of the three methods. The importance of the extraction step is illustrated by a comparison that we made between the DNA yields obtained by the fluorometric method and by the procedures of Tsai and Olson (45) and of Thiel and Higgins (43) (both used a fluorometric determination). The results obtained with the Tsai and Olson (45) method, specific for sedimentary DNA isolation, were very close to those of our fluorometric method (Fig. 4a), while the simple mortar homogenization in the Thiel and Higgins (43) method yielded 10 to 20-times-lower DNA concentrations (data not shown) even though it most likely causes the least damage to DNA molecules. Leff et al. (28) compared different extraction procedures (23, 34, 45) on stream sediments and recommended the Tsai and Olson (45) procedure if purity and the presence of eukaryotic DNA are of concern (as in our case) but found highest yields with the method of Ogram et al. (34). The percentages of internal DNA standard recovered by our spectrophotometric and fluorometric methods were almost equal, suggesting that there is little difference between the extraction efficiencies of dissolved (i.e., standard) DNA. This, however, does not imply that the methods are also equally efficient in the release of bound DNA (in cells or particles). However, because we found similar DNA values with the Tsai and Olson (45) method and our fluorometric method, there is little reason to assume that our method is not efficient in the release of DNA. Therefore, extraction alone cannot explain the lower fluorometric yields.
FIG. 4.
Comparison of two methods for fluorometric determination of DNA. (a) Comparison with the Tsai and Olson (45) extraction procedure; (b) comparison between DNA measured with thiazole orange after treatment with RNase (HTO-DNA) and with Hoechst without RNase treatment (HO-DNA).
A more likely explanation for the significant DNA yield discrepancies is that while the diphenylamine and HPLC determinations are relatively unaffected by DNA base composition and DNA molecular structure (25), fluorescence yield of the Hoechst-DNA complex is strongly dependent on the composition and structure of DNA. Hoechst fluorescence is higher in AT-rich DNA than in GC-rich DNA (33). Therefore, when DNA is quantified with Hoechst, concentrations appear to be affected by differences between the base composition of the sample DNA and the standard DNA (calf thymus; 60% A + T [7]). In this regard, Paul and Myers (37) assumed for natural heterogeneous bacterial communities the same percent A + T of the calf thymus DNA. However, if the actual microbial A + T content in our sediments were lower, this method would underestimate the concentration of microbial DNA.
Furthermore, Hoechst is a groove-binding DNA ligand that becomes brightly fluorescent when it binds to the double-stranded B form of the DNA (29) that represents the biologically active form of DNA. Paul (36) reported that denatured DNA gives a low contribution to the Hoechst-DNA fluorescence, while Fara et al. (17) found that DNA from seawater samples, after treatment with DNase, produced a negligible fluorescence with Hoechst and an 80% reduced fluorescence with thiazole orange. We measured the Hoechst fluorescence yields with sedimentary DNA extracted in perchloric acid (as in the spectrophotometric protocol in which the diphenylamine reacts with hydrolyzed DNA) and adjusted to pH 7.8, and we found a fluorescence similar to the background signal, meaning that Hoechst did not bind the hydrolyzed DNA. It is therefore likely that when the DNA molecule structure is altered, formation of the Hoechst-DNA complex is compromised and thus the fluorescence is greatly reduced.
The different affinities of Hoechst and diphenylamine with respect to hydrolyzed DNA could explain differences found in our samples and in other natural seawater samples assessed fluorometrically and spectrophotometrically (25, 37, 38). Therefore, it might be expected that in natural samples containing large amounts of denatured, damaged, and/or hydrolyzed DNA (i.e., detrital DNA), Hoechst measurements give yields lower than those obtained by the diphenylamine assay or by HPLC.
Comparison of methods for RNA estimates.
Estimates of total RNA in natural samples are difficult because of the lability of the RNA molecule (35). Since RNA concentrations determined by fluorometric and spectrophotometric methods are calculated by difference, their RNA estimates could be biased by the TNA and DNA estimates. By contrast, the HPLC method, because of independent assessments of RNA and DNA, is not biased by the DNA determination. RNA concentrations obtained with spectrophotometric and HPLC methods were not significantly different at most stations (ANOVA; F = 2.28, P = 0.14); only at depths of 100 and 200 m were spectrophotometric values significantly higher than HPLC values (ANOVA; F = 5.17, P < 0.05). By contrast, RNA concentrations determined fluorometrically were higher than those obtained by HPLC (ANOVA; F = 6.878, P < 0.05) but not significantly different from those obtained by the spectrophotometric method (ANOVA; F = 3.284, P = 0.08). As for DNA, we tested the fluorometric method for the recovery of RNA internal standards. This resulted in a mean recovery of 80% of the added RNA, indicating that no major loss or destruction of RNA occurred during the extraction procedure. Therefore, no special protocols for RNA stability (i.e., use of guanidium isothiocyanate and diethyl pyrocarbonate treatment of glassware and solutions) appear to be necessary. However, a complication may arise in the RNA determination with the fluorometric method because of the different affinities of Hoechst and thiazole orange for altered or damaged DNA. Figure 4b shows the DNA concentrations in extracts of the same sample, one stained with Hoechst and the other stained with thiazole orange (the latter previously treated with RNase). Staining with Hoechst clearly gave much lower DNA yields. As a consequence, RNA concentrations would be overestimated in this case. As we do not know the contribution of the damaged DNA to the fluorescence detected with thiazole orange, thiazole orange fluorescence cannot be used for correction of the DNA and RNA estimates.
Finally, RNA concentrations measured fluorometrically may be particularly affected by the kind of standard used for calibration. In this regard, Mordy and Carlson (33) reported that especially when low RNA concentrations are present, different RNA standards produce different fluorometric yields: the use of mRNA standards may produce overestimates compared to the use of rRNA or underestimates compared to the use of tRNA. However, since changes in the relative significance of ribosomal, transfer, and messenger RNAs may occur in marine sediments, as a function of the metabolic state of the living cells, these indications must be further investigated.
Comparison of methods for assessing the detrital fraction of DNA.
Large amounts of DNA are supplied to the benthos through sedimentation of particles produced in the photic layer (1, 48). This “rain” of particle-associated DNA can serve as a potentially important source of labile organic N and P and/or may be used as a reservoir of nucleic acid precursors that are energetically expensive to synthesize ex novo (39). Especially in deep-sea benthic habitats, characterized by low inputs of labile organic matter, detrital DNA could represent a suitable and high-quality food source for heterotrophic metabolism. To evaluate the ecological role of the detrital DNA, quantitative estimates of this fraction are needed. However, the actual amounts of detrital DNA are not easy to assess. Winn and Karl (49) at various Pacific Ocean locations and Bailiff and Karl (1) in the Antarctic Peninsula region provided the first estimates for the relative significance of particulate detrital DNA to the total DNA (75 to 90 and 0 to 93%, respectively). These estimates were obtained indirectly by converting both total DNA (as detrital plus living organic matter) and ATP (living organic matter) concentrations into carbon equivalents and subsequently subtracting these quantities to obtain estimates of detrital DNA. Using the same approach and conversion factors, we calculated the contribution of the living fraction to the total DNA pool (ATP data were summarized from reference 46), but we found extremely low values (on average 1.6%, ranging from 0.18 and 3.3% at depths of 100 and 900 m, respectively). The estimated amount of living carbon depends on the conversion factor C/ATP (here 250, used also by Bailiff and Karl [1]), but Graf and Linke (20) reported that C/ATP ratios in deep-sea sediments may range from <200 to >2,000. Therefore, the use of the C/ATP conversion factor appears too variable to provide accurate estimates.
An alternative approach is to estimate the detrital DNA by difference between total DNA and the contribution of the living components (i.e., microscopically visible intact and stainable bacteria and protozoa). We calculated microbial DNA contribution to the total DNA pool for the following reasons: (i) bacteria and protozoa account for more 90% of the total biomass in deep-sea samples of the Cretan Sea (11, 47), and (ii) macroscopic metazoans were removed from our samples before analysis. We found average microbial DNA contributions to the total DNA pool of 16 and 13%, respectively, for spectrophotometric and HPLC total DNA determinations (Table 3). Hence, with these methods, 84 and 87% of the DNA extracted from the sediment was either from organisms that were not counted microscopically or from the detrital pool.
By contrast, with fluorometric determinations, bacterial and protozoan DNA from intact cells below 100-m depth generally accounted for the whole DNA pool (Table 3), whereas at shallow depths (i.e., 40 and 100 m) they accounted on average for only 17.5%. These results demonstrate that when benthic biomass is dominated by microbes (i.e., bacteria and protozoa together accounting for >90% of total biomass, such as below 100-m depth [11]), Hoechst-DNA concentrations are very close to those estimated from the microscopic counts of intact cells. Conversely, at shallow depths, other DNA pools contributed to the fluorometric DNA estimates.
Estimates of detrital DNA calculated by difference between total and microbial DNA pools must be viewed with caution for the following reasons: (i) the estimates may be biased by the use of conversion factors (though to a much lower extent than for ATP); (ii) since no conversion factors are actually available for benthic communities, we used factors obtained from planktonic bacteria and protozoa; (iii) the conversion factors depend on cell biomass, whereas we estimated DNA content from cell number (though from similar cell sizes); and (iv) microbial assemblages display seasonal changes in DNA content (27).
Finally, to provide additional support for these estimates of detrital DNA, we calculated the percentage contribution of the fluorometric DNA concentrations (assumed to represent DNA of intact cells) to the DNA pool measured spectrophotometrically and by HPLC (assumed to represent the microbial-plus-detrital DNA fraction). DNA fluorometric yields accounted on average for about 30% of the spectrophotometric or HPLC DNA concentrations (Table 2). The resulting 70% of DNA concentration not detected fluorometrically was roughly close to 84 to 87% of the total spectrophotometric and HPLC DNA pool unaccounted by microbes. The major differences between these estimates were observed in the continental shelf at shallow depths (<100 m) where the fluorometric DNA concentrations gave highest yields, accounting on average for about 65% of spectrophotometric or HPLC DNA concentrations. Such a high contribution seems to confirm that at depths of 40 and 100 m, relatively large amounts of nonmicrobial DNA are present (as suggested by the much higher meiofauna and macrofauna biomass [11, 47]).
Further investigations are needed to confirm these data, but so far our results indicate that the combined use of a spectrophotometric or HPLC and Hoechst fluorometric DNA assessment could represent an alternative approach to estimate the detrital DNA fraction in the sediments.
RNA/DNA ratio in relation to environmental constraints.
The decrease of RNA concentrations along the Cretan shelf and slope reflects a drop in the benthic metabolic activity; Duineveld et al. (15), from synoptic measurements on SCOC, demonstrated a clear decrease of the respiratory activity with increasing depth. Since below 100-m depth meiofauna and macrofauna accounted together for less than 10% of the total benthic biomass (11, 47), most of the respiratory activity in deeper sediments will be microbial (15, 40). The dominance of microbes is consistent with the very high proportion of microbial RNA, which generally accounted for almost the entire sedimentary RNA pools below 100-m depth (Table 3). This finding implies that changes in the microbial metabolic activity in the sediment should be reflected by sedimentary RNA concentrations measured by chemical methods.
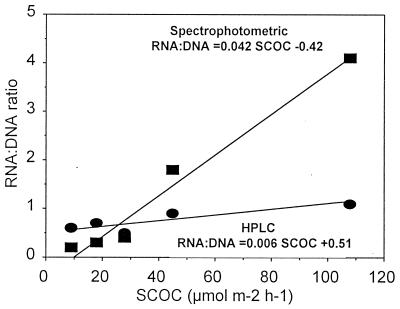
The RNA/DNA ratio in bacteria and phytoplankton has long been thought to be proportional to levels of metabolic activities and growth rates (12, 13). In a study on natural water samples, Jeffrey et al. (24) found that RNA and DNA were strongly correlated with bacterial numbers but also that the RNA/DNA ratio did not correlate with either bacterial growth or metabolic activity and concluded that this ratio cannot be used as biochemical indicator of activity in the environment. Correlation between RNA/DNA ratio and bacterial metabolic activity in natural samples could be biased by the presence of detrital DNA because its proportion is not constant in time and space. To test the reliability of the use of the RNA/DNA ratio as an indicator of the benthic metabolic activity, we tested the correlation between SCOC and the ratios of RNA to DNA, calculated between RNA determined spectrophotometrically or by HPLC (as fluorometric values might be overestimated as previously discussed) and DNA determined fluorometrically (which is assumed to represent the biologically active fraction of the total DNA pool). Figure 5 illustrates the significant correlation found between the two RNA/DNA ratios and SCOC below 100-m depth (P < 0.01). When the shallowest stations (i.e., 40- and 100-m depth) are included, the correlation between SCOC and RNA/DNA ratios becomes weak. This appears to be due to the higher meiofauna and macrofauna oxygen consumption at shallow stations (11, 47). Interestingly, a highly significant correlation between sedimentary RNA content (measured spectrophotometrically and by HPLC) and SCOC was found (r = 0.875, P < 0.01 [spectrophotometric] and r = 0.997, P < 0.01 [HPLC]), indicating that RNA concentrations alone might predict better than the RNA/DNA ratio the benthic metabolic activity.
FIG. 5.
Relationships between SCOC and RNA/DNA ratios (based on spectrophotometric or HPLC determinations of RNA and fluorometric determinations of DNA) in the deep-sea sediments (>100-m depth) of the Cretan Sea.
These data suggest that the RNA/DNA ratio, calculated as described above, could be used as an indicator of the metabolic activity of heterogeneous benthic microbial assemblages. However, the use of this ratio as indicator of benthic metabolism appears to be hampered when other (nonmicrobial) contributions to the DNA are not negligible.
ACKNOWLEDGMENTS
We thank the crew and officers of the R.V. Philia for help during sampling. We thank A. Tselepides and A. Eleftheriou (Institute of Marine Biology of Crete) for great collaboration and providing laboratory facilities. We thank also Rita Colwell (Baltimore) and Elisa Berdalet (Barcelona) and two anonymous referees for suggestions on improving an early draft of the report.
This research was undertaken in the framework of the CINCS project. We acknowledge support from the European Commission’s Marine Science and Technology Program (MAST II) under contract MAS2-CT-940092.
REFERENCES
- 1.Bailiff D M, Karl D M. Dissolved and particulate DNA dynamics during a spring bloom in the Antarctic Peninsula region, 1986–87. Deep-Sea Res. 1991;38:1077–1095. [Google Scholar]
- 2.Bak R P M, Nieuwland G. Seasonal fluctuations in benthic protozoan populations at different depths in marine sediments. Neth J Sea Res. 1989;24:37–44. [Google Scholar]
- 3.Berdalet E, Dortch Q. New double-staining technique for RNA and DNA measurement in marine phytoplankton. Mar Ecol Prog Ser. 1991;73:295–305. [Google Scholar]
- 4.Berdalet E, Estrada M. Relationships between nucleic acid concentrations and primary production in the Catalan Sea (Northwestern Mediterranean) Mar Biol. 1993;117:163–170. [Google Scholar]
- 5.Buckley L J, Lough R G. Recent growth, biochemical composition, and prey field of larval haddock (Melanogrammus aeglefinus) and Atlantic cod (Gadus morhua) on George’s Bank. Can J Fish Aquat Sci. 1987;44:14–25. [Google Scholar]
- 6.Bulow F J. RNA-DNA ratios as indicators of growth in fish: a review. In: Summerfelt R C, Hall G E, editors. The age and growth of fish. Ames, Iowa: Iowa State University Press; 1987. pp. 45–64. [Google Scholar]
- 7.Comings D E. Mechanisms of chromosome banding. VIII. Hoechst 33258-DNA interaction. Chromosoma. 1975;52:229–243. doi: 10.1007/BF00332113. [DOI] [PubMed] [Google Scholar]
- 8.Coppela S J, Acheson C M, Dhurjati P. Isolation of high-molecular weight nucleic acids for copy number analysis using high-performance liquid chromatography. J Chromatogr. 1987;402:189–199. doi: 10.1016/0021-9673(87)80017-1. [DOI] [PubMed] [Google Scholar]
- 9.Danovaro R, Fabiano M, Della Croce N. Labile organic matter and microbial biomasses in deep-sea sediments (Eastern Mediterranean Sea) Deep-Sea Res. 1993;40:953–965. doi: 10.1007/s002489900080. [DOI] [PubMed] [Google Scholar]
- 10.Danovaro R. Detritus-bacteria-meiofauna interactions in a seagrass bed (Posidonia oceanica) of the NW Mediterranean. Mar Biol. 1996;127:1–13. [Google Scholar]
- 11.Danovaro R, Tselepides A, Della Croce N, Otegui A, Dell’Anno A, Ferrando M, Martorano D. Meiofaunal community structure of the continental shelf, slope and deep-sea basin of the Cretan Sea. In: Tselepides A, Papadopoulou K-N, Polychronaki T, editors. CINCS: pelagic benthic coupling in the oligotrophic Cretan Sea. Heraklion, Greece: IMBC; 1996. pp. 157–166. [Google Scholar]
- 12.Dortch Q F, Roberts T L, Clayton J R, Ahmed S I. RNA:DNA ratios and DNA concentrations as indicators of growth rate and biomass in planktonic organisms. Mar Ecol Prog Ser. 1983;13:61–71. [Google Scholar]
- 13.Dortch Q F, Clayton J R, Thoresen S S, Ahmed S I. Nitrogen storage and the use of biochemical indices to assess nitrogen deficiency and growth rate in natural plankton populations. J Mar Res. 1985;43:437–464. [Google Scholar]
- 14.Dugdale R C, Wilkerson F P. Nutrient sources and primary production in the Eastern Mediterranean. Oceanol Acta. 1988;9:178–184. [Google Scholar]
- 15.Duineveld G C A, Tselepides A, Berghuis E M, Van Derwelle J, Kok A. Oxygen consumption by the sediment communities in the oligotrophic Cretan Sea. In: Tselepides A, Papadopoulou K-N, Polychronaki T, editors. CINCS: pelagic benthic coupling in the oligotrophic Cretan Sea. Heraklion, Greece: IMBC; 1996. pp. 145–153. [Google Scholar]
- 16.Fabiano M, Danovaro R, Crisafi E, La Ferla R, Povero P, Acosta Pomar L. Particulate matter composition and bacterial distribution in Terra Nova Bay (Antarctica) during summer 1989–1990. Polar Biol. 1995;15:393–400. [Google Scholar]
- 17.Fara A, Berdalet E, Arin L. Determination of RNA and DNA concentrations in natural plankton samples using Thiazole Orange in combination with DNase and RNase digestions. J Phycol. 1996;32:1074–1083. [Google Scholar]
- 18.Fontvieille D, Fevotte G. DNA content of the sediment in relation to self-purification in streams polluted by organic wastes. Verth Int Verein Limnol. 1981;21:221–226. [Google Scholar]
- 19.Fuhrman J A, Azam F. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar Biol. 1982;66:109–120. [Google Scholar]
- 20.Graf G, Linke P. Adenosine nucleotides as indicators of deep–sea benthic metabolism. In: Rowe G T, Pariente V, editors. Deep-sea food chains and the global carbon cycle. Dordrecht, The Netherlands: Kluwer; 1992. pp. 237–243. [Google Scholar]
- 21.Holm-Hansen O, Sutcliffe W H, Sharp J. Measurement of deoxyribonucleic acid in the ocean and its ecological significance. Limnol Oceanogr. 1968;13:507–514. [Google Scholar]
- 22.Kerkhof L, Ward B B. Comparison of nucleic acid hybridization and fluorometry for measurement of the relationship between RNA/DNA ratio and growth rate in a marine bacterium. Appl Environ Microbiol. 1993;59:1303–1309. doi: 10.1128/aem.59.5.1303-1309.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen C S, Rasmussen O F. Development and application of a new method to extract bacterial DNA from soil based on separation of bacteria from soil with cation-exchange resin. Appl Environ Microbiol. 1992;58:2458–2462. doi: 10.1128/aem.58.8.2458-2462.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffrey W H, Von Haven R, Hoch M P, Coffin R B. Bacterioplankton RNA, DNA, protein content and relationships to rates of thymidine and leucine incorporation. Aquat Microb Ecol. 1996;10:87–95. [Google Scholar]
- 25.Jones D R, Karl D M, Laws E A. DNA:ATP ratios in marine microalgae and bacteria: implications for growth rate estimates based on rates of DNA synthesis. J Phycol. 1995;31:215–223. [Google Scholar]
- 26.Lee L G, Chen C-H, Chiu L A. Thiazole Orange: a new dye for reticulocyte analysis. Chytometry. 1986;7:508–517. doi: 10.1002/cyto.990070603. [DOI] [PubMed] [Google Scholar]
- 27.Lee S, Kemp P F. Single-cell RNA content of natural marine planktonic bacteria measured by hybridization with multiple 16S rRNA-targeted fluorescent probes. Limnol Oceanogr. 1994;39:869–879. [Google Scholar]
- 28.Leff L G, Dana J R, McArthur J V, Shimkets L J. Comparison of methods of DNA extraction from stream sediments. Appl Environ Microbiol. 1995;61:1141–1143. doi: 10.1128/aem.61.3.1141-1143.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loontiens F G, McLaughlin L W, Diekmann S, Clegg R M. Binding of Hoechst 33258 and 4′,6-diamidino-2-phenylindole to self-complementary decadeoxynucleotides with modified exocyclic base substituents. Biochemistry. 1991;30:182–189. doi: 10.1021/bi00215a027. [DOI] [PubMed] [Google Scholar]
- 30.Mandel M. Nucleic acids of protozoa. In: Kidder G W, editor. Chemical zoology. 1. Protozoa. New York, N.Y: Academic Press; 1967. pp. 541–568. [Google Scholar]
- 31.Montagna P A. Sampling design and enumeration statistics for bacteria extracted from marine sediments. Appl Environ Microbiol. 1982;43:1366–1372. doi: 10.1128/aem.43.6.1366-1372.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moran M A, Torsvik V L, Torsvik T, Hodson R E. Direct extraction and purification of rRNA for ecological studies. Appl Environ Microbiol. 1993;59:915–918. doi: 10.1128/aem.59.3.915-918.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mordy C W, Carlson D J. An evaluation of fluorescence techniques for measuring DNA and RNA in marine microorganisms. Mar Ecol Prog Ser. 1991;73:283–293. [Google Scholar]
- 34.Ogram A, Sayler G S, Barkay T. The extraction and purification of microbial DNA from sediments. J Microbiol Methods. 1987;7:57–66. [Google Scholar]
- 35.Ogram A, Sun W, Brockman F J, Fredrickson J K. Isolation and characterization of RNA from low-biomass deep-subsurface sediments. Appl Environ Microbiol. 1995;61:763–768. doi: 10.1128/aem.61.2.763-768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paul J H. Use of Hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982;43:939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul J H, Myers B. Fluorometric determination of DNA in aquatic microorganisms by use of Hoechst 33258. Appl Environ Microbiol. 1982;43:1393–1399. doi: 10.1128/aem.43.6.1393-1399.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul J H, Carlson D. Genetic material in the marine environment implication for bacterial DNA. Limnol Oceanogr. 1984;29:1091–1097. [Google Scholar]
- 39.Paul J H, Jeffrey W H, DeFlaun M F. Dynamics of extracellular DNA in the marine environment. Appl Environ Microbiol. 1987;53:170–179. doi: 10.1128/aem.53.1.170-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rowe G T, Boland G S, Escobar Briones E G, Cruz-Kaegi M E, Newton A, Piepemburg D, Walsh I, Deming J. Sediment community biomass and respiration in the Northeast Water Polynia, Greenland: a numerical simulation of benthic lander and spade core data. J Mar Syst. 1997;10:497–515. [Google Scholar]
- 41.Simon M, Azam F. Protein content and protein synthesis rates of planktonic bacteria Mar. Ecol Prog Ser. 1989;51:201–213. [Google Scholar]
- 42.Sutcliffe W H. Relationship between growth rate and ribonucleic acid concentration in some invertebrates. J Fish Res Bd Can. 1970;27:606–609. [Google Scholar]
- 43.Thiel H, Higgins R P. Introduction to the study of meiofauna. Washington, D.C: Smithsonian Institution Press; 1988. pp. 94–96. [Google Scholar]
- 44.Thoresen S S, Clayton J R, Dortch Q F, Ahmed S I. A rapid technique for the determination of RNA and DNA in marine phytoplankton. J Plankton Res. 1983;5:253–261. [Google Scholar]
- 45.Tsai Y-L, Olson B H. Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tselepides A, Danovaro R, Della Croce N, Duineveld G, Polychronaki T, Dell’Anno A, Dafnomili E, Plaiti W, Akoumianaki I, Martorano D. Seasonal variability of chloroplastic pigments, TOC, TON, ATP and labile organic matter (carbohydrates, lipids, proteins and nucleic acids) over the continental margin and deep-sea sediments of the oligotrophic Cretan Sea. In: Tselepides A, Papadopoulou K-N, Polychronaki T, editors. CINCS: pelagic benthic coupling in the oligotrophic Cretan Sea. Heraklion, Greece: IMBC; 1996. pp. 113–130. [Google Scholar]
- 47.Tselepides A, Papadopoulou K-N, Podaras D, Plaiti W, Pantazoglou F. Macrofaunal community structure of the continental shelf, slope and deep sea basin of the Cretan Sea. In: Tselepides A, Papadopoulou K-N, Polychronaki T, editors. CINCS: pelagic benthic coupling in the oligotrophic Cretan Sea. Heraklion, Greece: IMBC; 1996. pp. 171–183. [Google Scholar]
- 48.Turley C M, Mackie P J. Bacterial and cyanobacterial flux to the deep NE Atlantic on sedimenting particles. Deep-Sea Res. 1995;42:1453–1474. [Google Scholar]
- 49.Winn C D, Karl D M. Diel nucleic acid synthesis and particulate DNA concentrations: conflicts with division rate estimates by DNA accumulation. Limnol Oceanogr. 1986;31:637–645. [Google Scholar]
- 50.Zachleder V. Optimization of nucleic acids assay in green and blue-green algae: extraction procedures and the light-activated diphenylamine reaction for DNA. Arch Hydrobiol. 1984;67:313–328. [Google Scholar]