Abstract
The worldwide prevalence of autoimmune diseases that have limited treatment options and preventive strategies is rapidly rising. There is growing evidence that the microbiota and the integrity of the intestinal barrier play a role in autoimmune diseases. The potential to evaluate intestinal barrier integrity for susceptible individuals and to determine whether restoring intestinal junction integrity impacts autoimmune diseases is an important area of research that requires further attention. In the intestinal permeability model of autoimmune diseases, the breakdown of the intestinal tight junction proteins (zonulin/occludin) allows bacteria, toxins, undigested dietary proteins, and other antigens to pass into the lumen, thereby increasing the number of inflammatory reactions and the activation of immune cells throughout the body. In this study, we investigate the relationship between zonulin/occludin antibodies, which are used to determine intestinal permeability, with autoantibodies used to diagnose autoimmunity. Our investigation may identify significant levels of circulating autoantibodies in human subjects with intestinal permeability compared to those without intestinal permeability. Furthermore, we identified that significant positive linear correlations between serum occludin/zonulin antibodies and circulating autoantibodies could be used to determine autoimmune diseases.
Keywords: intestinal permeability, zonulin, autoimmunity, zonulin/occludin antibodies, leaky gut
1. Introduction
The worldwide incidence and prevalence of virtually all autoimmune diseases has risen steadily over the past 30 years [1]. There is growing evidence that the imbalances in the gut microbiota and impaired integrity of intestinal tight junctions may play a role in the development of autoimmune disease [2,3]. In the intestinal permeability model of autoimmune disease, the breakdown of the intestinal tight junctions allows bacteria, toxins, undigested dietary proteins, and other antigens to pass into the lumen, thereby increasing inflammatory reactions within the gastrointestinal environment and throughout the body [4,5]. The loss of proper macromolecule trafficking can induce immune dysregulation, impair tolerance, and lead to several mechanisms that set the stage for the expression of autoimmune diseases in susceptible individuals [6,7].
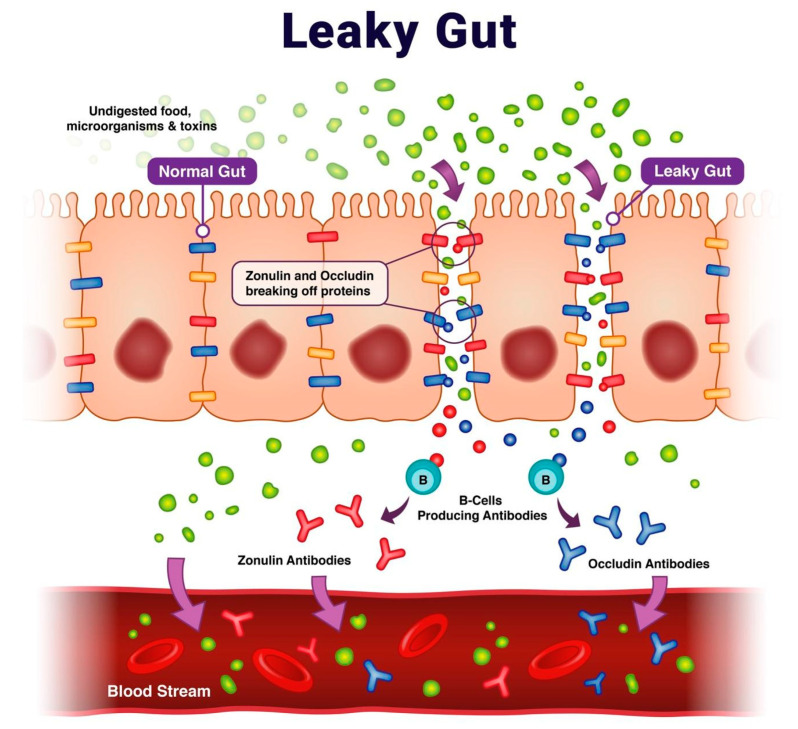
The intestinal epithelium maintains its impermeability from large undigested protein macromolecules and various pathogens with occludin junctional adhesion molecules. These tight junction proteins are regulated by zonulin. Intestinal cells synthesize zonulin, which is used to reversibly regulate intestinal permeability [8]. Serum antibodies against intestinal tight junction proteins, zonulin, and occludin develop with intestinal permeability and are found to be reliable, stable, and reproducible biomarkers for identifying intestinal permeability (Figure 1) [9,10].
Figure 1.
Intestinal permeability referred to as “leaky gut” pathophysiology and the formation of occludin/zonulin antibodies.
In this study, our objective was to assess the relationship of autoantibody markers of autoimmunity with intestinal permeability as clinically determined by the presence of elevated zonulin/occludin antibodies. This includes comparing mean autoantibodies in human subjects with and without intestinal permeability and comparing autoimmunity risk for human subjects with and without intestinal permeability.
2. Results
There were statistically significant elevations in mean autoimmune antibodies in subjects with intestinal permeability (zonulin/occludin positive) as compared to subjects without intestinal permeability (zonulin/occludin negative) for 17 out of 24 autoimmune target proteins (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6). With logistic regression analyses, there was a 3- to 30-fold increase in the odds of detecting elevated autoimmune target protein antibodies in subjects with intestinal permeability compared to the odds of developing autoimmune target proteins in subjects without intestinal permeability (Table 1). There were also statistically significant positive linear correlations with zonulin/occludin antibodies and autoimmune target protein antibodies (Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11). The correlations coefficients were small to moderate (Table 2).
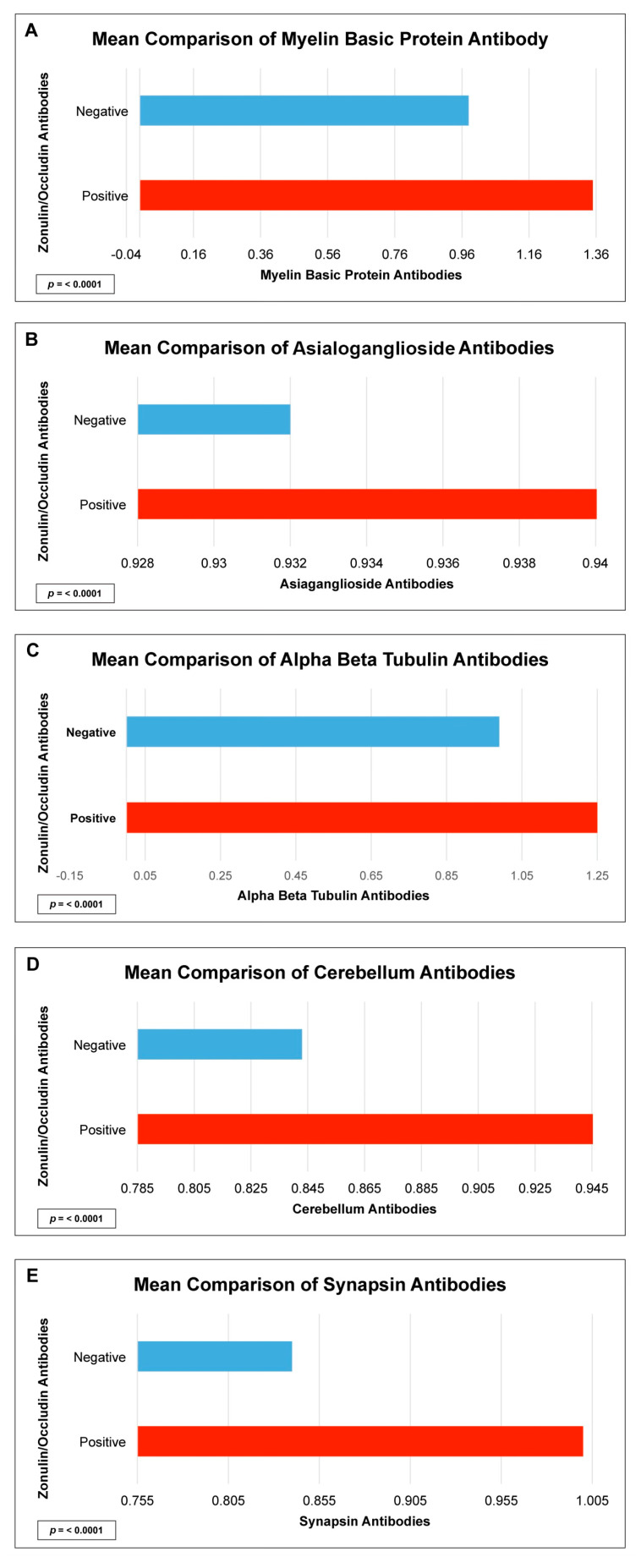
Figure 2.
Comparison of subjects with positive and negative serum zonulin/occludin antibody levels and neurological tissue antibodies; (A) myelin basic protein, (B) asialoganglioside, (C) alpha/beta tubulin, (D) cerebellum, and (E) synapsin. Positive zonulin/occludin antibodies were defined as levels greater than two (2) standard deviations from the mean. The p-value for all comparisons were <0.0001.
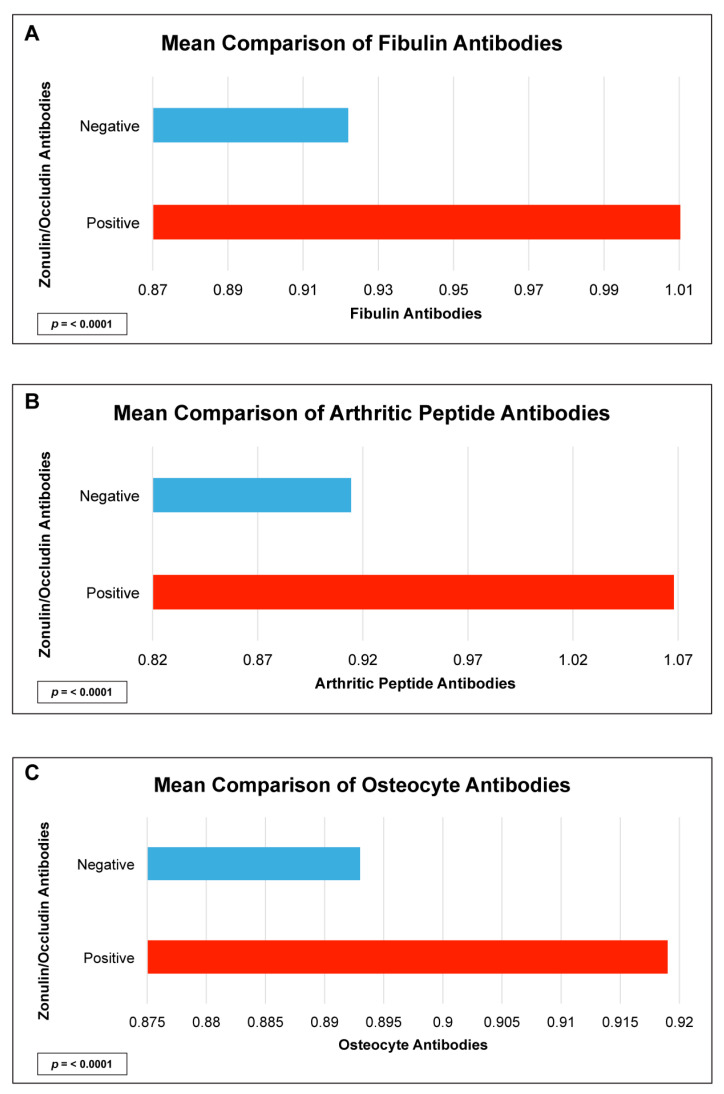
Figure 3.
Comparison of subjects with positive and negative zonulin/occludin antibody levels and various joint tissue antibodies: (A) fibulin, (B) arthritic peptide, and (C) osteocyte. Positive zonulin/occludin antibodies were defined as levels greater than two (2) standard deviations from the mean. The p-values for all comparisons were <0.0001.
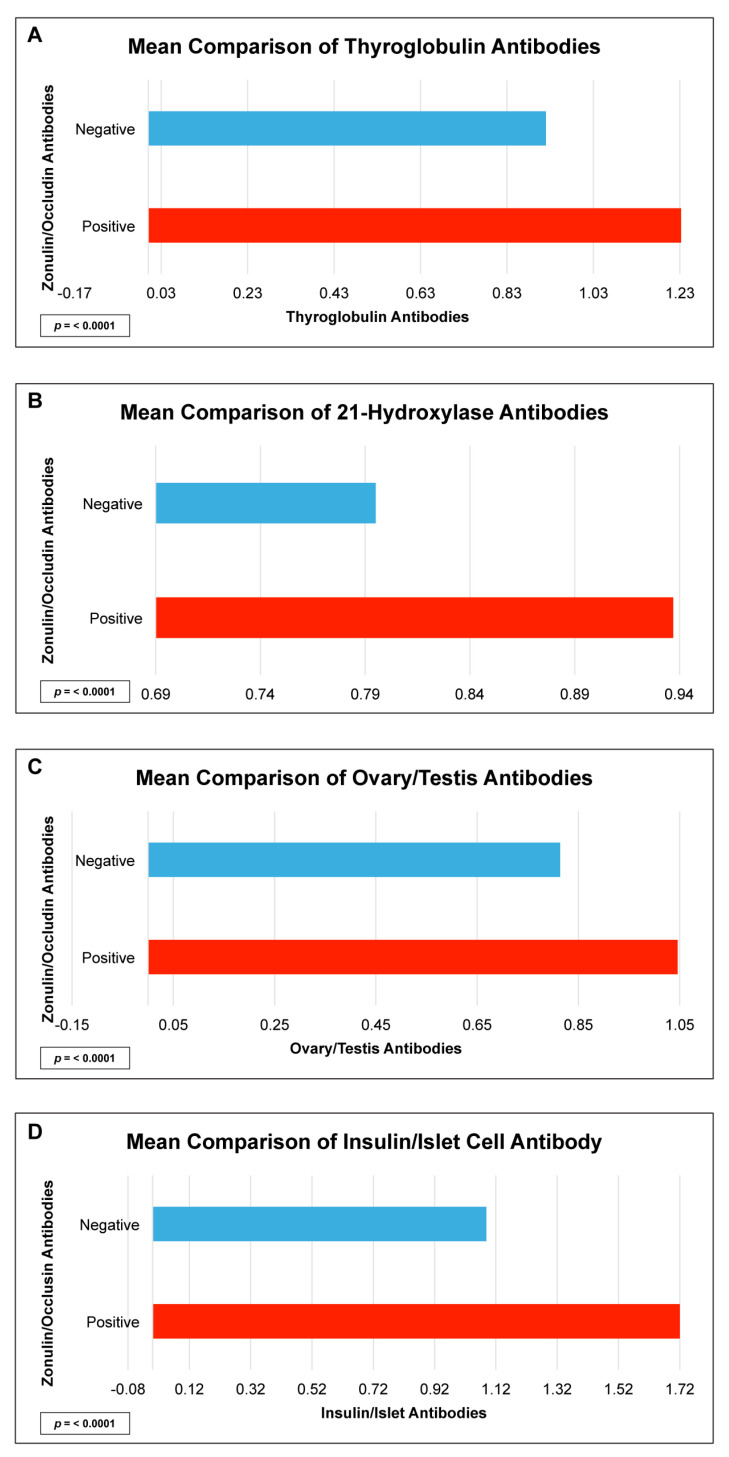
Figure 4.
Comparison of subjects with positive and negative zonulin/occludin antibody levels and endocrine tissue antibodies: (A) thyroglobulin, (B) 21-hydroxylase, (C) ovary/testis, and (D) insulin/islet cell. Positive zonulin/occludin antibodies were defined as levels greater than two (2) standard deviations from the mean. The p-values for all comparisons were <0.0001.
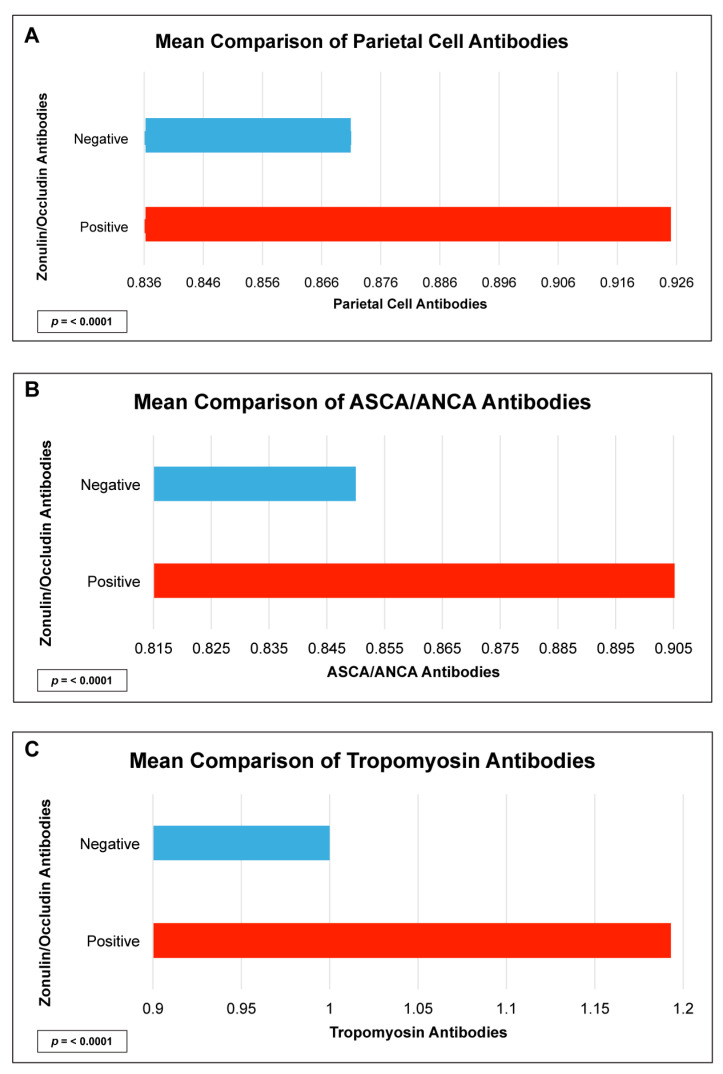
Figure 5.
Comparison of subjects with positive and negative zonulin/occludin antibody levels and intestinal tissue antibodies: (A) parietal cell, (B) ASCA/ANCA, and (C) tropomyosin. Positive zonulin/occludin antibodies were defined as levels greater than two (2) standard deviations from the mean. The p-values for all comparisons were <0.0001.
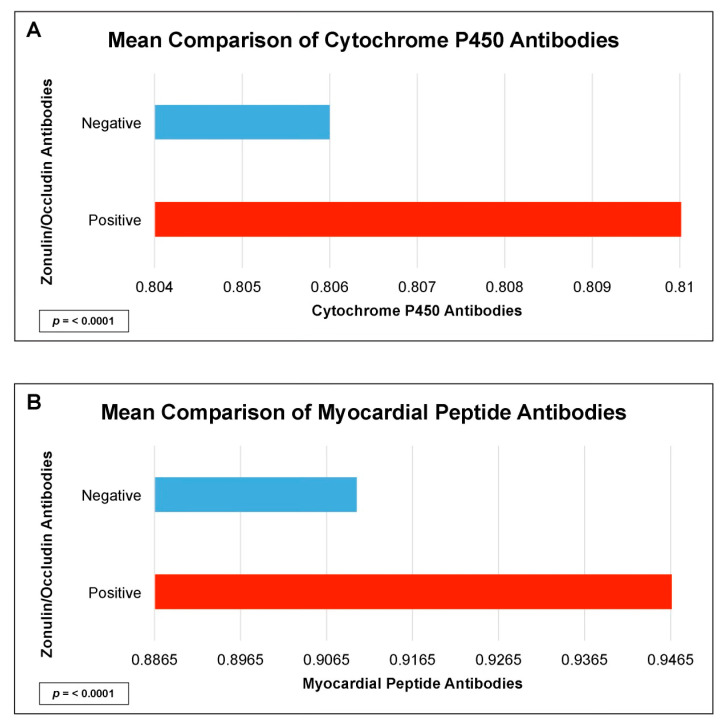
Figure 6.
Comparison of subjects with positive and negative zonulin/occludin antibody levels and tissue antibodies: (A) myocardial peptide, and (B) cytochrome P450. Positive zonulin/occludin antibodies were defined as levels greater than two (2) standard deviations from the mean. The p-values for all comparisons were <0.0001.
Table 1.
Odds ratio for developing elevated autoimmune target protein antibodies with occludin/zonulin positive subjects compared to the odds of developing barrier protein antibodies with occludin/zonulin negative subjects. The statistically significant p-value with a Bonferroni adjustment for multiple comparisons is <0.002.
| Autoantibody | Odd Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Parietal Cell + ATPase IgG + IgA | 5.8 | 2.6–12.8 | <0.0001 |
| Intrinsic Factor IgG + IgA | 1.7 | 1.1–2.7 | 0.025 |
| ASCA + ANCA IgG + IgA | 8.4 | 3.5–20.2 | <0.0001 |
| Tropomyosin IgG + IgA | 7.0 | 3.3–15.0 | <0.0001 |
| Thyroglobulin IgG + IgA | 1.6 | 0.3–1.0 | <0.034 |
| Thyroid Peroxidase IgG + IgA | 2.4 | 1.4–4.2 | 0.002 |
| 21 Hydroxylase IgG + IgA | 28.0 | 8.7–89.0 | <0.0001 |
| Myocardial Peptide IgG + IgA | 9.0 | 4.1–19.2 | <0.0001 |
| Alpha-Myosin IgG + IgA | 10.4 | 4.5–23.1 | <0.0001 |
| Phospholipid IgG + IgA | 2.5 | 1.3–5.0 | 0.009 |
| Platelet Glycoprotein IgG + IgA | 5.4 | 2.3–13.0 | <0.0001 |
| Ovary/Testis IgG + IgA | 7.1 | 3.0–17.7 | <0.0001 |
| Fibulin IgG + IgA | 7.2 | 3.0–17.0 | <0.0001 |
| Collagen Complex IgG + IgA | 2.2 | 1.3–4.0 | 0.005 |
| Arthritic Peptide IgG + IgA | 30.0 | 10.0–77.8 | <0.0001 |
| Osteocyte IgG + IgA | 6.6 | 2.8–15.7 | <0.0001 |
| Cytochrome P450 IgG + IgA | 22.3 | 8.0–64.4 | <0.0001 |
| Insulin + Islet Cell Antibody IgG + IgA | 0.9 | 0.6–1.6 | <0.0001 |
| Glutamic Acid Decarboxylase-65 IgG + IgA | 10.0 | 4.2–23.3 | <0.0001 |
| Myelin Basic Protein IgG + IgA | 6.9 | 3.2–15.0 | <0.0001 |
| Asialoganglioside IgG + IgA | 6.2 | 2.9–13.3 | <0.0001 |
| Alpha + Beta Tubulin IgG + IgA | 2.7 | 1.6–4.6 | <0.0001 |
| Cerebellar IgG + IgA | 22.0 | 8.0–59.9 | <0.0001 |
| Synapsin IgG + IgA | 8.7 | 3.3–22.5 | <0.0001 |
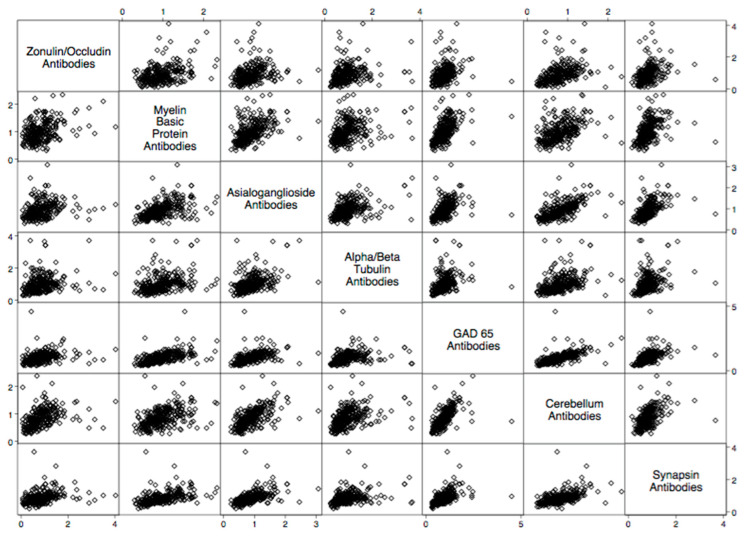
Figure 7.
Scatter matrix of positive linear relationships with neurological tissue antibodies and zonulin/occludin antibodies.
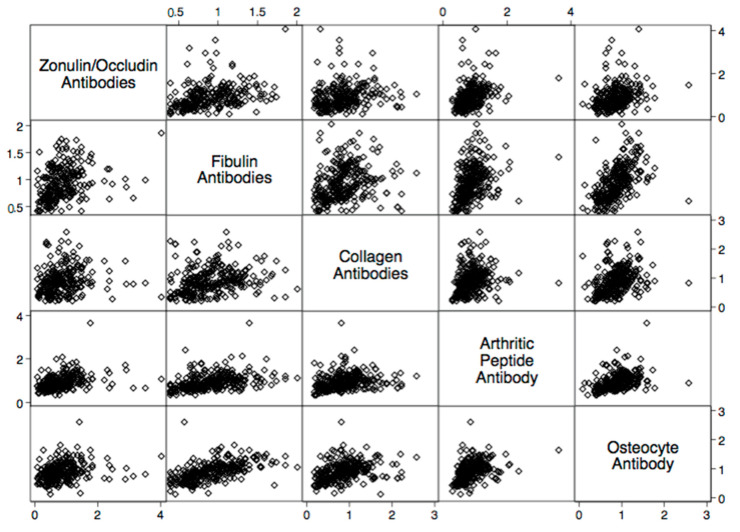
Figure 8.
Scatter matrix of positive linear relationships with joint tissue antibodies and zonulin/occludin antibodies.
Figure 9.
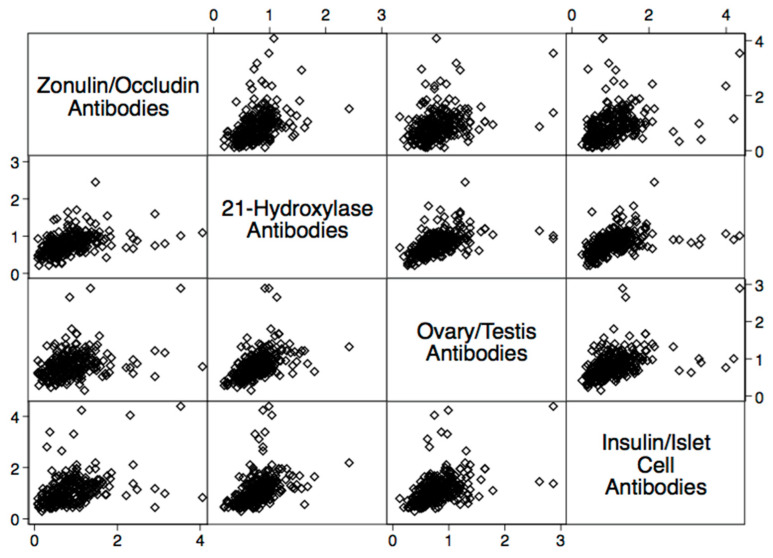
Scatter matrix of positive linear relationships with endocrine tissue antibodies and zonulin/occludin antibodies.
Figure 10.
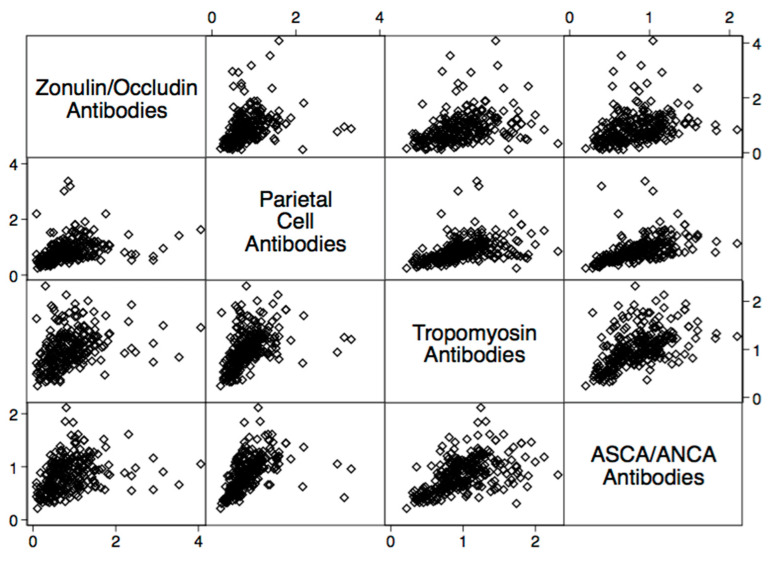
Scatter matrix of positive linear relationships with gastrointestinal tissue antibodies and zonulin/occludin antibodies.
Figure 11.
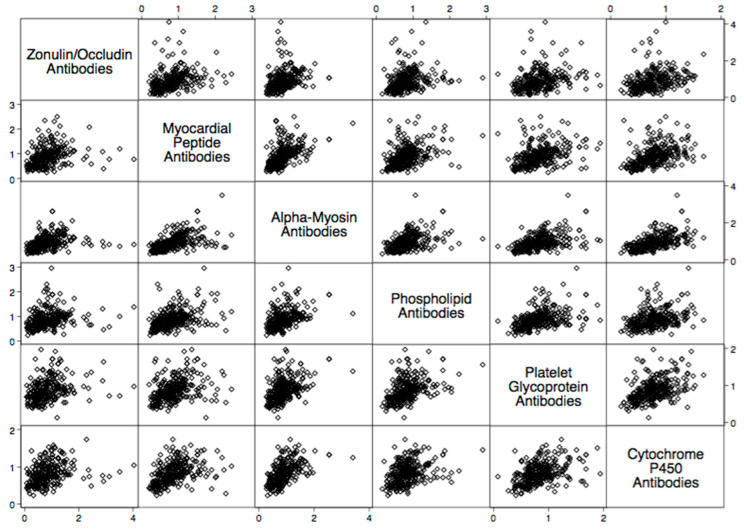
Scatter matrix of positive linear relationships with tissue antibodies throughout the body and zonulin/occludin antibodies.
Table 2.
Correlation coefficients between zonulin/occludin IgG antibodies and autoimmune target protein antibodies. The statistically significant p-value with a Bonferroni adjustment for multiple comparisons is <0.002.
| Autoantibody | Correlation Coefficient | p-Value |
|---|---|---|
| Parietal Cell + ATPase IgG + IgA | 0.4 | <0.0001 |
| Intrinsic Factor IgG + IgA | 0.3 | <0.0001 |
| ASCA + ANCA IgG + IgA | 0.4 | <0.0001 |
| Tropomyosin IgG + IgA | 0.4 | <0.0001 |
| Thyroglobulin IgG + IgA | 0.2 | 0.02 |
| Thyroid Peroxidase IgG + IgA | 0.2 | 0.001 |
| 21 Hydroxylase IgG + IgA | 0.4 | <0.0001 |
| Myocardial Peptide IgG + IgA | 0.3 | <0.0001 |
| Alpha-Myosin IgG + IgA | 0.5 | <0.0001 |
| Phospholipid IgG + IgA | 0.3 | <0.0001 |
| Platelet Glycoprotein IgG + IgA | 0.3 | <0.0001 |
| Ovary/Testis IgG + IgA | 0.4 | <0.0001 |
| Fibulin IgG + IgA | 0.3 | <0.0001 |
| Collagen Complex IgG + IgA | 0.2 | <0.0001 |
| Arthritic Peptide IgG + IgA | 0.2 | 0.0005 |
| Osteocyte IgG + IgA | 0.2 | 0.004 |
| Cytochrome P450 IgG + IgA | 0.3 | <0.0001 |
| Insulin + Islet Cell Antibody IgG + IgA | 0.2 | 0.0004 |
| Glutamic Acid Decarboxylase-65 IgG + IgA | 0.4 | <0.0000 |
| Myelin Basic Protein IgG + IgA | 0.3 | <0.0000 |
| Asialoganglioside IgG + IgA | 0.3 | <0.0001 |
| Alpha + Beta Tubulin IgG + IgA | 0.3 | <0.0001 |
| Cerebellar IgG + IgA | 0.4 | <0.0001 |
| Synapsin IgG + IgA | 0.3 | <0.0001 |
3. Discussion
Our investigation may identify significant levels of circulating autoantibodies in human subjects with intestinal permeability compared to those without intestinal permeability. Furthermore, we identified that significant positive linear correlations between serum occludin/zonulin antibodies and circulating autoantibodies could be used to determine autoimmune diseases. An important finding of our study indicates that intestinal permeability has a generalized role in autoimmune diseases, involving the brain, the endocrine gland, joints, smooth muscles, the cardiovascular system, etc. These findings support the notion that autoimmune diseases throughout the body may share a centralized role, involving the integrity of intestinal junctions.
Specifically, we found elevated neurological antibodies with human subjects that had elevated levels of tight junction proteins in addition to statistically significant correlations between tight junction antibodies and neurological antibodies (Figure 2 and Table 2). During their breakdown, tight junction proteins in the intestinal barrier also have similar relationships with tight junction proteins in the blood–brain barrier, and the dysfunction of tight junction proteins has been theorized to play a role in neuroinflammatory conditions. Tight junction proteins have been found to play a role in immunological responses in the brain and are associated with elevations in the surrogate markers of blood–brain barrier permeability [10,11,12,13,14]. Patients suffering from relapsing–remitting multiple sclerosis were found to have a relatively high proportion of intestinal permeability compromise when compared to matched controls, suggesting that disturbance in the integrity of microbiota may play a role in the pathophysiology of multiple sclerosis. Intestinal permeability has been found to be a potential target site for the therapeutic treatment of multiple sclerosis, and the disruption of the intestinal tight junctions could lead to the early detection of autoimmune encephalomyelitis in animal models [15]. Our data support these previous studies and suggest a specific list of neurological autoimmune target proteins antibodies that may have a relationship with intestinal permeability.
We identified numerous autoimmune target antibodies to joint and bone sites that are correlated and elevated in subjects with elevated tight junction proteins (Figure 3 and Table 2). The integrity of the intestinal microbiota is found to be significantly different in patients with early rheumatoid arthritis [16,17]. Recent studies have suggested that intestinal permeability can lead to the circulation of arthritogenic bacteria and cause inflammation in synovial tissues [18,19,20]. Furthermore, inflammatory conditions in the bowel, involving the translocation of bacterial products against the endothelial gut barrier have been found to cause inflammation in the bone and impact what is known as the gut–microbiota–bone axis [21,22]. Our data provide further support that intestinal permeability may play a role in autoimmune and inflammatory reactions involving the protein target sites of both bone and synovial tissues.
The relationship between intestinal permeability and autoimmune diseases of the endocrine system has become an area of great interest for researchers. Several case–control studies have identified increased intestinal permeability in patients with type 1 diabetes compared to healthy controls [23,24,25,26]. Our study supports the findings of these previous studies and has determined that subjects with elevated tight junction antibody levels may have mean insulin/islet cell antibodies compared to those without elevated tight junction antibody levels. We also identified several other autoimmune antibody elevations that could be found with autoimmune thyroid disease (thyroglobulin Abs), Addison’s disease (21-hydroxylase Abs), and autoimmunity associated ovarian/testicular failure (ovary/tes-190 tis Abs) (Figure 4 and Table 2). The findings of our study suggest that intestinal permeability may have systemic autoimmune responses, involving a diverse list of autoimmune target proteins, and may potentially play a role in polyglandular autoimmune disease.
Our study may also indicate relationships between intestinal permeability/autoimmunity and gastrointestinal and hepatic autoimmune target proteins, such as parietal cell antibodies, ASCA antibodies, ANCA antibodies, cytochrome p450, and tropomyosin antibodies 196 (Figure 5 and Figure 6, and Table 2). These autoantibodies are found with primary sclerosing cholangitis, autoimmune hepatitis, gastric autoimmunity, and chronic inflammatory bowel diseases. Researchers have speculated that intestinal permeability may play a role in these specific autoantibodies and associated diseases [4,27,28,29].
4. Methods and Materials
4.1. Data Set
To study the relationship between intestinal permeability and autoimmunity in human subjects, the investigators contacted a clinical autoimmune specialty laboratory and requested a non-identifiable data set containing 200 or more human subjects that included ELISA (enzyme-linked immunosorbent assay) measurements of antibodies, both for intestinal permeability and antibodies as well as autoimmune target proteins. The laboratory is CLIA (Clinical Laboratory Improvement Amendments of 1988)-certified and monitored for proficient testing, compliance with laboratory guidelines, and standards of ELISA testing. The laboratory provided our investigation with a data set of 266 random human subjects containing the quantification of 24 different autoimmune target protein antibodies of the occludin/zonulin antibody (but not measures of occludin and zonulin antibodies separately). The collection of data was approved by the Institutional Review Board of Partners Healthcare at Massachusetts General Hospital. Age and gender data were provided but no further clinical or demographic data were made available.
Serum samples used in the data set were collected at multiple medical centers in the United States. Licensed physicians clinically selected subjects to be screened for intestinal permeability and autoimmunity based on the subject’s medical records and medical findings in each clinician’s files. The ages of subjects ranged between 19 and 87 years, with a mean age of 50. Sixty-five percent of the subjects were female and thirty-fix percent of the subjects were male. No further medical or demographic information was provided about the subjects to the autoimmune specialty laboratory or provided to us in the data set.
4.2. Statistical Analysis
Statistical analysis was performed using STATA software package 14.2. Logistic regression, Pearson’s correlation coefficients, and t-tests were conducted to analyze the data. Subjects with mean occludin/zonulin IgG antibodies that were two (2) or more standard deviations above the mean were classified as positive for intestinal permeability. This was carried out by converting continuous data from optical density measurements of zonulin/occludin antibodies to the binary classification of intestinal permeability as either “positive” or “negative”. Our analyses were conducted using a Bonferroni correction to adjust for a type I error with the significant p-value set to 0.002.
5. Conclusions
The worldwide prevalence of autoimmune diseases is rapidly rising with limited treatment options and preventive strategies [30]. There is growing evidence that the microbiota and the integrity of the intestinal barrier may play a role in autoimmune diseases [31]. Understanding these relationships could lead to medications, lifestyle modifications, nutraceuticals, and dietary strategies that may attenuate autoimmune expression [7]. These relationships may play a role in a spectrum of autoimmune diseases [32]. The potential to evaluate intestinal barrier integrity for susceptible individuals and to determine whether restoring intestinal junction integrity impacts autoimmune disease will be an important area of research in autoimmune disease development and future treatment strategies [33,34].
Author Contributions
D.K., M.H. and J.L. helped develop the study design and participated in writing and editing the manuscript. D.K. completed the statistical analyses, figures, and tables. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Partners Healthcare at Massachusetts General Hospital (IRB Protocol #2017P002153/PHS, 3 January 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Martha Herbert and Jama Lambert have no conflict of interest. Datis Kharrazian is a consultant at Cyrex labs. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lerner A., Jeremias P., Matthias T. The world incidence and prevalence of autoimmune disease is increasing. Int. J. Celiac Dis. 2015;3:151–155. doi: 10.12691/ijcd-3-4-8. [DOI] [Google Scholar]
- 2.Mu Q., Kirby J., Reilly C.M., Luo X.M. Leaky gut as a danger signal for autoimmune diseases. Front. Immunol. 2017;23:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyauchi E., Shimokawa C., Steimle A., Desai M.S., Ohno H. The impact of the gut microbiome on extra-intestinal autoimmune diseases. Nat. Rev. Immunol. 2023;23:9–23. doi: 10.1038/s41577-022-00727-y. [DOI] [PubMed] [Google Scholar]
- 4.Fasano A., Shea-Donohue T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005;2:416–422. doi: 10.1038/ncpgasthep0259. [DOI] [PubMed] [Google Scholar]
- 5.Charoensappakit A., Sae-Khow K., Leelahavanichkul A. gut barrier damage and gut translocation of pathogen molecules in lupus, an impact of innate immunity (macrophages and neutrophils) in autoimmune disease. Int. J. Mol. Sci. 2022;23:8223. doi: 10.3390/ijms23158223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasano A. Leaky gut and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012;42:71–78. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 7.Paray B.A., Albeshr M.F., Jan A.T., Rather I.A. Leaky gut and autoimmunity: An intricate balance in individuals health and the diseased state. Int. J. Mol. Sci. 2020;21:9770. doi: 10.3390/ijms21249770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fasano A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012;1258:25–33. doi: 10.1111/j.1749-6632.2012.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simeonova D., Ivanovska M., Murdjeva M., Carvalho A.F., Maes M. Recognizing the leaky gut as a trans-diagnostic target for neuroimmune disorders using clinical chemistry and molecular immunology assays. Curr. Top. Med. Chem. 2018;18:1641–1655. doi: 10.2174/1568026618666181115100610. [DOI] [PubMed] [Google Scholar]
- 10.Vojdani A., Vojdani E., Kharrazian D. Fluctuation of zonulin levels in blood vs stability of antibodies. World J. Gastroenterol. 2017;23:5669–5679. doi: 10.3748/wjg.v23.i31.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skardelly M., Armbruster F.P., Meixensberger J., Hilbig H. Expression of Zonulin, c-kit, and glial fibrillary acidic protein in human gliomas. Transl. Oncol. 2009;2:117–120. doi: 10.1593/tlo.09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Díaz-Coránguez M., Segovia J., López-Ornelas A., Puerta-Guardo H., Ludert J., Chávez B., Meraz-Cruz N., González-Mariscal L. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS ONE. 2013;8:e60655. doi: 10.1371/journal.pone.0060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturgeon C., Fasano A. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384. doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buscarinu M.C., Romano S., Mechelli R., Pizzolato Umeton R., Ferraldeschi M., Fornasiero A., Reniè R., Cerasoli B., Morena E., Romano C., et al. Intestinal permeability in relapsing-remitting multiple sclerosis. Neurotherapeutics. 2018;15:68–74. doi: 10.1007/s13311-017-0582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nouri M., Bredberg A., Weström B., Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS ONE. 2014;9:e106335. doi: 10.1371/journal.pone.0106335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaahtovuo J., Munukka E., Korkeamäki M., Luukkainen R., Toivanen P. Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 2008;35:1500–1505. [PubMed] [Google Scholar]
- 17.Azzouz D.F., Silverman G.J. Is gut microbial LPS a potential trigger of juvenile idiopathic arthritis? J. Rheumatol. 2017;44:1569–1571. doi: 10.3899/jrheum.170791. [DOI] [PubMed] [Google Scholar]
- 18.Taneja V. Arthritis susceptibility and the gut microbiome. FEBS Lett. 2014;588:4244–4249. doi: 10.1016/j.febslet.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Picchianti-Diamanti A., Rosado M.M., D’Amelio R. Infectious agents and inflammation: The role of microbiota in autoimmune arthritis. Front. Microbiol. 2018;8:2696. doi: 10.3389/fmicb.2017.02696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pisetsky D.S. How the gut inflames the joints. Ann. Rheum. Dis. 2018;77:634–635. doi: 10.1136/annrheumdis-2018-212942. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez C.J., Guss J.D., Luna M., Goldring S.R. Links between the microbiome and bone. J. Bone Miner. Res. 2016;31:1638–1646. doi: 10.1002/jbmr.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Villa C.R., Ward W.E., Comelli E.M. Gut microbiota-bone axis. Crit. Rev. Food Sci. Nutr. 2017;57:1664–1672. doi: 10.1080/10408398.2015.1010034. [DOI] [PubMed] [Google Scholar]
- 23.Carratù R., Secondulfo M., de Magistris L., Iafusco D., Urio A., Carbone M.G., Pontoni G., Cartenì M., Prisco F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J. Pediatr. Gastroenterol. Nutr. 1999;28:264–269. doi: 10.1097/00005176-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Kuitunen M., Saukkonen T., Ilonen J., Akerblom H.K., Savilahti E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity. 2002;35:365–368. doi: 10.1080/0891693021000008526. [DOI] [PubMed] [Google Scholar]
- 25.Sapone A., de Magistris L., Pietzak M., Clemente M.G., Tripathi A., Cucca F., Lampis R., Kryszak D., Cartenì M., Generoso M., et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–1449. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 26.Secondulfo M., Iafusco D., Carratù R., deMagistris L., Sapone A., Generoso M., Mezzogiomo A., Sasso F.C., Cartenì M., De Rosa R., et al. Ultrastructural mucosal alterations and increased intestinal permeability in non-celiac, type I diabetic patients. Dig. Liver Dis. 2004;36:35–45. doi: 10.1016/j.dld.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Terjung B., Spengler U. Atypical p-ANCA in PSC and AIH: A hint toward a “leaky gut”? Clin. Rev. Allergy Immunol. 2009;36:40–51. doi: 10.1007/s12016-008-8088-8. [DOI] [PubMed] [Google Scholar]
- 28.Lin R., Zhou L., Zhang J., Wang B. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int. J. Clin. Exp. Pathol. 2015;8:5153–5160. [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi T., Iwaki M., Nakajima A., Nogami A., Yoneda M. Current research on the pathogenesis of NAFLD/NASH and the gut-liver axis: Gut microbiota, dysbiosis, and leaky-gut syndrome. Int. J. Mol. Sci. 2022;23:11689. doi: 10.3390/ijms231911689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fugger L., Jensen L.T., Rossjohn J. Challenges, progress, and prospects of developing therapies to treat autoimmune diseases. Cell. 2020;181:63–80. doi: 10.1016/j.cell.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Kinashi Y., Hase K. Partners in leaky gut syndrome: Intestinal dysbiosis and autoimmunity. Front. Immunol. 2021;12:673708. doi: 10.3389/fimmu.2021.673708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martel J., Chang S.H., Ko Y.F., Hwang T.L., Young J.D., Ojcius D.M. Gut barrier disruption and chronic disease. Trends Endocrinol. Metab. 2022;33:247–265. doi: 10.1016/j.tem.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Camilleri M., Vella A. What to do about the leaky gut. Gut. 2022;71:424–435. doi: 10.1136/gutjnl-2021-325428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christovich A., Luo X.M. Gut microbiota, leaky gut, and autoimmune diseases. Front. Immunol. 2022;13:946248. doi: 10.3389/fimmu.2022.946248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.