Abstract
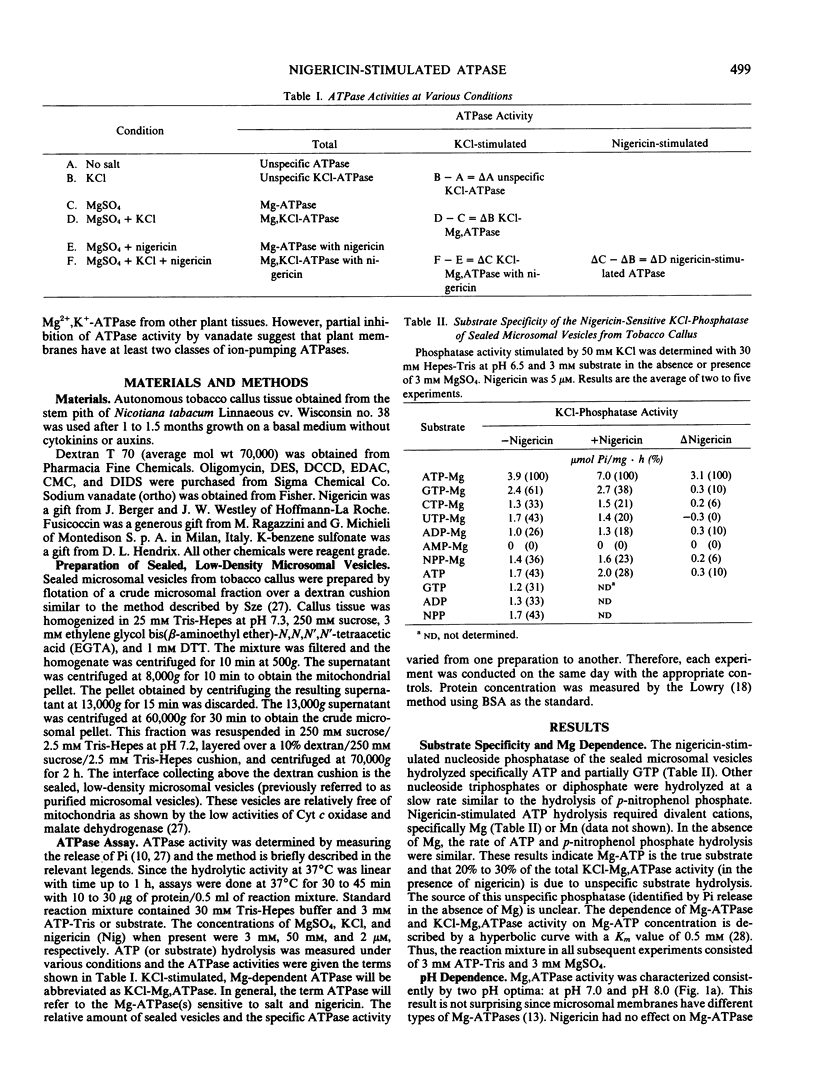
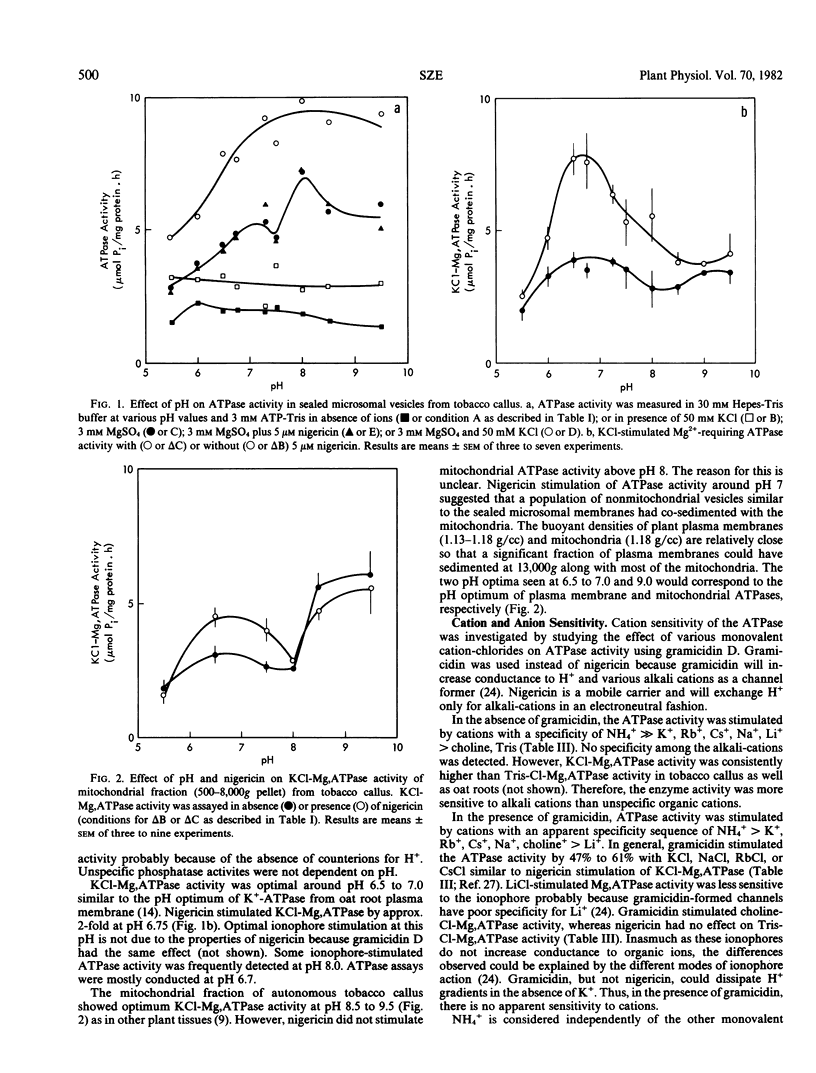
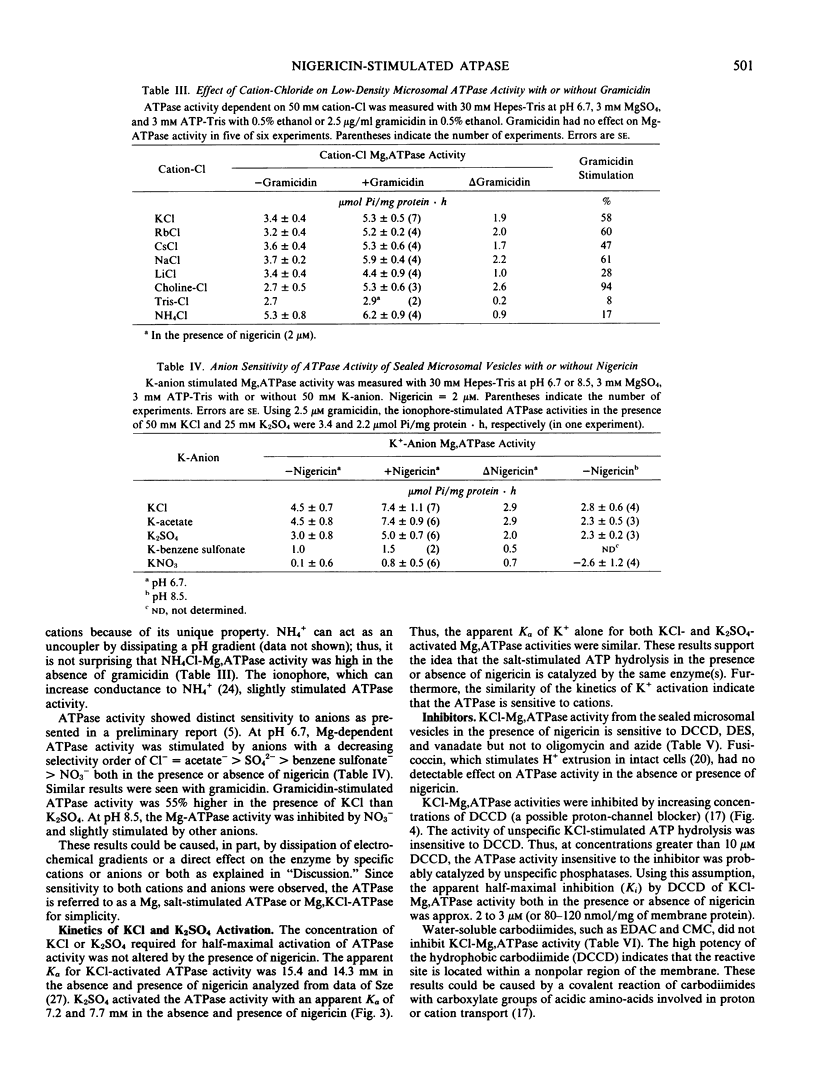
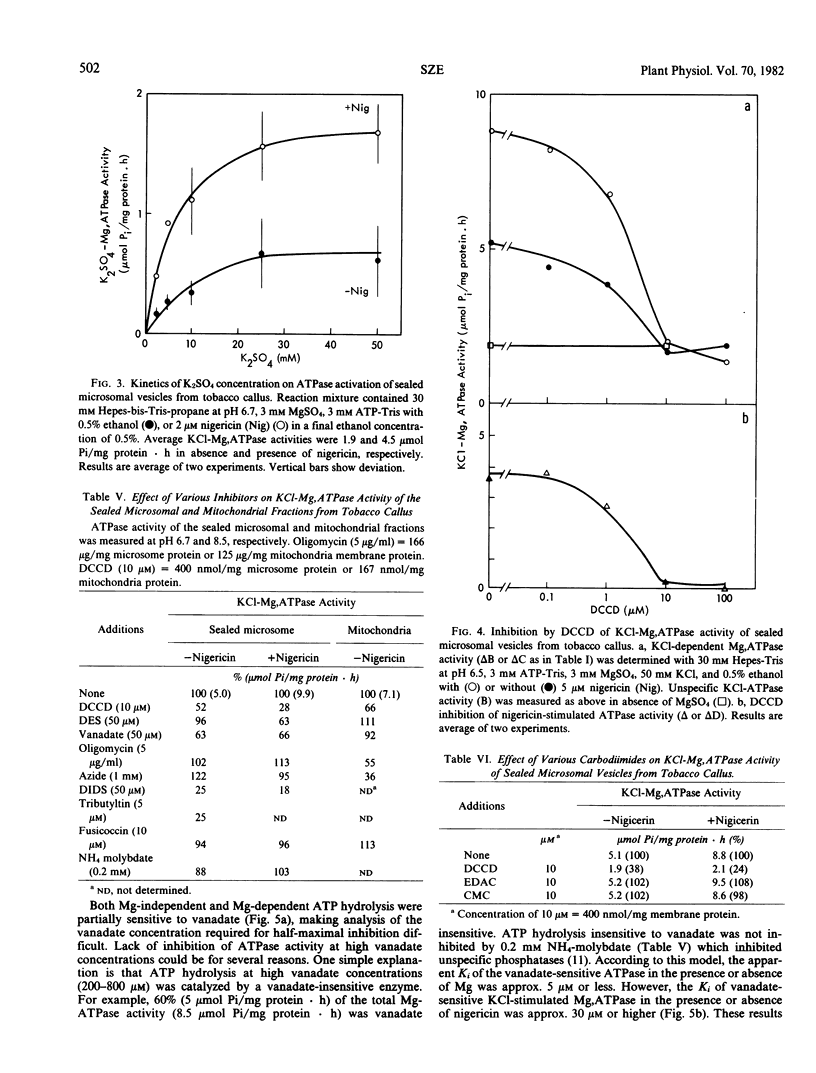
To understand the function and membrane origin of ionophore-stimulated ATPases, the activity of nigericin-stimulated ATPase was characterized from a low-density microsomal fraction containing sealed vesicles of autonomous tobacco (Nicotiana tabacum Linnaeous cv. Wisconsin no. 38) callus. The properties of KCl-stimulated, Mg-requiring ATPases (KCl-Mg,ATPase) were similar in the absence or presence of nigericin. Nigericin (or gramicidin) stimulation of a KCl-Mg,ATPase activity was optimum at pH 6.5 to 7.0. The enzyme was inhibited completely by N,N′-dicyclohexylcarbodiimide (10 μm), tributyltin (5 μm), and partially by vanadate (200 μm), but it was insensitive to fusicoccin and mitochondrial ATPase inhibitors, such as azide (1 mm) and oligomycin (5 μg/ml). The ATPase was more sensitive to anions than cations. Cations stimulated ATPase activity with a selectivity sequence of NH4+ > K+, Rb+, Cs+, Na+, Li+ > Tris+. Anions stimulated Mg, ATPase activity with a decreasing sequence of Cl− = acetate > SO42− > benzene sulfonate > NO3−. The anion stimulation was caused partly by dissipation of the electrical potential (interior positive) by permeant anions and partly by a specific ionic effect. Plant membranes had at least two classes of nigericin-stimulated ATPases: one sensitive and one insensitive to vanadate. Many of the properties of the nigericin-sensitive, salt-stimulated Mg,ATPase were similar to a vanadate-sensitive plasma membrane ATPase of plant tissues, yet other properties (anion stimulation and vanadate insensitivity) resembled those of a tonoplast ATPase. These results support the idea that nigericin-stimulated ATPases are mainly electrogenic H+ pumps originated in part from the plasma membrane and in part from other nonmitochondrial membranes, such as the tonoplast.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Inhibition of adenosine triphosphatase activity of the plasma membrane fraction of oat roots by diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):48–52. doi: 10.1104/pp.63.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Slayman C. W. The effects of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979 Apr 25;254(8):2928–2934. [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Ohsumi Y., Anraku Y. Properties of H+-translocating adenosine triphosphatase in vacuolar membranes of SAccharomyces cerevisiae. J Biol Chem. 1981 Nov 10;256(21):10859–10863. [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnett P. E., Beechey R. B. Inhibitors of the ATP synthethase system. Methods Enzymol. 1979;55:472–518. doi: 10.1016/0076-6879(79)55061-7. [DOI] [PubMed] [Google Scholar]
- Sze H., Churchill K. A. Mg/KCl-ATPase of plant plasma membranes is an electrogenic pump. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5578–5582. doi: 10.1073/pnas.78.9.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H., Hodges T. K. Selectivity of alkali cation influx across the plasma membrane of oat roots: cation specificity of the plasma membrane ATPase. Plant Physiol. 1977 Apr;59(4):641–646. doi: 10.1104/pp.59.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze H. Nigericin-stimulated ATPase activity in microsomal vesicles of tobacco callus. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5904–5908. doi: 10.1073/pnas.77.10.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]


