Abstract
Ornithine decarboxylase and arginine decarboxylase activities were measured in roots and buds of tomato (Lycopersicon esculentum Mill. cv. Pearson ms-35) and potato (Solanum tuberosum cv. Desire) plants. In both tomato and potato, the activity of ornithine decarboxylase was the highest at the root tip, decreasing proximally. The same was true for potato buds. In vegetative buds of tomato, the highest activity was found in the youngest leaves. The older the leaf, the lower was orithine decarboxylase activity. Arginine decarboxylase, on the other hand, did not display a similar gradient. These findings are in accordance with the suggestion that in tomato and potato elevated ornithine decarboxylase activity is associated with intense mitotic activity.
Full text
PDF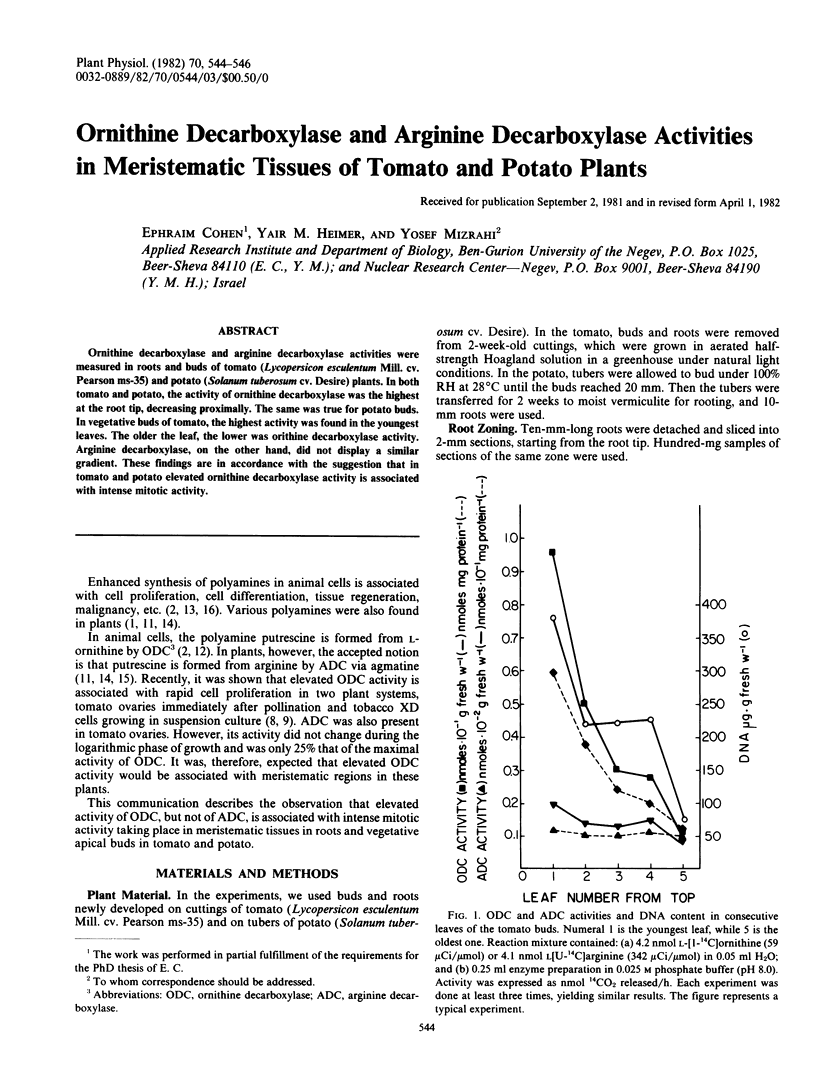


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleksijevic A., Grove J., Schuber F. Studies on polyamine biosynthesis in Euglena gracilis. Biochim Biophys Acta. 1979 Nov 22;565(1):199–207. doi: 10.1016/0005-2787(79)90096-0. [DOI] [PubMed] [Google Scholar]
- Bachrach U. Metabolism and function of spermine and related polyamines. Annu Rev Microbiol. 1970;24:109–134. doi: 10.1146/annurev.mi.24.100170.000545. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cocucci S., Eagni N. Polyamine-induced activation of protein synthesis in ribosomal preparation from Helianthus tuberosus tissue. Life Sci II. 1968 Feb 15;7(4):113–120. doi: 10.1016/0024-3205(68)90294-4. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Mizrahi Y., Bachrach U. Ornithine decarboxylase activity in rapidly proliferating plant cells. FEBS Lett. 1979 Aug 1;104(1):146–148. doi: 10.1016/0014-5793(79)81102-3. [DOI] [PubMed] [Google Scholar]
- Heimer Y. M., Mizrahi Y. Characterization of ornithine decarboxylase of tobacco cells and tomato ovaries. Biochem J. 1982 Feb 1;201(2):373–376. doi: 10.1042/bj2010373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: I. Changes in Intracellular Content and Rates of Synthesis. Plant Physiol. 1978 Sep;62(3):430–433. doi: 10.1104/pp.62.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prouty W. F. Ornithine decarboxylase inactivation in HeLa cells. J Cell Physiol. 1976 Sep;89(1):65–76. doi: 10.1002/jcp.1040890107. [DOI] [PubMed] [Google Scholar]
- Russell D. H. The roles of the polyamines, putrescine, spermidine, and spermine in normal and malignant tissues. Life Sci. 1973 Dec 16;13(12):1635–1647. doi: 10.1016/0024-3205(73)90111-2. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. 1,4-Diaminobutane (putrescine), spermidine, and spermine. Annu Rev Biochem. 1976;45:285–306. doi: 10.1146/annurev.bi.45.070176.001441. [DOI] [PubMed] [Google Scholar]


