Abstract
Background: Metabolic syndrome (MetS) is a group of metabolic abnormalities characterised by central obesity, hypertension, dyslipidaemia, and dysregulation of blood glucose, which is associated with the risk of diabetes, cardiovascular disease, and overall mortality. White blood cell count is a selective marker of acute infection and inflammation, which could provide information on the metabolic status of subjects. This study aims to provide the best evidence on the association between MetS and white blood cell count by determining the effect size of this biomarker. Methods: A systematic review and meta-analysis of studies indexed in the PubMed and Scopus databases were performed. Methodological quality was assessed using the STROBE tool, overall risk of bias using RevMan (Cochrane Collaboration), and quality of evidence using Grade Pro. Results: We included 14 articles comparing leukocyte concentrations in 21,005 subjects with MetS and 66,339 controls. Subjects with MetS had a higher mean leukocyte count, 0.64 cells ×109/L; CI95% 0.55–0.72; p < 0.00001; I2 = 93%. Conclusions: An in-depth evaluation of the relationship of leukocytes in the pathophysiological process of MetS could lead to new insights into early diagnosis.
Keywords: metabolic syndrome, leukocytes, white blood cells, biologic marker
1. Introduction
Metabolic syndrome (MetS) is a group of metabolic abnormalities that includes central obesity, hypertension, dyslipidaemia, and blood glucose disorders. This condition is associated with an increased risk of developing diabetes, cardiovascular disease, and a raised overall mortality rate [1]. In addition, the incidence and prevalence of MetS have increased globally, making this non-communicable disease a major public health hazard [2,3]. Therefore, early diagnosis and prevention of MetS are essential. The underlying pathophysiology involves insulin resistance (IR), chronic low-grade inflammation, and oxidative stress, playing a crucial role in the pathogenesis of MetS [4,5].
Inflammatory markers are generally increased in patients with MetS, but the link between inflammation and the development of MetS is less well established. However, evidence suggests that changes in haematological parameters related to inflammatory processes, such as white blood cell count (WBC) and prothrombotic markers, may be associated with MetS [6,7]. WBC, neutrophils, and lymphocytes are common, inexpensive, and widely used markers of inflammation in the clinical setting [8]. These markers activate the main cell types involved in acute and chronic inflammation [9]. Additionally, white blood cells altered by chronic inflammatory risk factors are more likely to bind and adhere to vascular endothelium, which can cause capillary leukocytosis and eventually lead to vasoconstriction and hypertension [10].
Likewise, WBC count is directly associated with insulin resistance and, inversely, with insulin secretion. Concerning this, WBC count has been shown to predict both worsening insulin sensitivity and the incidence of type 2 diabetes [11]. Furthermore, due to hypertrophy-induced inflammation and leukocyte infiltration, adipose tissue loses sensitivity to insulin, resulting in increased lipolysis and impaired lipid storage, augmenting its dysfunctionality. As a result, free fatty acids and triglycerides are mobilised into the circulation, accumulating lipid derivatives in skeletal muscle, liver, and pancreatic B-cells, leading to impaired tissue function and systemic insulin resistance [12].
Thus, increased WBC may be directly involved in the pathogenesis of MetS by increasing the movement of inflammatory cells into adipose tissue. Prolonged maintenance or worsening of this metabolically dysfunctional state further perpetuates dysregulation of lipid metabolism and immune responses, increasing the individual’s risk of developing a wide range of chronic diseases [13,14].
In addition, previous studies have shown a significant relationship between WBC and MetS [6,15]. In this regard, it has been observed that the number of immune cell subtypes, specifically, the total number of leukocytes, lymphocytes, and monocytes, is higher in individuals with MetS [16]. Therefore, since chronic subclinical inflammation is implicated in the genesis of MetS and WBC can be used as a marker of inflammation, assessing the association between WBC count and the development of MetS may generate a new parameter to aid in its detection.
The primary aim of this systematic review and meta-analysis is to offer the most robust evidence regarding the correlation between Metabolic Syndrome (MetS) and leukocyte levels, ascertaining the magnitude of this biomarker’s impact.
2. Materials and Methods
2.1. Search Strategy and Eligibility Criteria
This systematic review and meta-analysis were conducted according to the criteria established by the PRISMA statement [17] (Supplementary Materials). The search was performed in the PubMed and Scopus databases, covering January 2017 to January 2022. The search methodology was formulated by amalgamating the following Medical Subject Headings (MeSH) descriptors: “metabolic syndrome”, “leukocytes”, and “white blood cells” with the Boolean operator AND. Cross-sectional and longitudinal studies investigating the association between MetS and leukocytes or articles collecting data related to both parameters were included. In addition, the results had to include the mean and standard deviation. Only manuscripts in English and Spanish and those collecting data on subjects older than 18 years were considered. Papers from subjects previously diagnosed with diabetes, obesity or active infections that could increase the level of leukocytes in their study groups were excluded. The systematic review was registered in PROSPERO with ID CRD42022228327.
2.2. Selection of Papers
E.R.C. and M.R.S. conducted independent reviews of all the articles retrieved in the search to remove duplicates. Subsequently, R.J.M., R.M.L., J.M.G.G., and G.M.R., four other authors, individually examined the titles and abstracts, applying eligibility criteria to select the articles that ultimately made it into the review. Lastly, M.V.A., the fifth author, served as a judge in the event of any discrepancies.
2.3. Data Extraction
One researcher (E.R.C) extracted the data, verified by a second investigator (R.J.M). A third researcher (M.R.S) decided in case of disagreement between them. Cohen’s Kappa index was used to assess the degree of agreement. We collected the following information from each study: citation, characteristics of the study population (including age and gender), study methodology, duration of follow-up, sample size, as well as the average and standard deviation of leukocyte levels in individuals with Metabolic Syndrome (MetS+) and those without Metabolic Syndrome (MetS−). In addition, the mean and standard deviation were extracted for reports collecting neutrophil, lymphocyte, and monocyte data.
2.4. Evaluation of the Qualitative Synthesis
A team of four authors (R.M.L, R.J.M, E.R.C, and G.M.R) conducted a thorough qualitative synthesis assessment through a triple analysis:
-
(a)
Methodological quality evaluation was performed using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement [18] for observational studies.
-
(b)
Risk of bias evaluation was conducted using the Cochrane Collaboration tool [19] integrated into the REVMAN 5.4.2 software (Cochrane Collaboration, Copenhagen, Denmark). This analysis assessed risks related to selection, conduct, detection, attrition, and reporting.
-
(c)Evaluating the evidence quality. Utilizing the Grade Pro tool (McMaster University and Evidence Prime), we constructed the evidence profile table, assigning specific levels as outlined [20]:
- High: Strong assurance in aligning the actual and estimated effect;
- Moderate: Reasonable confidence in the estimated effect. The actual effect may differ significantly;
- Low: Restricted confidence in the estimated effect. The actual effect may deviate substantially from the estimate;
- Very Low: Minimal confidence in the estimated effect. The actual effect is highly likely to vary extensively from the estimate.
2.5. Statistical Analysis (Evaluation of Quantitative Synthesis or Meta-Analysis)
The statistical computations and generation of forest and funnel plots for the meta-analysis were conducted using the Cochrane Review Manager software (RevMan 5.4.2). Given the variation in effect sizes among the included studies, a meta-analysis was executed utilizing the Mantel–Haenszel random-effects approach, following the DerSimonian and Laird model. The difference between arithmetic means with a 95% confidence interval was used to measure effect size. Leukocyte count was measured in cells ×109/L. Publication bias risk was evaluated through an examination of the funnel plot. Heterogeneity was assessed by computing the Chi-square test and the inconsistency index (I2). Following the Cochrane Collaboration tool, heterogeneity was categorized as follows: unimportant (0–40%), moderate (30–60%), substantial (50–90%), and considerable (75–100%).
3. Results
3.1. Characteristics of the Studies
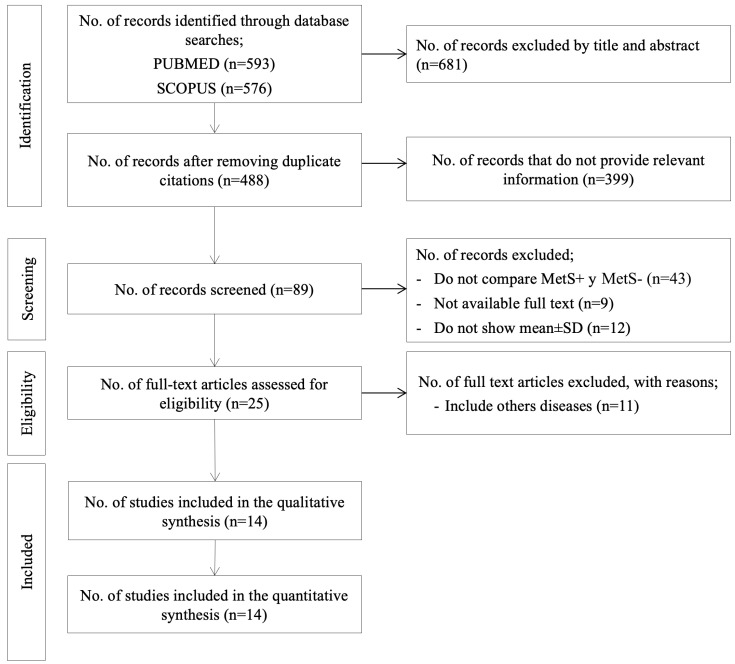
The search yielded 89 records, of which 25 were identified for full-text review (Figure 1). Of these, 14 met the inclusion criteria and were therefore selected for systematic review and meta-analysis.
Figure 1.
PRISMA flow chart. MetS, metabolic syndrome; SD, standard deviation.
Regarding the research design, all studies were observational: 10 cross-sectional studies [10,21,22,23,24,25,26,27,28,29], 3 cohort studies [9,30,31], and 1 case–control study [32]. In total, the 14 papers compared leukocyte concentrations between 21,005 MetS+ and 66,339 MetS− subjects. The ages of the participants ranged from 18 to 85 years. Most of the papers (57.14%) [9,22,24,26,27,28,30,32] included participants of both sexes, but analysed the data globally; 3 studies (21.4%) included only men [21,23,25], and 3 others collected data from men and women separately [10,29,31]. In relation to provenance, half of the articles found were developed in the Chinese population [9,10,22,26,28,29,30,31]. In addition, neutrophil data were extracted from 7 articles [9,22,26,27,28,29,30], lymphocyte data from 6 studies [9,22,24,27,31,32], monocyte data from 4 papers [28,29,30,32], and eosinophil and basophil data from 2 manuscripts [28,29].
MetS was defined according to the National Cholesterol Education Program (NCEP-ATP III) third report criteria [33] in 7 research studies [22,23,24,27,29,31,32]; 3 studies [10,21,28] assessed MetS using the International Diabetes Federation (IDF) definition [34]; 2 studies [25,26] used harmonised criteria [35]; and 2 articles [9,30] as defined by the Chinese Diabetes Society [36].
The in-depth features of the chosen studies can be found in Table 1.
Table 1.
Characteristics of included studies (n = 14).
| Author, Year, Country | Study Design | STROBE18 Reporting Guidelines | Age of Participants | No. of Subjects MetS+/MetS− | MetS Criteria | Results | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmadzadeh et al., 2017, Iran [21] | Cross-sectional study | 19 | Men MetS+ 41.4 ± 9.9 MetS− 36.4 ± 9.6 |
Men 3203/7911 Total 11,114 |
IDF | Increased WBC (p < 0.001) is related to a higher number of MetS criteria. Men MetS+ 7.2 ± 1.7 (WBC) MetS− 6.7 ± 1.7 (WBC) |
|||||
| Chen et al., 2019, China [22] | Cross-sectional study | 20 | MetS+ 56.5 ± 0.5 MetS− 47.6 ± 0.4 |
254/598 Total 852 |
NCEP ATP III | Elevated WBC levels in MetS+ subjects. MetS+ 7.03 ± 0.1 (WBC) MetS− 6.4 ± 0.06 (WBC) |
|||||
| Chen et al., 2020, China [10] | Cross-sectional study | 19 | Women MetS+ 60.7 ± 10.0 MetS− 52.6 ± 12.7 Men MetS+ 57.2 ± 10.5 MetS− 54.8 ± 13.5 |
Women 277/641 Total 918 Men 140/343 Total 483 |
IDF | Haematological parameters, including WBC and subtypes, correlate with the occurrence of MetS. | |||||
| Women MetS+ 6.69 ± 1.67 (WBC) MetS− 6.1 ± 1.53 (WBC) |
Men MetS+ 7.24 ± 1.66 (WBC) MetS− 6.87 ± 1.59 (WBC) |
||||||||||
| Hoi et al., 2017, Japan [23] | Cross-sectional study | 21 | Men MetS+ 49.5 ± 6.5 MetS− 48.8 ± 6.1 |
Men 251/474 Total 725 |
NCEP ATP III | Significantly higher white blood cell count in MetS+ subjects. Men MetS+ 6.57 ± 1.55 (WBC) MetS− 5.95 ± 1.44 (WBC) |
|||||
| Li et al., 2019, China [30] | Retrospective cohort study | 19 | MetS+ 52.5 ± 13.6 MetS− 41.1 ± 13.3 |
120/1948 Total 2068 |
Chinese Diabetes Society | The MetS+ group had higher TSH and inflammation levels, indicated by higher WBC, LY, and Mo/HDL. | |||||
| MetS+ 7.1 ± 2.11 (WBC) MetS− 6.4 ± 1.6 (WBC) MetS+ 2.57 ± 0.79 (Lymphocyte) MetS− 2.25 ± 0.61 (Lymphocyte) |
MetS+ 3.89 ± 1.52 (Neutrophil) MetS− 3.57 ± 1.2 (Neutrophil) MetS+ 0.43 ± 0.15 (Monocyte) MetS− 0.39 ± 0.13 (Monocyte) |
||||||||||
| Lin et al., 2021, China [9] | Cohort study | 20 | MetS+ 45 ± 11.6 MetS− 44.9 ± 13.18 |
179/1363 Total 1542 |
Chinese Diabetes Society | Subjects with MetS+ have higher levels of leukocytes, neutrophils, and total lymphocytes. Elevated levels of leukocytes, neutrophils, and lymphocytes increased the incidence of MetS. | |||||
| MetS+ 6.6 ± 1.4 (WBC) MetS− 6.21 ± 1.3 (WBC) |
MetS+ 3.6 ± 1.03 (Neutrophil) MetS− 3.39 ± 0.94 (Neutrophil) |
MetS+ 2.39 ± 0.68 (Lymphocyte) MetS− 2.25 ± 0.56 (Lymphocyte) |
|||||||||
| Liu C et al., 2019, Taiwan [24] | Cross-sectional study. | 19 | MetS+ 50.4 ± 11.1 MetS− 45.6 ± 11.1 |
10,475/23,538 Total 34,013 |
NCEP ATP III | Inflammatory biomarkers (WBC, CRP, and Hs-CRP), lipid markers (total cholesterol, triglycerides, and LDL-cholesterol), and glycaemic markers (fasting glucose, HbA1c, insulin, HOMA-IR, and SUA) were on average higher in the MetS+ group than in MetS− (p < 0.001). MetS+ 6.83 ± 1.72 (WBC) MetS− 6.05 ± 1.45 (WBC) |
|||||
| Mauss et al., 2020, Germany [25] | Cross-sectional study | 19 | Men MetS+ 49.5 ± 8.1 MetS− 44.5 ± 9.9 |
Men 137/552 Total 689 |
Harmonised criteria | Total leukocyte count and CRP were higher in the MetS+ group, while leukocyte ratios showed no significant differences. Men MetS+ 7.1 ± 1.81 (WBC) MetS− 6.44 ± 1.68 (WBC) |
|||||
| Meng et al., 2017, China [26] | Cross-sectional study | 21 | MetS+ 52.7 ± 9.7 MetS− 48.9 ± 9.7 |
2292/4020 Total 6312 |
Harmonised criteria | They observe that leukocyte, neutrophil, and lymphocyte concentrations are associated with MetS. | |||||
| MetS+ 5.84 ± 1.46 (WBC) MetS− 5.32 ± 1.29 (WBC) |
MetS+ 3.29 ± 0.97 (Neutrophil) MetS− 2.98 ± 0.97 (Neutrophil) |
MetS+ 1.98 ± 0.49 (Lymphocyte) MetS− 1.77 ± 0.65 (Lymphocyte) |
|||||||||
| Tanaka et al., 2020, China [31] | Cohort study | 19 | Women MetS+ 55.2 ± 10.4 MetS− 44.8 ± 9.8 Men MetS+ 50.3 ± 9.4 MetS− 44.8 ± 9.7 |
Women 401/8035 Total 8436 Men 1184/10,542 Total 11,726 |
NCEP ATP III | Higher levels of WBC are observed in the MetS group. | |||||
|
Women MetS+ 6.0 ± 1.5 (WBC) MetS− 5.3 ± 1.4 (WBC) |
Men MetS+ 6.6 ± 1.7 (WBC) MetS− 5.7 ± 1.5 (WBC) |
||||||||||
| Uslu et al., 2018, Turkey [32] |
Case–control study | 19 | MetS+ 47 ± 13.5 MetS− 44 ± 15.2 |
147/134 Total 281 |
NCEP ATP III | MHR is a useful inflammatory marker to assess MetS and disease severity. | |||||
| MetS+ 7.96 ± 2.63 (WBC) MetS− 6.69 ± 1.58 (WBC) |
MetS+ 0.59 ± 0.26 (Monocyte) MetS− 0.48 ± 0.16 (Monocyte) |
||||||||||
| Vahit et al., 2017, Turkey [27] |
Cross-sectional study | 20 | MetS + 57.4 ± 8.8 MetS− 56.3 ± 9.1 |
371/391 Total 762 |
NCEP ATP III | MRLs such as MHR may be novel and valuable indicators in MetS. | |||||
| MetS+ 7.55 ± 1.66 (WBC) MetS− 7.49 ± 1.69 (WBC) |
MetS + 4.32 ± 1.34 (Neutrophil) MetS− 4.51± 1.36 (Neutrophil) |
||||||||||
| Xie et al., 2021, China. [28] |
Cross-sectional study | 19 | MetS+ 26.1 MetS− 25.7 |
655/2189 Total 2844 |
IDF | Lasso’s logistic regression algorithm helped to identify MetS with high accuracy in an occupational population. | |||||
| MetS+ 7.37 ± 1.79 (WBC) MetS− 6.68 ± 1.65 (WBC) MetS+ 0.42 ± 0.15 (Monocyte) MetS− 0.39 ± 0.13 (Monocyte) MetS+ 0.17 ± 0.13 (Eosinophil) MetS− 0.18 ± 0.18 (Eosinophil) |
MetS+ 2.45 ± 0.69 (Lymphocytes) MetS− 2.39 ± 0.71 (Lymphocytes) MetS+ 4.32 ± 1.42 (Neutrophil) MetS− 3.71 ± 1.25 (Neutrophil) MetS+ 0.07 ± 0.16 (Basophil) MetS− 0.05 ± 0.11 (Basophil) |
||||||||||
| Yang et al., 2020, China. [29] |
Cross-sectional study | 19 | ≥60 years | Women 608/1771 Total 2379 Men 311/1889 Total 2200 |
NCEP ATP III | They observe interactions between leukocytes, monocytes, neutrophils, and sex in MetS. | |||||
|
Women MetS+ 5.68 ± 1.31 (WBC) MetS− 5.15 ± 1.28 (WBC) MetS+ 1.8 ± 0.57 (Lymphocytes) MetS− 1.61 ± 0.51 (Lymphocytes) MetS+ 0.3 ± 0.1 (Monocyte) MetS− 0.28 ± 0.1 (Monocyte) MetS+ 3.41 ± 0.99 (Neutrophil) MetS− 3.1 ± 1.01 (Neutrophil) MetS+ 0.13 ± 0.11 (Eosinophil) MetS− 0.13 ± 0.13 (Eosinophil) MetS+ 0.03 ± 0.02 (Basophil) MetS− 0.03 ± 0.02 (Basophil) |
Men MetS+ 5.87 ± 1.43 (WBC) MetS− 5.48 ± 1.53 (WBC) MetS+ 1.75 ± 0.53 (Lymphocytes) MetS− 1.56 ± 0.62 (Lymphocytes) MetS+ 0.35 ± 0.16 (Monocyte) MetS− 0.34 ± 0.13 (Monocyte) MetS+ 3.56 ± 1.14 (Neutrophil) MetS− 3.4 ± 1.21 (Neutrophil) MetS+ 0.16 ± 0.15 (Eosinophil) MetS− 0.14 ± 0.14 (Eosinophil) MetS+ 0.04 ± 0.02 (Basophil) MetS− 0.03 ± 0.02 (Basophil) |
||||||||||
CRP, C-reactive protein; HbA1c, haemoglobin A1c; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; hsCRP, high-sensitivity C-reactive protein; IDF, International Diabetes Federation; LY, lymphocytes; LMR, lymphocyte-to-monocyte ratio, MetS, metabolic syndrome; MHR, monocyte to high-density lipoprotein cholesterol ratio; Mo/HDL, monocyte/high-density lipoprotein; NCEP ATP III, National Cholesterol Education Program Adult Treatment Panel III; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; SUA, serum uric acid; TSH, thyroid-stimulating hormone; WBC, white blood cells.
3.2. Methodological Quality Assessment
Every report scored 19 or higher out of the 22 items outlined in the STROBE reporting guidelines [18], placing them in the highest tercile. No articles were excluded for poor methodological quality. In Table 1, you can observe the individual scores assigned to each paper.
3.3. Bias Risk Analysis
Overall (Figure 2), it can be seen that the main biases were random sequential generation, allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. Only one of the included articles collected data randomly with allocation concealment [30]. Figure 3 represents the individual assessment of the included studies.
Figure 2.
Overall risk of bias observed in the studies.
Figure 3.
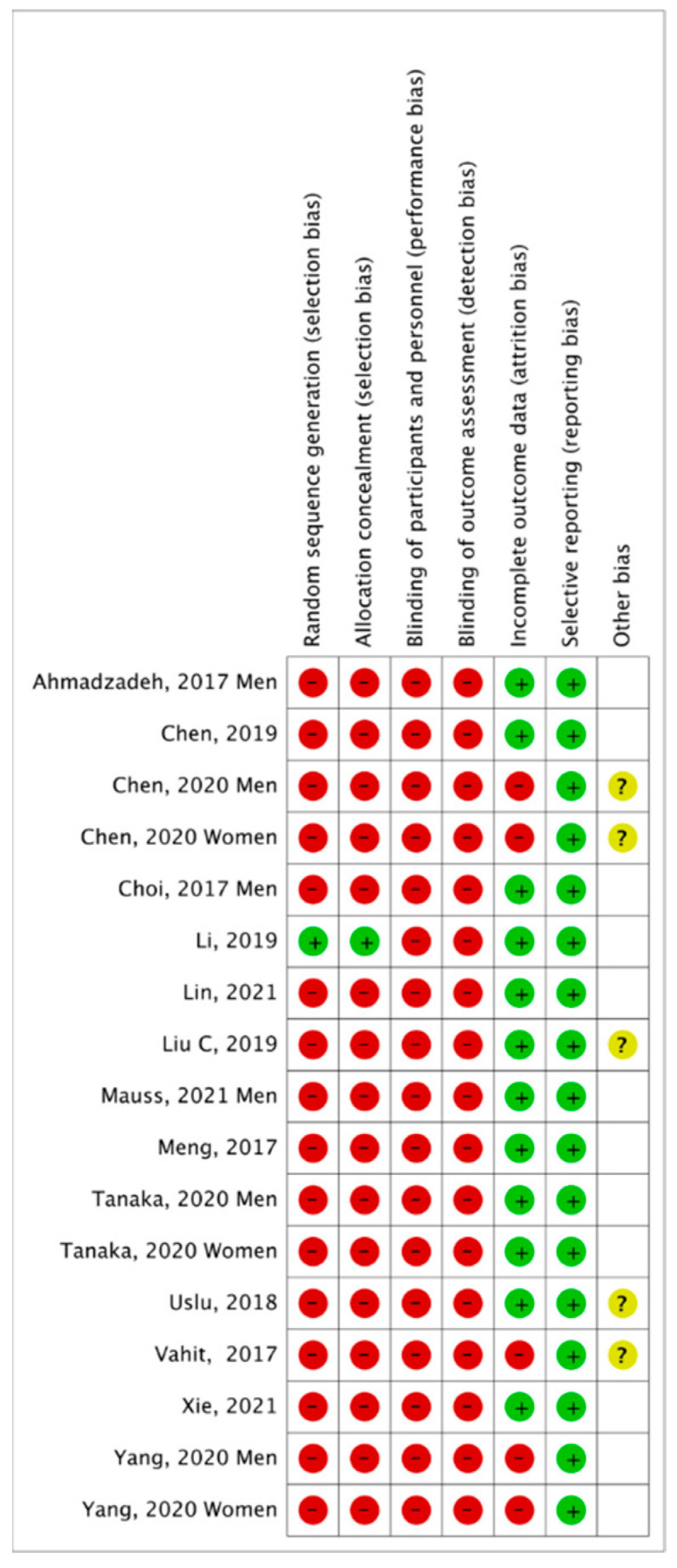
Summary of risk of bias by study [9,10,21,22,23,24,25,26,27,28,29,30,31,32].
3.4. Quantitative Analysis and Meta-Analysis
Figure 4 shows the Forest Plot, including the results for both sexes from the 14 review articles. MetS+ subjects showed a higher mean leukocyte count, namely the mean difference was 0.64 cells ×109/L (CI95% 0.55–0.72; p < 0.00001; I2 = 93%), compared to MetS− subjects.
Figure 4.
Results and summary statistics of studies analysing leukocyte levels in the total population with and without metabolic syndrome (MetS) [9,10,21,22,23,24,25,26,27,28,29,30,31,32].
The Funnel Plot (Figure 5) shows a low risk of publication bias. The sensitivity analysis did not show that any study significantly affected the heterogeneity of the meta-analysis; therefore, no articles were excluded.
Figure 5.
Funnel plot.
MetS+ subjects showed a higher mean number of neutrophils, specifically, the mean difference was 0.28 cells ×109/L (CI95% 0.2–0.36; p < 0.00001; I2 = 88%), compared to MetS− subjects (Figure 6).
Figure 6.
Results and summary statistics of studies analysing neutrophil levels in the total population with and without metabolic syndrome (MetS) [9,22,26,27,28,29,30].
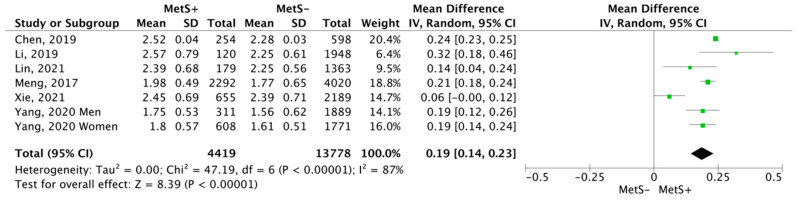
In relation to lymphocytes (Figure 7), MetS+ subjects showed a higher mean, the mean difference was 0.19 cells ×109/L (CI95% 0.14–0.23; p < 0.00001; I2 = 87%), compared to MetS− subjects.
Figure 7.
Results and summary statistics of studies analysing lymphocyte levels in the total population with and without metabolic syndrome (MetS) [9,22,26,28,29,30].
3.5. Quality of Evidence
Table 2 shows the evidence profile of the meta-analysis, providing specific information regarding the overall certainty of the evidence of the studies included in the comparison, the magnitude of the studies examined, and the sum of the data available for the outcomes assessed.
Table 2.
Evidence profile with GRADE pro for the meta-analyses.
| Certainty Assessment | No. of Subjects | Size of the Effect | Quality of Evidence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirect Evidence | Imprecision | Other Considerations | MetS+ | MetS− | Mean Difference (95% CI) | |
| Meta-analysis White blood cells | ||||||||||
| n = 14 | Observational studies | serious | Very serious | It is not serious | It is not serious | dose-response gradient | 21,005 | 66,339 | 0.64 (0.55–0.72) | ⨁◯◯◯ Very low |
| Meta-analysis Neutrophils | ||||||||||
| n = 7 | Observational studies | serious | Very serious | It is not serious | It is not serious | dose-response gradient | 4790 | 14,169 | 0.28 (0.2–0.36) | ⨁◯◯◯ Very low |
| Meta-analysis Lymphocytes | ||||||||||
| n = 6 | Observational studies | serious | Very serious | It is not serious | It is not serious | dose-response gradient | 4419 | 13,778 | 0.19 (0.14–0.23) | ⨁◯◯◯ Very low |
MetS, metabolic syndrome; CI, confidence interval.
4. Discussion
A comprehensive review and meta-analysis were performed to examine the latest evidence regarding the association between Metabolic Syndrome (MetS) and leukocyte levels. Fourteen articles were selected to quantify the size effect and the limitations that have conditioned their results. All demonstrated sufficient reliability and methodological quality regarding the association between leukocytes and MetS.
The present meta-analysis shows the relationship between the level of leukocytes and MetS. The leucocyte concentration in the 21,005 MetS+ subjects was significantly higher than in the group of 66,339 controls (mean difference (MD): 0.64 cells ×109/L; CI95% 0.55–0.72; p < 0.00001).
The results of this review support how elevated white blood cell count is closely related to MetS. The mechanisms that explain this association are not entirely clear, but some possibilities have been suggested. On the one hand, IR, defined as the decreased capability of insulin to stimulate glucose uptake by muscle and adipose tissues and to suppress hepatic glucose production [37], may contribute to metabolic disturbances and accumulation of inflammatory markers, such as total leukocytes and other inflammatory factors [29].
On the other hand, MetS indicates metabolic dysregulation or dysfunction, strongly associated with atherosclerotic cardiovascular disease and often accompanied by chronic low-grade inflammation [4,38]. This inflammation can induce the synthesis of several groups of cytokines and proteolytic enzymes and decrease the formation of prostacyclin and nitric oxide, which can cause impaired endothelial integrity and functional impairment, leading to an increase in white blood cells and their subtypes [9,11,13]. Furthermore, TNF-a has been shown to be consistently expressed in adipose tissue, and these proinflammatory cytokines lead to elevated leukocyte levels [39]. This increase may lead to hypertension and loss of vasodilatory capacity [40]. The study by Marques P et al. [41] reports that neutralising chemokine axes partially inhibit leukocyte adhesion through altered adhesiveness of proinflammatory monocytes to dysfunctional endothelium, suggesting a potential link between the systemic inflammatory response and the development of CVD in MetS.
In addition, Lorenzo et al. note that elevated total white blood cell, neutrophil, and lymphocyte counts can be detected in people at increased risk of diabetes due to insulin sensitivity and low-grade inflammation [14]. Metabolic alterations and inflammation enter a vicious cycle of T-cell activation, senescence, and proinflammatory cytokine production that worsens pathological conditions [42].
Our results are consistent with reported associations between leukocytes and MetS. Previous longitudinal and cross-sectional studies have associated WBC with the incidence and prevalence of MetS [6,43]. The cross-sectional study by Babio et al. [44] demonstrates that WBC count was associated with increased risk and prevalence of MetS and concluded that WBC count is positively associated with three parameters used as defining criteria for MetS: hyperglycaemia, HDL-cholesterol, and hypertriglyceridaemia. Therefore, circulating white blood cells could represent a critical factor in the study of obesity and its associated comorbidities, such as MetS and CVD [45]. In addition, the study by Wang et al. [46] confirms that monitoring longitudinal changes in leukocyte markers may help provide a strategy for primary prevention of future cardiovascular events. Thus, cardiometabolic risk factors contribute to developing and worsening this proinflammatory and prothrombotic state associated with MetS, leading to detrimental metabolic conditions. Many of these conditions are acquired through lifestyle and are modifiable, indicating the importance of prevention and treatment methods to improve cardiometabolic risk factors to reduce their impact on MetS [47,48].
5. Limitations and Strengths
In this kind of research, evaluating the potential biases in study methodologies is a crucial concern under PRISMA guidelines. Studies with similar methodologies but discrepancies in quality may have biased results. The quality of the evidence obtained is “very low” because observational studies have been analysed. These study designs pose a high bias risk and show a very high inconsistency (heterogeneity). The authors were unable to thoroughly examine the impact of adjustment for all known and potential risk factors due to the varying degrees of adjustment for confounding factors across individual studies. One of the main strengths of this review is a large sample size of subjects with and without MetS was included, which increased the study’s statistical power. However, analysing the findings in this systematic review and meta-analysis should be conducted with caution, considering some limitations. Firstly, non-randomised comparisons in observational studies may suffer from biases, which could affect the results and thus weaken the strength of the evidence. Secondly, the different criteria or definitions used to diagnose MetS in the included studies may influence the determination and identification of affected individuals. Also, the treatment approach and health objectives may change depending on the definition. Third, with increasing age, there is decreased adaptive immunity and increased inflammation or immunoaging, which affects the levels of proinflammatory cytokines that can alter the leukocyte profile [49]. Fourth, most studies come from the Far East region, making it difficult to generalize the results to other countries. Fifth, further research is required to identify the importance of increased neutrophils and lymphocytes in MetS and other cardiovascular diseases. Finally, another limitation was that no additional strategies were used in the current search to locate unpublished reviews (grey literature).
6. Conclusions
The results have shown that subjects with MetS have higher levels of leukocytes (0.64 cells ×109/L; CI95% 0.55–0.72; p < 0.00001), neutrophils (0.28 cells ×109/L; CI95% 0.2–0.36; p < 0.00001), and lymphocytes (0.19 cells ×109/L; CI95% 0.14–0.23; p < 0.00001). These results provide a rationale for further evaluation of the relationship of leukocytes in the pathophysiological process of MetS. They could lead to new insights in early diagnosis, identification of new biomarkers, and discovery of new therapeutic targets for pharmacological interventions. Further research is therefore required to identify the importance of white blood cell counts in MetS or other cardiovascular diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12227044/s1, PRISMA 2020 Checklist [50].
Author Contributions
Study conception and design: E.R.-C., M.R.-S. and R.M.-L.; data collection: E.R.-C., R.J.-M. and R.M.-L.; analysis and interpretation of results: E.R.-C., M.R.-S. and R.M.-L.; draft manuscript preparation: E.R.-C. and M.R.-S.; writing—review and editing: E.R.-C., M.R.-S., R.J.-M., R.M.-L., J.M.G.-G., M.V.-A. and G.M.-R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Materials. The primary findings of the study are incorporated in the article; for additional information, please contact the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Laaksonen D., Lakka H.M., Niskanen L., Kaplan G.A., Salonen J., Lakka T. Metabolic syndrome and development of diabetes mellitus: Application and validation of recently suggested definitions of the metabolic syndrome in a prospective cohort study. Am. J. Epidemiol. 2002;156:1070–1077. doi: 10.1093/aje/kwf145. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCracken E., Monaghan M., Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018;36:14–20. doi: 10.1016/j.clindermatol.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Gluvic Z., Zaric B., Resanovic I., Obradovic M., Mitrovic A., Radak D., Isenovic E. Link between metabolic syndrome and insulin resistance. Curr. Vasc. Pharmacol. 2017;15:30–39. doi: 10.2174/1570161114666161007164510. [DOI] [PubMed] [Google Scholar]
- 5.Lugrin J., Rosenblatt-Velin N., Parapanov R., Liaudet L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014;39:203–230. doi: 10.1515/hsz-2013-0241. [DOI] [PubMed] [Google Scholar]
- 6.Yang H., Fu Y.-Q., Yang B., Zheng J.-S., Zeng X.-Y., Zeng W., Fan Z.-F., Chen M., Wang L., Li D. Positive association between the metabolic syndrome and white blood cell counts in Chinese. Asia Pac. J. Clin. Nutr. 2017;26:141–147. doi: 10.6133/apjcn.102015.13. [DOI] [PubMed] [Google Scholar]
- 7.Karaman A., Aydin H., Geckinli B., Cetinkaya A., Karaman S. DNA damage is increased in lymphocytes of patients with metabolic syndrome. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2015;782:30–35. doi: 10.1016/j.mrgentox.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Fadini G., Marcuzzo G., Marescotti M.C., Kreutzenberg S., Avogaro A. Elevated white blood cell count is associated with prevalence and development of the metabolic syndrome and its components in the general population. Acta Diabetol. 2012;49:445–451. doi: 10.1007/s00592-012-0402-5. [DOI] [PubMed] [Google Scholar]
- 9.Lin H.Y., Zhang X.J., Liu Y.M., Geng L.Y., Guan L.Y., Li X.H. Comparison of the triglyceride glucose index and blood leukocyte indices as predictors of metabolic syndrome in healthy Chinese population. Sci. Rep. 2021;11:10036. doi: 10.1038/s41598-021-89494-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T., Chen H., Xiao H., Tang H., Xiang Z., Wang X., Wang X., Zou H. Comparison of the Value of Neutrophil to High-Density Lipoprotein Cholesterol Ratio and Lymphocyte to High-Density Lipoprotein Cholesterol Ratio for Predicting Metabolic Syndrome Among a Population in the Southern Coast of China. Diabetes Metab. Syndr. Obes. 2020;13:597–605. doi: 10.2147/DMSO.S238990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horne B.D., Anderson J.L., John J.M., Weaver A., Bair T.L., Jensen K.R., Renlund D.G., Muhlestein J.B. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 12.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar A., Klein B.E., Klein R. Relationship between white blood cell count and incident hypertension. Am. J. Hypertens. 2004;17:233–239. doi: 10.1016/j.amjhyper.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo C., Hanley A.J., Haffner S.M. Differential white cell count and incident type 2 diabetes: The Insulin Resistance Atherosclerosis Study. Diabetologia. 2014;57:83–92. doi: 10.1007/s00125-013-3080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdel Moneim A., Mahmoud B., Sultan E.A., Mahmoud R. Relationship of leukocytes, platelet indices and adipocytokines in metabolic syndrome patients. Diabetes Metab. Syndr. 2019;13:874–880. doi: 10.1016/j.dsx.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Ter Horst R., Van den Munckhof I.C., Schraa K., Aguirre-Gamboa R., Jaeger M., Smeekens S.P., Brand T., Lemmers H., Dijkstra H., Galesloot T.E., et al. Sex-Specific Regulation of Inflammation and Metabolic Syndrome in Obesity. Arterioscler. Thromb. Vasc. Biol. 2020;40:1787–1800. doi: 10.1161/ATVBAHA.120.314508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urrutia G., Bonfill X. PRISMA statement: A proposal to improve the publication of systematic reviews and meta-analyses. Med. Clin. 2010;135:507–511. doi: 10.1016/j.medcli.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Von E.E., Altman D.G., Egger M., Pocock Stuart J., Gøtzsche C., Vandenbroucke P. STROBE (Strengthening the Reporting of Observational studies in Epidemiology) Initiative Statement: Guidelines for reporting observational studies. Gac. Sanit. 2008;22:144–150. doi: 10.1157/13119325. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C., et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Ahmadzadeh J., Mansorian B., Attari M.M.-A., Mohebbi I., Naz-Avar R., Moghadam K., Ghareh-Bagh S.A.K. The Association between Hematological Parameters and Metabolic Syndrome in Iranian Men: A Single Center Large-Scale Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2018;12:17–21. doi: 10.1016/j.dsx.2017.07.044. [DOI] [PubMed] [Google Scholar]
- 22.Chen H., Xiong C., Shao X., Ning J., Gao P., Xiao H., Chen Y., Zou Z., Hong G., Li X., et al. Lymphocyte To High-Density Lipoprotein Ratio As A New Indicator Of Inflammation And Metabolic Syndrome. Diabetes Metab. Syndr. Obes. 2019;14:2117–2123. doi: 10.2147/DMSO.S219363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi E.S., Cho S.H., Kim J.H. Relationship between rectus abdominis muscle thickness and metabolic syndrome in middle-aged men. PLoS ONE. 2017;12:e0185040. doi: 10.1371/journal.pone.0185040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Zhang L., Huang Y., Yang P., Xu W. A Mechanism Exploration of Metabolic Syndrome Causing Nodular Thyroid Disease. Int. J. Endocrinol. 2019;2019:9376768. doi: 10.1155/2019/9376768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu C.-C., Ko H.-J., Liu W.-S., Hung C.-L., Hu K.-C., Yu L.-Y., Shih S.-C. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine. 2019;98:e17537. doi: 10.1097/MD.0000000000017537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mauss D., Herr R.M., Jarczok M.N., Motoc I., Fischer J.E., Bosch J.A. The association of cortisol levels with leukocyte distribution is disrupted in the metabolic syndrome. Obes. Res. Clin. Pract. 2020;15:78–84. doi: 10.1016/j.orcp.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Meng G., Zhu Q., Shao J., Zhang Q., Liu L., Wu H., Xia Y., Bao X., Gu Y., Wang H., et al. Comparing the diagnostic ability of inflammatory markers in metabolic syndrome. Clin. Chim. Acta. 2017;475:1–6. doi: 10.1016/j.cca.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka M., Okada H., Hashimoto Y., Kumagai M., Nishimura H., Fukui M. Combined effect of hemoglobin and mean corpuscular volume levels on incident metabolic syndrome: A population-based cohort study. Clin. Nutr. ESPEN. 2020;40:314–319. doi: 10.1016/j.clnesp.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Uslu A.U., Sekin Y., Tarhan G., Canakcı N., Gunduz M., Karagulle M. Evaluation of Monocyte to High-Density Lipoprotein Cholesterol Ratio in the Presence and Severity of Metabolic Syndrome. Clin. Appl. Thromb. Hemost. 2018;24:828–833. doi: 10.1177/1076029617741362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vahit D., Akboga M.K., Samet Y., Hüseyin E. Assessment of monocyte to high density lipoprotein cholesterol ratio and lymphocyte-to-monocyte ratio in patients with metabolic syndrome. Biomark. Med. 2017;11:535–540. doi: 10.2217/bmm-2016-0380. [DOI] [PubMed] [Google Scholar]
- 31.Xie Q.Y., Wang M.W., Hu Z.Y., Cao C.J., Wang C., Kang J.Y., Fu X.-Y., Zhang X.-W., Chu Y.-M., Feng Z.-H., et al. Screening the Influence of Biomarkers for Metabolic Syndrome in Occupational Population Based on the Lasso Algorithm. Front. Public Health. 2021;9:743731. doi: 10.3389/fpubh.2021.743731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang X.J., Tian S., Ma Q.H., Sun H.P., Xu Y., Pan C.W. Leukocyte-related parameters in older adults with metabolic syndrome. Endocrine. 2020;68:312–319. doi: 10.1007/s12020-020-02243-2. [DOI] [PubMed] [Google Scholar]
- 33.Expert Panel on Detection, Evaluation Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 34.Alberti G., Zimmet P., Shaw J. The metabolic syndrome a new worldwide definition. IDF epidemiology task force consensus group. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 35.Alberti K.G.M.M., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.-C., James W.P.T., Loria C.M., Smith S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/circulationaha.109.192644. [DOI] [PubMed] [Google Scholar]
- 36.Chinese Medical Association . The Suggestion on Chinese Metabolic Syndrome. Chinese Medical Association; Shanghai, China: 2004. [Google Scholar]
- 37.Mansyur M.A., Bakri S., Patellongi I.J., Rahman I.A. The association between metabolic syndrome components, low-grade systemic inflammation and insulin resistance in non-diabetic Indonesian adolescent male. Clin. Nutr. ESPEN. 2020;35:69–74. doi: 10.1016/j.clnesp.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Esser N., Legrand Poels S., Piette J., Scheen A.J., Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105:141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Kawanishi N., Yano H., Yokogawa Y., Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in highfat-diet-induced obese mice. Exerc. Immunol. Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 40.Su B.Y., Tian C.F., Gao B.L., Tong Y.H., Zhao X.H., Zheng Y. Correlation of the leucocyte count with traditional and non-traditional components of metabolic syndrome. Postgrad. Med. 2016;128:805–809. doi: 10.1080/00325481.2016.1243980. [DOI] [PubMed] [Google Scholar]
- 41.Marques P., Collado A., Martinez-Hervás S., Domingo E., Benito E., Piqueras L., Real J.T., Ascaso J.F., Sanz M.-J. Systemic Inflammation in Metabolic Syndrome: Increased Platelet and Leukocyte Activation, and Key Role of CX3CL1/CX3CR1 and CCL2/CCR2 Axes in Arterial Platelet-Proinflammatory Monocyte Adhesion. J. Clin. Med. 2019;8:708. doi: 10.3390/jcm8050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corrado M., Pearce E.L. Targeting memory T cell metabolism to improve immunity. J. Clin. Investig. 2022;132:e148546. doi: 10.1172/JCI148546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li P.-F., Chen J.-S., Chang J.-B., Chang H.-W., Wu C.-Z., Chuang T.-J., Huang C.-L., Pei D., Hsieh C.-H., Chen Y.-L. Association of complete blood cell counts with metabolic syndrome in an elderly population. BMC Geriatr. 2016;16:10. doi: 10.1186/s12877-016-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Babio N., Ibarrola-Jurado N., Bulló M., Martínez-González M., Wärnberg J., Salaverría I., Ortega-Calvo M., Estruch R., Serra-Majem L., Covas M.I., et al. White blood cell counts as risk markers of developing metabolic syndrome and its components in the PREDIMED study. PLoS ONE. 2013;8:e58354. doi: 10.1371/journal.pone.0058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryder E., Diez Ewald M., Mosquera J., Fernández E., Pedreañez A., Vargas R., Peña C., Fernández N. Association of obesity with leukocyte count in obese individuals without metabolic syndrome. Diabetes Metab. Syndr. 2014;8:197–204. doi: 10.1016/j.dsx.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Guo Q., Zhou L., Li W., Yuan Y., Lei W., Liu K., Xu M., Diao T., Gao H., et al. Associations of Baseline and Changes in Leukocyte Counts with Incident Cardiovascular Events: The Dongfeng-Tongji Cohort Study. J. Atheroscler. Thromb. 2022;29:1040–1058. doi: 10.5551/jat.62970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lender D., Sysko S.K. The metabolic syndrome and cardiometabolic risk: Scope of the problem and current standard of care. Pharmacotherapy. 2006;26:3S–12S. doi: 10.1592/phco.26.5part2.3S. [DOI] [PubMed] [Google Scholar]
- 48.Ghafouri A., Estêvão M.D., Alibakhshi P., Pizarro A.B., Kashani A.F., Persad E., Heydari H., Hasani M., Heshmati J., Morvaridzadeh M. Sumar fruit supplemetentation improve glycemic parameters in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis. Phytomedicine. 2021;90:153661. doi: 10.1016/j.phymed.2021.153661. [DOI] [PubMed] [Google Scholar]
- 49.Kasten-Jolly J., Lawrence D.A. Differential blood leukocyte populations based on individual variances and age. Immunol. Res. 2022;70:114–128. doi: 10.1007/s12026-021-09257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Supplementary Materials. The primary findings of the study are incorporated in the article; for additional information, please contact the corresponding author.