Abstract
Cardiac resynchronization therapy (CRT) via biventricular pacing (BiVP-CRT) is considered a mainstay treatment for symptomatic heart failure patients with reduced ejection fraction and wide QRS. However, up to one-third of patients receiving BiVP-CRT are considered non-responders to the therapy. Multiple strategies have been proposed to maximize the percentage of CRT responders including two new physiological pacing modalities that have emerged in recent years: His bundle pacing (HBP) and left bundle branch area pacing (LBBAP). Both pacing techniques aim at restoring the normal electrical activation of the ventricles through the native conduction system in opposition to the cell-to-cell activation of conventional right ventricular myocardial pacing. Conduction system pacing (CSP), including both HBP and LBBAP, appears to be a promising pacing modality for delivering CRT and has proven to be safe and feasible in this particular setting. This article will review the current state of the art of CSP-based CRT, its limitations, and future directions.
Keywords: cardiac resynchronization therapy, His bundle pacing, left bundle branch pacing, conduction system pacing
1. Introduction
Cardiac resynchronization therapy (CRT) is an established treatment for patients with heart failure (HF), wide QRS, and impaired LV systolic function despite optimal medical treatment [1]. It was first described by Cazeau et al. [2] in 1994 who used four-chamber pacing (biauricular and biventricular pacing [BiVP]) for the treatment of a patient with advanced HF and a left bundle branch block (LBBB) assuming that the electromechanical dyssynchrony induced by the LBBB could be counteracted by this new pacing modality. The standard CRT technique was thereafter refined and consisted of the transvenous implantation of a right atrial lead, an RV lead, and a left ventricular (LV) lead implanted in a tributary branch of the coronary sinus (CS) in order to obtain BiVP. Since the initial description, the technique rapidly evolved and multiple observational non-randomized studies first showed significant acute hemodynamic improvements [3,4,5,6]. Subsequently, the first randomized trials demonstrated BiVP-CRT’s benefits in terms of functional capacity, peak oxygen consumption, LV ejection fraction (LVEF) improvement, and a reduction in HF hospitalizations [7,8,9,10,11,12,13]. Finally, over the next decade, multiple large randomized controlled trials showed that CRT delivered through BiVP significantly decreased mortality and HF hospitalizations [14,15,16,17,18,19,20,21,22,23,24,25]. As a result, current guidelines consider BiVP-CRT as a mainstay therapy for patients with symptomatic HF with a reduced ejection fraction and wide QRS in spite of optimal medical therapy (Figure 1).
Figure 1.
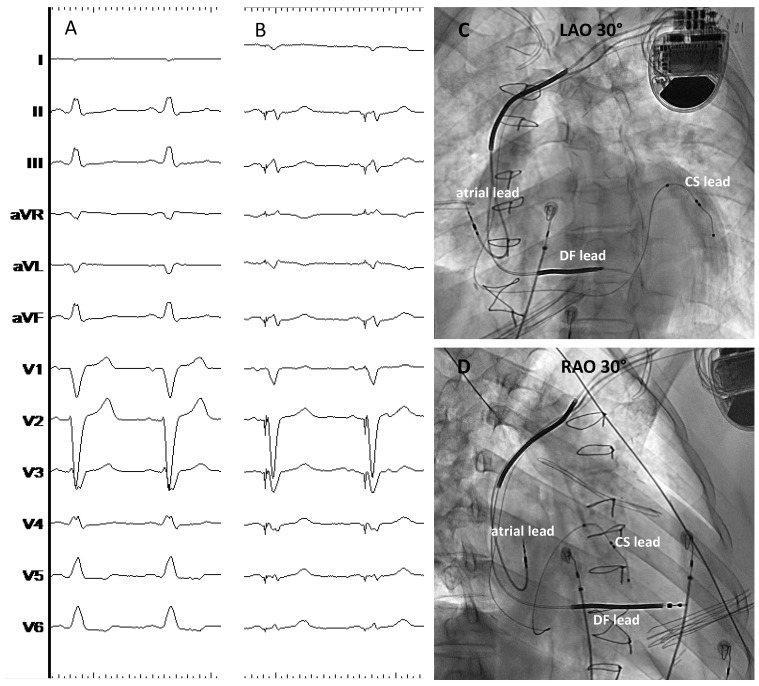
Conventional BiVP-CRT using a quadripolar CS lead in a patient with ischemic cardiomyopathy. Panel (A) shows the baseline QRS with LBBB; panel (B) shows the final paced QRS obtained with BiVP; panels (C,D) show the final lead position in the 30° LAO and RAO views, respectively. CS: Coronary sinus; DF: Defibrillation; LAO: Left anterior oblique view; RAO: Right anterior oblique view. ECG sweep speed 25 mm/s.
However, approximately one-third of patients implanted with a BiVP-CRT device show no clinical or echocardiographic improvement and are considered non-responders to the therapy. Moreover, and in spite of the improvement in implant tools and device technology, there is still a small percentage of patients in which either the implant of a CS lead is not successful or, once implanted, optimal resynchronization is hampered by a high pacing threshold or by the presence of phrenic nerve stimulation [26,27,28]. As a result, different strategies have emerged in order to reduce the percentage of BiVP-CRT non-responders including the use of quadripolar LV leads and the optimization of atrio-ventricular (AV) and interventricular delay (VV) intervals, among many others [29].
Concomitantly, in recent years, a renewed interest in His bundle pacing (HBP) has emerged [30,31,32]. This physiological pacing modality aims for the restoration of the normal electrical cardiac activation sequence through the intrinsic conduction system and has been used for patients with bradycardia pacing indications [30,31,32,33,34]. Different studies have shown that HBP is able to correct intraventricular conduction disturbances including the right bundle branch block (RBBB) and LBBB. In the same manner, but more recently, LBBAP has been described as a second conduction system pacing modality (CSP) [35,36,37,38], and both techniques have been proposed as potential alternative methods for delivering CRT. This article will review the state of the art on CSP-based CRT.
2. Physiopathology Associated with Asynchronous LV Activation
The electromechanical dyssynchrony induced by the presence of LBBB or by conventional right ventricular (RV) myocardial pacing is the cornerstone explaining CRT’s effectiveness from a physiopathology point of view [39,40,41,42,43]. In patients with normal QRS, the myocardium is activated uniformly and the electrical waveform rapidly spreads through the His–Purkinje system and the bundle branches, resulting in a synchronized depolarization of the ventricles. The normal activation sequence includes an early transseptal activation with apex-to-base, posterior-to-anterior, and endocardial-to-epicardial electrical wave propagation. However, in the presence of an LBBB, the ventricular activation pattern changes starting in the RV as the right bundle branch function is preserved. Then, the activation waveform travels through the interventricular septum from the RV endocardium to the left ventricular (LV) endocardium, finally propagating to the endocardium of the posterolateral LV and completing a significantly slower LV ventricular activation as the electrical waveform travels through myocardial fibers not using the rapidly conducting Purkinje system.
Preclinical studies have shown that both LBBB and RV myocardial pacing are associated with poorer acute hemodynamic parameters in comparison with the normal activation observed with narrow QRS as a result of the mechanical dis-coordination leading to structural, electrical, and contractile remodeling [39,40]. At the cellular level, the dyssynchronous heart typically shows an increase in the apoptosis markers (tumor necrosis factor alpha (TNFα), caspases, and DNA fragmentation), with the development of fibrosis (increasing expression of collagen, matrix metalloproteases (MMPs), transforming growth factor beta (TGFβ), connective tissue growth factor (CTGF), and osteopontin (OPN)) and hypertrophy (increased levels of B-type natriuretic peptide (BNP), myosin heavy chain alpha (MHCα), and CTGF with a reduction in miR133) [44,45]. As a result, LBBB is associated with cardiac adverse remodeling, worsening of systolic and diastolic function, and progressive HF. BiVP plays a key role in correcting the LBBB-induced asynchrony by reducing the interventricular and intraventricular dyssynchrony.
In patients with permanent conventional RV myocardial pacing, a specific entity called “pacemaker-induced cardiomyopathy” (PICM) has also been defined to describe the detrimental effects of the asynchronous activation of the LV due to chronic RV pacing [42,43,46]. PICM has a variable incidence ranging between 10 and 30% depending on the series, and during the last 20 years, different pacing strategies aimed at physiological pacing have emerged, including algorithms to reduce unnecessary RV pacing in patients with preserved intrinsic conduction. However, these strategies are not useful in patients who need permanent RV pacing, and BiVP or CSP-based CRT could play a role in this particular scenario [47,48].
3. The Potential Role of CSP in CRT Candidates
HBP is a physiological pacing modality first described in 1999 by Deshmunk et al. [49]. The objective of this pacing modality is to place a pacing lead in the His bundle area in order to capture the conduction system and restore the physiological activation of the ventricles through the specific conduction system and not in a cell-to-cell fashion as with conventional myocardial RV pacing [50]. HBP was initially evaluated in patients with chronic atrial fibrillation undergoing AV node ablation and thus requiring permanent RV pacing. Subsequently, the safety and feasibility of HBP have also been demonstrated in other conduction disturbances including supra-Hisian and infra-Hisian AV block, and have also shown the capacity to correct both RBBB and LBBB in a variable percentage of patients [30,31,32,33,34]. For this reason, HBP has been proposed as an alternative or complementary technique for CRT.
More recently, a second physiological pacing modality has been described, namely left LBBAP, which includes both left bundle branch pacing (LBBP) and left ventricular septal pacing (LVSP). LBBP was first described by Huang et al. in 2017 [51], and since this initial description, observational studies have demonstrated its safety and feasibility in different scenarios including conventional bradycardia pacing indications [35,36,37,38]. Interestingly, LBBAP has been also tested in patients with wide QRS, demonstrating a high percentage of bundle branch correction with higher implantation success (85–95%) and lower complication rates when compared with HBP. Moreover, acute and mid-term electrical parameters are also superior to those previously described with HBP including lower pacing thresholds and higher R wave sensing amplitudes. As a result, LBBAP has also been investigated as an alternative or complementary technique for CRT.
Using non-invasive epicardial electrocardiographic imaging, Arnold et al. [52] identified CRT candidates in which HBP shortened the left ventricular activation time (LVAT) (18/23, 78%) and then compared the hemodynamic effects of both HBP and conventional BiVP in those patients, showing that HBP was associated with a greater reduction in QRS duration, LVAT, and the left ventricular dyssynchrony index, as well as a better hemodynamic response than conventional BiVP. In the same way, Sussenbek and colleagues recently used ultra-high-frequency electrocardiography (UHF-ECG) to compare ventricular activation patterns during BiVP and LBBAP in patients with baseline LBBB and CRT indication using two principal parameters: e-DYS (the time difference between the first and last activation in V1–V8 leads) and Vdmean (the average of V1–V8 local depolarization durations) [53]. LBBAP was associated with shorter e-DYS and shorter Vdmean than BiVP in spite of a significant reduction in the paced QRS duration in both groups, although this was greater for the LBBAP group, indicating more physiological ventricular activation with LBBAP in comparison with BiVP.
4. Key Concepts and Definitions for CSP-Based CRT
HBP implies the capture of the proximal or distal His bundle resulting in a normal ventricular activation in the presence of a normal conduction through the right and left bundle branches. When no adjacent myocardium is captured, selective HB pacing is defined (S-HBP), while non-selective HBP (NS-HBP) implies the capture of both the HB and part of the surrounding myocardium [54]. Both capture patterns have been associated with comparable benefits in terms of electromechanical resynchronization. However, in patients with CRT indications, baseline wide QRS is usually present due to intraventricular conduction disturbances, typically LBBB. In this particular scenario, it is not enough to have HB capture (either selective or non-selective) but is mandatory to obtain the correction of the bundle branch block with subsequent QRS narrowing in order to be able to restore electrical synchrony (Figure 2). Thus, during HBP-CRT, up to five different capture patterns can be described including S- and NS-HBP, both with or without bundle branch correction, as well as myocardial-only capture. Every HBP-CRT capture pattern will be associated with a particular pacing threshold that should be clearly detailed in order to facilitate adequate device programming and follow-up as only bundle branch correction thresholds (either selective or non-selective) are useful to obtain cardiac resynchronization.
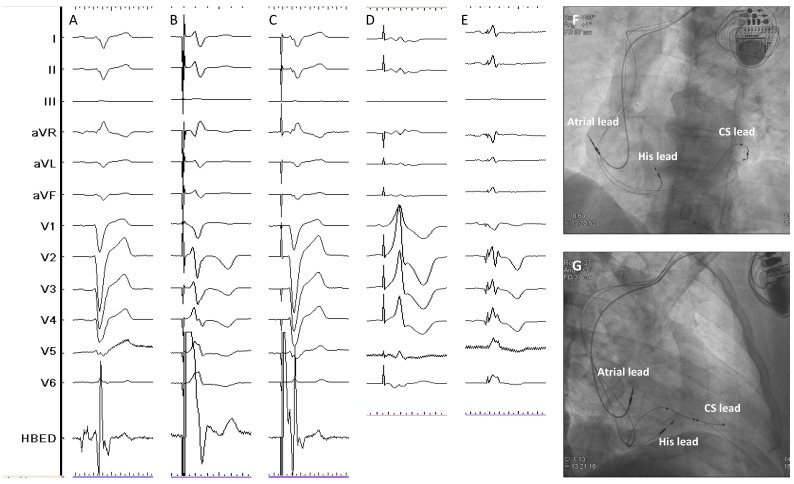
Figure 2.
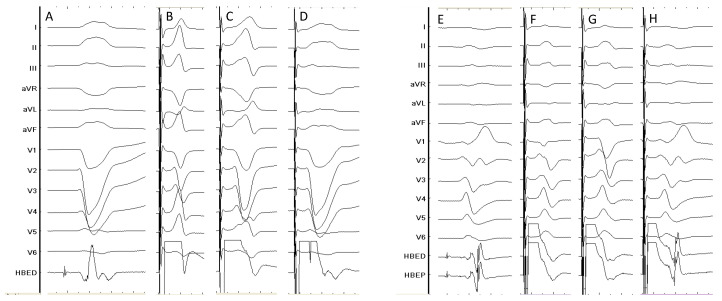
Different capture patterns during HBP-CRT. The left side of the figure shows a patient with baseline LBBB (panel (A)); HBP results in LBBB correction at high outputs (panel (B)), partial LBBB correction at intermediate output (panel (C)) and selective HB capture but without LBBB correction at lower output (panel (D)). The right side shows a patient with baseline RBBB (panel (E)) shows complete RBBB correction at high output (panel (F)), partial correction at intermediate outputs (panel (G)) and selective HB capture without RBBB correction at lower outputs (panel (H)). HBED: His bundle electrogram (distal); HBEP: His bundle electrogram (proximal). Sweep speed 100 mm/s.
On the other hand, LBBP is defined by the direct capture of the LBB or any of its fascicles together with a variable amount of the surrounding myocardium whereas LVSP is characterized by the capture of the LV septal subendocardium with subsequently rapid engagement of the left conduction system [55]. Both concepts are included under the term LBBAP and require the intraseptal implantation of a pacing lead reaching the subendocardium of the left ventricular septum. As the conduction system is captured distally to the right bundle branch during LBBAP, a delay in RV activation is typically seen with this pacing modality expressed by the characteristic r prime wave present in lead V1 (Figure 3). As both the distal and proximal dipoles of the LBBAP lead are usually within the interventricular septum, bipolar pacing may result in anodal capture, which implies that the right side of the septum is being also captured during pacing resulting in a faster activation of the RV with this particular pacing configuration and, thus, a potential benefit in terms of QRS narrowing and better electrical resynchronization. Finally, the RV activation delay induced by LBBAP-CRT can be also compensated by fusing the intact intrinsic conduction through the right bundle branch present in patients with baseline LBBB with the LBBAP wavefront adjusting the device-programmed AV interval.
Figure 3.
Different capture patterns during LBBAP-CRT in a patient with non-ischemic cardiomyopathy and baseline wide QRS (panel (A)). During the procedure, LVSP (panel (B)), NS-LBBP (panel (C)) and S-LBBP (panel (D)) could be observed during unipolar pacing at different outputs. Bipolar pacing with AV Interval adjusted to favor intrinsic conduction through the RBB resulted in further QRS narrowing (panel (E)). Panels (F,G) show the final lead position in the LAO (40°) and RAO (35°) projections, respectively. COI: Current of injury; DF: Defibrillation; LB: Left bundle; LVSP: Left ventricular septal pacing; NS-LBBP: Non-selective left bundle branch pacing; S-LBBP: Selective left bundle branch pacing. Sweep speed 100 mm/s.
5. Clinical Evidence of HBP-CRT
HBP is theoretically the most physiological pacing modality as it can restore the normal electrical activation pattern of the ventricles. In CRT candidates with a typical LBBB, HBP with bundle branch correction would eliminate the asynchronous activation associated with the intraventricular conduction defect. In 2013, Barba et al. [56] described the first series of 16 patients with CRT indications who underwent HBP after a failed CS lead implantation attempt. In this series, LBBB correction was temporally obtained in 81% of the cases, but permanent LBBB correction was finally achieved only in 56% due to difficulties in HBP lead fixation. The mean LBBB correction threshold at implant was high (3.09 V ± 0.44) and tended to increase at the last follow-up (3.7 V ± 0.54) with no cases of lead dislodgment. LV diameters and LVEF significantly improved during a follow-up of 31.33 ± 21.45 months. Subsequently, other mostly observational studies have evaluated the potential utility of HBP for CRT [57,58,59,60,61,62,63,64] (Table 1). Sharma et al. [58] published the largest multicenter, observational, and retrospective study of HBP in patients with different indications for CRT (primary CRT strategy, previous failed CS lead implantation, non-responders to conventional CRT) including 106 patients with a successful implant in 95 (90%). The mean BBB correction threshold was 2 ± 1.2 V at 1 ms. During a mean follow-up of 14 months, there was a significant improvement in LVEF and functional class with 6.6% of lead-related complications. In patients with a baseline LVEF < 35%, mean LVEF went from 25% at baseline to 40% at the last follow-up (p = 0.0001) and the NYHA functional class significantly increased from 2.8 ± 0.5 to 1.8 ± 0.6 (p = 0.0001). Other small, observational, single-center studies have shown similar results with significant improvement of LVEF and NYHA class [59,60].
Table 1.
Principal studies reporting data about HBP-CRT.
| Study | Design | Patients’ Allocation | BBB Correction Rate | HBP Threshold at Implant (V) * | HBP Threshold at Follow-Up (V) * | Mean Follow-Up (Months) | Outcomes # | HBP Lead Related Complications (%) # |
|---|---|---|---|---|---|---|---|---|
| Barba et al. [56] Europace, 2013 | observational, retrospective, single-centre |
HBP: 16 | 81% temporarily 56% permanently |
3.1 ± 0.4 | 3.7 ± 0.5 | 31 | QRS narrowing, LVEF improvement and reduction in LVEDD and LVESD | 0 |
| Lutsgarten et al. [57] Heart Rhythm, 2015 | randomized, crossover, multicentre | HBP: 29 BiVP: 29 |
72% | 1.3 ± 2.2 | 2.4 ± 4.5 | 12 | LVEF, NYHA class, 6MWT and QoL significantly improved with both HBP and BiVP | 10.3 |
| Sharma et al. [58] Heart Rhythm, 2018 | observational, retrospective, multicentre |
HBP: 106 | 90% | 1.4 ± 0.9 | 2.0 ± 1.2 | 14 | QRS narrowing, LVEF and NYHA class improvement | 6.6 |
| Huang et al. [59] Heart, 2019 | observational, prospective, single-centre | HBP: 74 | 97% temporarily 76% permanently |
1.9 ± 1.1 | 2.3 ± 0.9 | 37 | QRS narrowing, LVEF and NYHA class improvement | 0 |
| Moriña-Vázquez et al. [60] Europace, 2020 | observational, prospective, single-centre |
HBP: 48 | 81% | 1.6 (0.9–1.9) | 0.9 (0.7–2) | 6 | QRS narrowing, LVEF and dyssynchrony parameters improvement | 0 |
| Upadhyay et al. [61] Heart Rhythm, 2019 | randomized, prospective, multicentre | HBP: 21 BiVP: 20 |
52% | 2.75 (1.3–3.4) | 2 (1–3.3) | 12 | QRS narrowing, trend towards higher echo response with HBP vs. BiVP | 0 |
| Vinther et al. [62] JACC EP, 2021 | randomized, prospective, single-centre | HBP: 25 BiVP: 25 |
72% | 2.2 ± 1.2 | 2.4 ± 1.6 | 6 | LVEF significantly higher and LVESV significantly lower in HBP group at 6 months | 5.3 |
| Huang et al. [63] Heart Rhythm, 2022 |
randomized, prospective, multicentre, crossover |
HBP: 50 BiVP: 50 |
N/A, patients with baseline narrow QRS undergoing AV node ablation | 0.9 ± 0.6 | 0.9 ± 0.6 | 9 | significant improvement in LVEF with HBP vs. BiVP | 0 |
| Whinnet et al. [64] Eur J Heart Fail, 2023 | randomized, crossover, multicentre | HBP: 167 | 93% | N/A | N/A | 6 | HBP did not increased peak O2 uptake but significantly improved QoL | 5.6 |
* HBP threshold refers to the BBB correction threshold. Note that HB pacing thresholds were measured at different pulse widths depending on the study. # In randomized studies, outcomes and HBP lead-related complications are reported as per-protocol analyses. BBB: Bundle branch block; BiVP: Biventricular pacing; HBP: His bundle pacing; LVEDD: Left ventricular end-diastolic diameter; LVEF: Left ventricular ejection fraction; LVESD: Left ventricular end-systolic diameter; LVESV: Left ventricular end-systolic volume; NYHA: New York Heart Association; QoL: Quality of life; 6MWT: 6-min walking test.
To date, only four randomized studies have directly compared conventional BiVP-CRT with HBP-CRT [57,61,62,63]. Lutsgarten et al. [57] conducted a randomized, crossover study including 29 patients with wide QRS (>130 ms) and CRT indication who received both an LV and an HB lead and were randomized after 1 month to HBP or BiVP during 6 months and then crossover to the alternative pacing mode for 6 additional months. The HBP implant success rate was 72%, and 12 patients completed the entire protocol showing significant and comparable improvements in LVEF, NYHA class, 6-min walking test distance, and quality of life (QoL) between HBP and BiVP. The His-SYNC pilot was a multicenter, prospective, randomized controlled trial comparing BiVP-CRT with HBP-CRT in patients with conventional CRT indications [61]. A total of 41 patients were enrolled in the study with 21 randomized to HBP-CRT and 20 to BiVP-CRT. In the treatment-received analysis, patients who received HBP-CRT showed a significantly greater QRS narrowing in comparison to BiVP-CRT (125 ± 22 ms vs. 164 ± 25 ms, p = 0.001). After a mean follow-up duration of 12.2 months, the echocardiographic response, defined by an LVEF improvement ≥5%, tended to be higher with HBP-CRT but did not reach statistical significance. Of note, up to 48% of patients allocated to HBP-CRT crossed over to BiVP-CRT while 26% of patients initially randomized to BiVP-CRT were finally implanted with HBP-CRT. The presence of non-specific intraventricular conduction disturbance (IVCD) was the principal reason for crossover from HBP-CRT to BiVP-CRT.
In the His-Alternative trial, Vinther et al. [62] randomized 50 patients with symptomatic HF, LVEF ≤ 35%, and LBBB according to Strauss criteria to HBP-CRT or BiVP-CRT in a 1:1 ratio and were followed for 6 months. LBBB correction was achieved in up to 72% of patients in the HBP-CRT group at implant. In the per-protocol analysis, there were no differences in the LVEF improvement at 6 months between the 2 groups and HBP thresholds were significantly higher than CS lead thresholds both at implant and at follow-up. However, 7 patients crossed over from the HBP-CRT group to the BiVP-CRT group at implant while only 1 patient crossed over from BiVP-CRT to HBP-CRT. In the treatment-received analysis, LVEF was significantly higher (48 ± 8% vs. 42 ± 8%, p < 0.05) and the LV end-systolic volume (LVESV) was lower (65 ± 22 mL vs. 83 ± 27 mL, p < 0.05) in the HBP-CRT group in comparison with the BiVP-CRT group.
HBP-CRT has been also compared to BiV-CRT in patients with atrial fibrillation and LVEF < 40% undergoing AV node ablation [63]. Using a crossover design, patients received both a CS lead and an HBP lead and were randomized to either HBP-CRT or BiVP-CRT during the first 9 months and then switched to the alternative pacing mode for another 9 months. Fifty patients were enrolled but only thirty-eight patients completed the two phases of the study and were included in the final analysis. HBP-CRT was associated with a significant improvement in LVEF in comparison to BiVP-CRT. In both groups, LVEDD, NYHA class, and B-type natriuretic peptide levels significantly improved.
In summary, HBP has been evaluated in lieu of CRT in small, observational, and mainly single-center studies with limited follow-up data. To date, only 125 patients have been allocated to HBP in randomized controlled trials and compared to BiVP in patients with conventional CRT indications. Two principal concerns arise when observing the currently published data in this particular setting. The first one is that the BBB correction rate with HBP is limited and highly variable, ranging from 52 to 93% in patients included in randomized studies with baseline wide QRS. The second one is that this HBP-BBB correction rate is achieved with high pacing thresholds and a relatively high incidence of lead-related complications (up to 10.3%) including the loss of HB capture or a significant increase in the BBB correction threshold during follow-up. Finally, it should be taken into consideration that all these data come from highly specialized centers with extensive experience in CSP, so the replication of these results may not be possible in other centers.
6. Clinical Evidence of LBBAP-CRT
The first description of LBBP by Huang et al. in 2017 was in a patient with dilated cardiomyopathy, HF, and LBBB in which both CS lead implantation and HBP lead implantation failed [51]. Posterior development of the technique, with the addition of left LVSP under the term LBBAP, revealed that this new physiological pacing modality appeared to be technically easier than HBP, with higher implant success rates and was associated with lower pacing thresholds at implant and during follow-up. Thus, taking into account these findings, LBBAP was considered a potential alternative for CRT (Figure 4).
Figure 4.
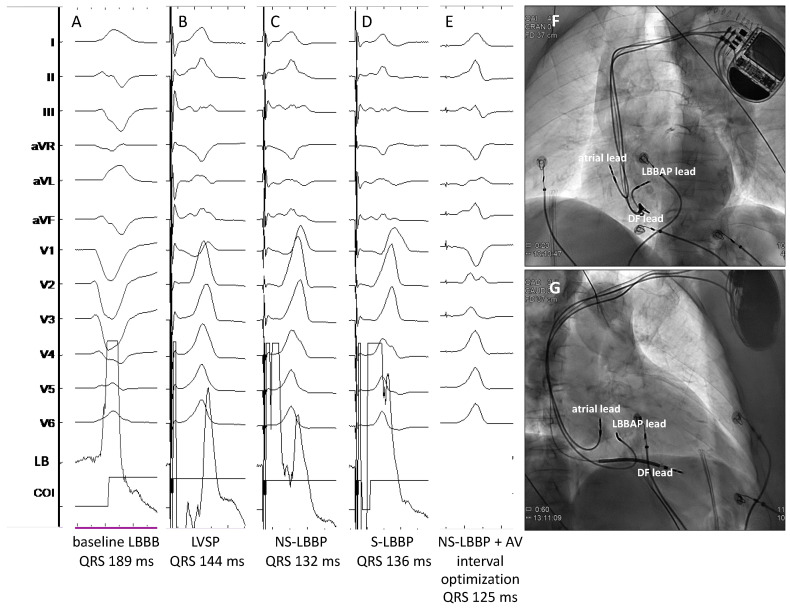
Patient with dilated cardiomyopathy undergoing LBBAP-CRT. Panel (A) shows the baseline QRS (188 ms); panel (B) shows NS-LBBP and panel (C) shows S-LBBP; panel (D) shows the final paced QRS after adjusting the programmed AV delay in the device to allow intrinsic conduction through the intact patient’s RBB resulting in further QRS narrowing; panels (E,F) show the RAO and LAO 30° view of the final lead location; panel (G) shows a four-chamber echocardiographic view with the LBBAP lead tip in the subendocardium of the left ventricular septum. COI: Current of injury; DF: Defibrillation; LAO: Left anterior oblique view; LB: Left bundle; LBBAP: Left bundle branch area pacing; RAO: Right anterior oblique view; RBB: Right bundle branch. Sweep speed 100 mm/s.
Li and colleagues published the first multicenter observational study evaluating LBBAP as a primary or rescue strategy after failed CS lead implantation in patients with conventional indications for CRT [65] (Table 2). They attempted LBBAP in 37 patients with successful implantation in 30, including 3 patients who received both an LBBAP lead and a CS lead, and compared the outcomes with 54 matched controls retrospectively recruited who had been previously treated with conventional BiVP-CRT. LBBAP-CRT resulted in significantly narrower paced QRS, a greater increase in LVEF, and greater echocardiographic response and super-response in comparison with conventional BiVP-CRT. A larger observational and retrospective series was published by Vijayaraman et al. [66] in 2021, including 325 patients with conventional indications for CRT who underwent LBBAP showing similar results: An implant success rate of 85%, optimal and stable electrical parameters, a significant reduction in paced QRS duration, and significant improvement of LVEF and NYHA class during a mean follow-up of 6 months. Other studies have consistently shown similar data in terms of a significant reduction in the paced QRS duration, optimal and stable electrical parameters during follow-up, and low lead-related complication rates associated with LBBAP-CRT [67,68].
Table 2.
Principal studies reporting data on LBBAP-CRT.
| Study | Design | Patients’ Allocation | Implant Success Rate | Pacing Threshold at Implant (V) | Pacing Threshold at Follow-Up (V) | Mean Follow-Up (Months) | Outcomes # | LBBAP/CS Lead Related Complications (%) # |
|---|---|---|---|---|---|---|---|---|
| Li et al. [65] ESC Heart Failure, 2020 | observational, prospective, multicentre | LBBAP: 37 BiVP: 54 |
LBBAP: 81% BiVP: N/A |
LBBAP: 0.81 ± 0.30BiVP: 1.22 ± 0.62 | LBBAP: 0.75 ± 0.31 BiVP: 1.43 ± 0.74 | 6 | narrower QRS, greater LVEF improvement, greater echocardiographic response and higher rate of super-responders with LBBAP vs. BiVP | LBBAP: 0 BiVP: N/A |
| Vijayaraman et al. [66] JACC EP, 2021 | observational, retrospective, multicentre | LBBAP: 325 | 85% | 0.6 ± 0.3 | 0.7 ± 0.3 | 6 | QRS narrowing, LVEF and NYHA class improvement | 2.5 |
| Jastrzębski et al. [67] Eur Heart J, 2022 | observational, retrospective, multicentre | LBBAP: 696 | 82% | N/A | N/A | 6.4 | N/A | N/A |
| Chen X et al. [68] Europace, 2022 | observational, prospective, multicentre | LBBAP: 49 BiVP: 51 |
LBBAP: 98% BiVP: 91% |
LBBAP: 0.92 ± 0.20 BiVP: 1.45 ± 0.39 |
LBBAP: 0.66 ± 0.17 BiVP: 1.42 ± 0.33 |
12 | narrower QRS, greater LVEF improvement and higher rate of super-responders with LBBAP vs. BiVP | LBBAP: 0 BiVP: 1.8 |
| Wang Y et al. [69] JACC EP, 2022 | randomized, prospective, multicentre | LBBAP: 20 BiVP: 20 |
LBBAP: 90% BiVP: 80% |
LBBAP: 0.69 ± 0.26 BiVP: 0.92 ± 0.40 |
LBBAP: 0.82 ± 0.20 BiVP: 1.12 ± 0.67 |
6 | higher LVEF improvement and greater reduction in LVESV and NT-proBNP with LBBAP | LBBAP: 0 BiVP: 5 |
| Pujol-López et al. [70] JACC EP, 2022 | randomized, prospective, single-centre | LBBAP *: 35 BiVP: 35 |
LBBAP: 77% BiVP: 94% |
LBBAP: 1.0 ± 0.4 BiVP: 1.2 ± 0.5 |
LBBAP: 0.8 ± 0.4 BiVP: 1.0 ± 0.3 |
6 | similar decrease in LVAT and LVESV; similar rates of mortality and HF hospitalization | LBBAP: 0 BiVP: 5 |
| Vijayaraman et al. [71] Heart Rhythm, 2022 | observational, retrospective, multicentre | HBP: 87 LBBAP: 171 BiVP: 219 |
CSP: 86% BiVP: 75% |
HBP: 1.1 ± 0.7 LBBAP: 0.8 ± 0.4 BiVP: 1.3 ± 0.6 |
HBP: 1.1 ± 0.7 LBBAP: 0.9 ± 0.5 BiVP: 1.4 ± 0.7 |
27 | greater improvement of LVEF with CS; combined outcome of death or HF hospitalization lower with CSP vs. BiVP | HBP: 2.3 LBBAP: 0.6 BiBP: 0.5 |
| Ezzedine et al. [72] Heart Rhythm, 2023 | observational, retrospective, multicentre | HBP: 69 LBBAP: 50 BiVP: 119 |
N/A | HBP: 1.29 ± 1 LBBAP: 0.92 ± 0.54 BiVP: N/A |
HBP: 1.46 ± 1.14 LBBAP: 0.86 ± 0.5 BiVP: N/A |
9 | greater proportion of CRT responders in CSP groups vs. BiVP. No differences in overall survival or time to first HF hospitalization | HBP: 11.1 LBBAP: 2.1 BiVP: 2.5 |
| Díaz et al. [73] JACC EP, 2023 | observational, prospective, multicentre | LBBAP: 128 BiVP: 243 |
LBBAP: 84.4% BiVP: 94.7% |
N/A | N/A | 11 | higher LVEF improvement with LBBAP; significant reduction in all-cause mortality or HF hospitalization with LBBAP | LBBAP: 7 BiVP: 6.2 |
| Vijayaraman et al. [74] JACC, 2023 | observational, retrospective, multicentre | LBBAP: 797 BiVP: 981 |
N/A | LBBAP: 0.72 ± 0.4 BiVP: 1.15 ± 0.7 |
LBBAP: 0.74 ± 0.3 BiVP: 1.31 ± 0.7 |
33 | higher LVEF improvement with LBBAP and higher proportion of patients with NYHA class improvement; significant reduction in time to death or HF hospitalization with LBBAP | LBAP: 1.3 BiVP: 2.5 |
* This study included 4 patients with HBP-CRT. # In randomized studies, pacing thresholds, outcomes, and HBP lead-related complications are reported as per-protocol analyses. BBB: Bundle branch block; BiVP: Biventricular pacing; CS: Coronary sinus; HBP: His bundle pacing; LBBAP: Left bundle branch area pacing; LVEF: Left ventricular ejection fraction; LVESV: Left ventricular end-systolic volume; NYHA: New York Heart Association.
The first multicenter, randomized controlled study comparing LBBAP-CRT with conventional BiV-CRT was published in 2022 by Wang et al. [69] A total of 40 patients with non-ischemic cardiomyopathy, LVEF ≤ 35%, and LBBB were randomized in a 1:1 fashion to LBBAP-CRT or BiVP-CRT. Two patients crossed over from LBBAP-CRT to BiVP-CRT whereas four patients randomized to BiVP-CRT finally underwent LBBAP-CRT. In the intention to treat analysis and after a follow-up of 6 months, LBBAP-CRT resulted in higher LVEF improvement, greater LVESV reduction, and greater reduction in NT-proBNP levels when compared with BiVP-CRT. However, rates of CRT response, paced QRS duration, changes in NYHA class, and 6-min walking test distance were comparable between LBBAP-CRT and BiVP-CRT. In the LEVEL-AT trial [70], 70 patients were randomized to BiVP-CRT (n = 35) or CSP-CRT (n = 35, 4 patients to HBP and 31 to LBBAP) showing a similar decrease in LVAT, LVESV, and similar rates of mortality and HF hospitalization at 6 months follow-up between the two groups in the intention-to-treat analysis.
Data on clinical outcomes comparing BiVP and LBBAP have begun to arise during the last year, principally from observational, non-randomized studies but constantly pointing towards a significant reduction in HF hospitalization with LBBAP-CRT when compared with BiVP-CRT, with no differences in overall mortality [71,72,73,74]. The largest multicenter, observational, and retrospective study published so far comparing LBBAP-CRT with BiVP-CRT included 1778 patients, 797 receiving LBBAP-CRT and 981 BiVP-CRT and provided data on clinical outcomes [74]. During a mean follow-up of 33 ± 16 months, both LBBAP-CRT and BiVP-CRT were associated with a significant increase in LVEF, but LBBAP-CRT showed a greater change in LVEF from baseline than BiVP-CRT (+13 ± 12% vs. +10 ± 12%, p < 0.001). The primary outcome of the study was a combined endpoint of time to death from any cause or the first episode of HF hospitalization and was significantly reduced with LBBAP-CRT compared to BiVP-CRT (20.8% vs. 28%; HR: 1.495; 95% CI: 1.213–1.842; p < 0.001). Secondary outcomes showed that mortality was comparable between the two groups but there was a significant reduction in HF hospitalizations in the LBBAP-CRT group (HR: 1.494; 95% CI: 1.159–1.927; p = 0.002).
In summary, both HBP-CRT and LBBAP-CRT are currently available techniques for delivering CRT and have been demonstrated to be safe and feasible. When directly compared to BiVP and HBP-CRT, LBBAP-CRT appears to be technically easier, with better electrical parameters and a low rate of lead-related complications [75,76,77] (Table 3). LBBAP-CRT and HBP-CRT are associated with a better acute hemodynamic response and a significantly greater improvement in LVEF than BiVP during follow-up when compared to BiVP-CRT. However, these direct comparisons arise from observational studies and should be taken cautiously. Data from randomized controlled trials are still required to draw definitive conclusions.
Table 3.
Comparison of procedural and follow-up outcomes with different CRT techniques. Estimation of the effect of the different pacing modalities has been obtained from pooled data from the references. Green has been added when both comparisons with the alternative groups are favorable; yellow shows if one of the comparisons is favorable and the other is neutral; orange shows one comparison is favorable and the other unfavorable; and red shows when both comparisons are unfavorable.
| BiVP-CRT | HBP-CRT | LBBAP-CRT | Reference | |
|---|---|---|---|---|
| Procedural time | lower than HBP higher than LBBAP |
higher than BiVP higher than LBBAP |
lower than BiVP lower than HBP |
[61,62,64,66,69,70,71,73,74] |
| Fluoroscopy time | higher than HBP higher than LBBAP |
lower than BiVP comparable to LBBAP |
lower than BiVP comparable to HBP |
[56,60,62,63,68,69,70,71,73,74] |
| Acute CS/CSP lead threshold | lower than HBP higher than LBBAP |
higher than BiVP higher than LBBAP | lower than BiVP lower than HBP |
[56,57,58,59,60,61,62,63,65,66,68,69,70,71,72,74] |
| Acute haemodynamic effects | worst than HBP worst than LBBAP |
better than BiVP comparable to LBBAP |
better than BiVP comparable to HBP |
[76] |
| Paced QRS duration | wider than HBP wider than LBBAP |
narrower than BiVP comparable to LBBAP |
narrower than BiVP comparable to HBP |
[75,76,77] |
| Change in LVEF | lower than HBP lower than LBBAP |
greater than BiVP comparable to LBBAP |
greater than BiVP comparable to HBP |
[75,76,77] |
| Follow-up CS/CSP lead threshold | lower than HBP higher than LBBAP |
higher than BiVP higher than LBBAP | lower than BiVP lower than HBP |
[56,57,58,59,60,61,62,63,65,66,68,69,70,71,72,74] |
| CS/CSP lead-related complications | lower than HBP comparable to LBBAP |
higher than BiVP higher than LBBAP |
comparable to BiVP lower than HBP |
[56,57,58,59,60,61,62,63,64,65,66,68,69,70,71,72,73,74] |
BiVP-CRT: Biventricular pacing cardiac resynchronization therapy; CS: Coronary sinus; CSP: Conduction system pacing; HBP-CRT: His bundle pacing cardiac resynchronization therapy; LBBAP-CRT: Left bundle branch area pacing cardiac resynchronization therapy; LVEF: Left ventricular ejection fraction.
7. Combination of CSP with CS Lead Pacing-CRT
There is a subset of patients in which CSP is not able to completely correct the baseline abnormal electrical activation of the ventricles. This can be explained by the presence of normal His–Purkinje activation even in the presence of a wide QRS, which reflects a primary myocardial disease and not an electrical disease. Upadhyay et al. [78] showed that among patients with LBBB patterns according to current guidelines [79], intact Purkinje activation was present in up to 36% of patients and no QRS narrowing could be obtained in this subset of patients even with demonstrated HB capture. In this scenario, and when CSP in patients with baseline wide QRS is not able to obtain a significant QRS narrowing, the combination of a CS lead with either HBP (His-optimized cardiac resynchronization therapy [HOT-CRT] or LBBAP (left bundle branch-optimized cardiac resynchronization therapy [LOT-CRT] may have beneficial effects in terms of electrical resynchronization [80,81,82,83,84] (Figure 5).
Figure 5.
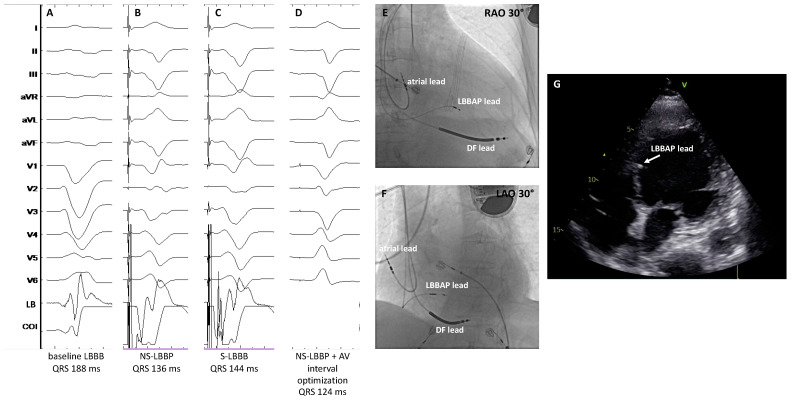
Patient with non-ischemic cardiomyopathy undergoing HOT-CRT. Baseline LBBB (panel (A)) could be only partially corrected with HBP (panel (B)). S-HBP without bundle branch correction could be seen at low outputs (panel (C)). Adding a CS lead and pacing from the His lead 20 ms earlier than from the CS lead, a further reduction in QRS duration could be obtained (panel (E)). Panel (D) shows the paced QRS morphology from the CS lead only. Panels (F,G) show the final lead locations in the LAO and RAO views, respectively. CS: Coronary sinus; HBED: His bundle electrogram (distal). HOT-CRT: His-Optimized cardiac resynchronization therapy. Sweep speed 25 mm/s.
Vijayaraman et al. [80] attempted HOT-CRT in 27 patients with CRT indication and different baseline conduction disease (LBBB in 17, intraventricular conduction defect in 5, and RV pacing in 5 patients) in an observational, multicenter, and retrospective study. HOT-CRT was successful in 93% and the paced QRS was further reduced with HOT-CRT (120 ± 16 ms) in comparison with BiVP (162 ± 17 ms) or HBP alone (151 ± 24 ms), p < 0.0001. Moreover, LVEF and NYHA class significantly improved during a mean follow-up of 14 ± 10 months with clinical and echocardiographic responses obtained at 84% and 92%, respectively.
LOT-CRT has been also evaluated in 112 CRT candidates in another observational study reporting an implant success rate of 81% [83]. LOT-CRT resulted in a significantly greater reduction in QRS duration (144 ± 22 ms) when compared with BiVP-CRT (170 ± 30 ms) and LBBAP-CRT (162 ± 23 ms), p < 0.0001. With a mean follow-up of 7.8 ± 2.3 months, there was a significant improvement in LVEF and a significant reduction in NT-proBNP levels. An echocardiographic response was obtained in 62.8% and a clinical response in 76% of patients.
Results from currently ongoing randomized controlled trials such as the HIS–Purkinje Conduction System Pacing Optimized Trial of Cardiac Resynchronization Therapy (HOT-CRT) (NCT04561778) or the Conduction System Pacing Optimized Therapy (CSPOT) study (NCT04905290) are expected to shed additional light on the potential utility of both HOT and LOT-CRT.
8. CSP-Based CRT in Other Clinical Scenarios
8.1. CSP-Based CRT in Patients with Non-LBBB
Current guidelines recommend CRT for patients with symptomatic HF in spite of optimal medical treatment, LVEF ≤ 35%, and non-LBBB morphology wide QRS with a lesser degree of recommendation with respect to patients with baseline LBBB (IIa if QRS ≥ 150 ms or IIb if QRS 130–149 ms according to the ESC Guidelines [1]). However, in the MADIT-CRT trial [85], no clinical benefit was observed in patients with non-LBBB (RBBB or intraventricular conduction disturbance (IVCD)), and the echocardiographic improvements were significantly higher in patients with LBBB. The prevalence of RBBB among HF patients has been estimated at around 6.1% with a non-negligible 1-year all-cause mortality rate of 11.9%, so there is still a significant number of HF patients with non-LBBB who could be potential targets for pacing therapy according to guidelines, but with limited support in terms of clinical benefit from currently published data [86].
HBP-CRT has been evaluated in patients with baseline RBBB and CRT indications in a multicenter observational study including 39 patients (implant success rate 95%) showing acceptable bundle branch correction pacing thresholds (1.4 ± 0.7 V at 1 ms), a significant QRS narrowing (from 158 ± 24 ms to 127 ± 17 ms, p = 0.0001), and a significant improvement in LVEF (from 31 ± 10% to 39 ± 13%, p = 0.004) and NYHA class (from 2.8 ± 0.6 to 2 ± 0.7, p = 0.0001) during a mean follow-up of 15 ± 23 months [87]. The utility of LBBAP-CRT has also been tested in an observational study including 121 patients with standard CRT indications and RBBB [85]. The implant success rate was 88%, and LBBAP-CRT resulted in a significant narrowing of the QRS (from 150 ± 20 ms at baseline to 150 ± 24 ms, p = 0.01) and a significant LVEF improvement (from 35 ± 9% to 43 ± 12, p < 0.01). Clinical and echocardiographic response was seen in 60% and 61%, respectively. Females and those patients with a greater reduction in QRS duration with pacing (≥10 ms) obtained the maximum benefit from LBBAP-CRT in this particular setting.
In contradistinction to HBP, QRS duration reduction with LBBAP in the presence of a RBBB is challenging as the activation of the left conduction system inevitably induces a delay in RV activation so most of the QRS duration narrowing observed during LBBAP in patients with baseline RBBB is due to the septal myocardial capture obtained during non-selective LBBAP. Using a bipolar pacing configuration, anodal capture, which implies simultaneous capture from the distal and proximal poles of the pacing lead tip both located within the interventricular septum, may enhance RV septal myocardial capture and, thus, reduce RV delayed activation. However, anodal capture thresholds are usually high (>3 V in 52% of patients in the Vijayaraman et al. [88] series) so cannot be used systematically in order to reduce QRS duration in patients with RBBB undergoing LBBAP-CRT.
8.2. CSP-Based CRT in Patients with HF Undergoing AV Node Ablation
Patients with atrial fibrillation, HF, and impaired LVEF are candidates for AV node ablation and CRT [63,89,90,91,92]. In this setting, CSP-based CRT is a new available pacing modality. In the ALTERNATIVE-AF [63], HBP-CRT showed a significant improvement in LVEF in comparison with BiVP with similar benefits in terms of NYHA class and BNP levels between both pacing modalities.
BiVP, HBP, and LBBAP have been latterly compared in an observational, retrospective study including 50 patients with refractory AF, symptomatic HF, impaired LVEF, and narrow QRS who underwent AV node ablation and implantation of a pacing device [92]. HBP (n = 25) and LBBAP (n = 10) were associated with a significant improvement in NYHA class and LVEF whereas no significant change in both parameters was registered with BiVP (n = 13). Moreover, Rijks and colleagues have recently demonstrated that performing LBBAP and AV node ablation in the same procedure is safe and feasible [93].
8.3. CSP-Based CRT in Coronary Venous Lead Failure or Non-Responders to BiVP-CRT
Both HBP and LBBAP-CRT have been shown to be suitable and effective alternatives for patients with CRT indications and previous CS lead failure implants. But one step forward is to consider the potential utility of CSP-based CRT for conventional BiVP-CRT non-responders. In a multicenter, observational study, Vijayaraman et al. [94] included 44 non-responders to previous BiVP-CRT patients who underwent LBBAP or LOT-CRT by adding a pacing lead in the LBB area. LVEF and volumes significantly improved with LBBAP/LOT-CRT. In this unfavorable scenario, LBBAP/LOT-CRT was able to obtain an echocardiographic response in 40%, a super-response in 9%, and a clinical response in 45% of these previously non-responder patients. The utility of this strategy to potentially increase the CRT response and the evaluation of the risks associated with an added intervention should be tested in large randomized controlled trials.
9. Current Recommendations and Future Directions
CSP-based CRT is currently a promising alternative for patients with CRT indications and failed CS lead implantation that has been demonstrated to be safe and feasible. Although HBP-CRT is theoretically the most physiological pacing modality, the difficulties in lead fixation, unreliable lead stability, the limited rate of bundle branch correction, and frequently high pacing thresholds are currently hindering the spread of the use of this physiological pacing modality in patients with CRT indications. In contrast, LBBAP-CRT has the advantage of better lead stability, with lower pacing thresholds and a higher implant success rate compared to HBP and, in spite of introducing some amount of RV activation delay, has been consolidated as the preferred CSP modality for patients requiring CRT. There are still important evidence gaps regarding CSP-based CRT including the lack of long-term performance, safety and complications data, and significant concerns about LBBAP lead extractability in the future. The development of a better and wider range of implant tools, with improvements in lead design and batteries and the introduction of specific algorithms for CSP-based CRT by the manufacturers are also critical aspects of the evolution of this technique and will surely result in patient benefits.
Meanwhile, current guidelines have prudently introduced CSP-based CRT into their recommendations [1,95]. The ESC Guidelines only consider HBP-CRT an alternative to conventional BiVP-CRT after unsuccessful CS lead implantation or as an alternative to BiVP in patients with AF and HF undergoing AVN ablation [1]. More recently, the 2023 HRS/APHRS/LAHRS guidelines on cardiac physiologic pacing and mitigation of HF have widely introduced both HBP and LBBAP as an alternative to BiVP-CRT in multiple scenarios (Table 4) [95].
Table 4.
Current CRT recommendations from the 2021 ESC guideline on cardiac pacing and CRT and the 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure.
| Clinical Scenarios | 2021 ESC Guideline on Cardiac Pacing and CRT [1] | Clinical Scenarios | 2023 HRS/APHRS/LAHRS Guideline on Cardiac Physiologic Pacing [95] |
|---|---|---|---|
| HF, SR, LVEF ≤ 35%, LBBB, QRS ≥ 150 ms | BiVP-CRT (I-A) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, LVEF ≤ 30%, NYHA class I | BiVP-CRT (2b, B-R) |
| HF, SR, LVEF ≤ 35%, LBBB, QRS 130–149 ms | BiVP-CRT (IIa-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, QRS ≥ 150 ms, LVEF ≤ 35%, NYHA class II-IV | BiVP-CRT (1, A) HBP or LBBAP if BiVP-CRT cannot be achieved (2a, C-LD) |
| HF, SR, LVEF ≤ 35%, non-LBBB, QRS ≥ 150 ms | BiVP-CRT (IIa-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, QRS 120–149 ms, LVEF ≤ 35%, NYHA class II-IV | BiVP-CRT (1, A) if female sex BiVP-CRT (2a, B-R) for the rest |
| HF, SR, LVEF ≤ 35%, non-LBBB, QRS 130–149 ms | BiVP-CRT (IIb-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, LBBB, QRS ≥ 150 ms, LVEF 36–50%, NYHA class II-IV | BiVP-CRT (2b, C-LD) HBP or LBBAP (2b, C-LD) |
| HF, AF, LVEF ≤ 35%, LBBB, QRS ≥ 130 ms, NYHA class III-IV | BiVP-CRT (IIa-C) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, non-LBBB, LVEF ≤ 35%, QRS 120–149 ms, NYHA class III-IV | BiVP-CRT (2b, B-NR) HBP or LBBAP (2b, C-LD) |
| HF, LVEF ≤ 35%, previous PM/ICD with high VP burden | BiVP-CRT (IIa-B) HBP if unsuccessful CS lead implantation (IIa-B) |
HF, non-LBBB, LVEF ≤ 35%, QRS ≥ 150 ms, NYHA class II | BiVP-CRT (2b, B-R) HBP or LBBAP (2b, C-LD) |
| Symptomatic AF, LVEF < 40% candidates for AVN ablation | BiVP-CRT (I-B) HBP if unsuccessful CS lead implantation (IIa-B) HBP (IIb-C) |
HF, non-LBBB, LVEF ≤ 35%, QRS ≥ 150 ms, NYHA class III-IV | BiVP-CRT (2a, A) HBP or LBBAP if BiVP-CRT cannot be achieved (2b, C-LD) |
| Symptomatic AF, LVEF 40–49% candidates for AVN ablation | BiVP-CRT (IIa-C) HBP if unsuccessful CS lead implantation (IIa-B) HBP (IIb-C) |
Pacemaker indication, LVEF 36–50% and anticipated high VP burden | BiVP-CRT (2a, B-R) HBP or LBBAP (2a, B-NR) |
| Symptomatic AF, LVEF ≥50% candidates for AVN ablation | BiVP-CRT (IIb-C) HBP if unsuccessful CS lead implantation (IIa-B) HBP (IIb-C) |
Pacemaker indication, LVEF 36–50%, LBBB and anticipated low VP burden | BiVP-CRT (2b, C-LD) HBP or LBBAP (2b, C-LD) |
| SR or AF, pacing indication for high degree AV block and LVEF < 40% | BiVP-CRT (I-A) HBP if unsuccessful CS lead implantation (IIa-B) |
PICM with HF and high burden RVP | BiVP-CRT (1, B-NR) HBP or LBBAP (2b, C-LD) |
| AF + AVN ablation + LVEF ≤ 50% | BiVP-CRT (2a, B-R) |
AF: Atrial fibrillation; AVN: Atrioventricular node; BiVP-CRT: Biventricular pacing cardiac resynchronization therapy; HBP: His bundle pacing; HF: Heart failure: SR: Sinus rhythm; LVEF: Left ventricular ejection fraction; LBBAP: Left bundle branch area pacing; LBBB: Left bundle branch block; NYHA: New York Heart Association; PICM: Pacemaker induced cardiomyopathy; RVP: Right ventricular pacing; VP: Ventricular pacing.
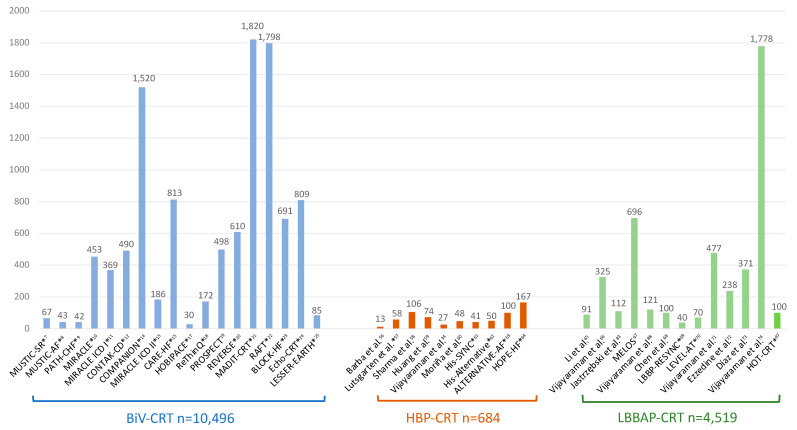
Anyway, the bulk of evidence about CRT benefits still favors conventional BiVP-CRT as shown in Figure 6 with up to 10,000 patients included in randomized controlled trials reporting data on hard clinical endpoints such as mortality and HF hospitalizations. On the other hand, CSP-based CRT is a relatively new and promising technique, and data from randomized studies are still scarce but are rapidly growing, especially with LBBAP-CRT. Large multicenter observational studies are consistently showing that LBBAP-CRT is associated with a greater LVEF improvement and significantly higher reduction in HF hospitalizations in comparison to BiVP-CRT. Multiple ongoing randomized clinical trials are expected to provide more evidence in the coming years to underpin CSP-based CRT as an alternative to conventional BiVP-CRT (Table 5).
Figure 6.
Principal studies reporting data on BiVP-CRT, HBP-CRT, and LBBAP-CRT with the total number of patients included. Asterisks indicate randomized studies [7,8,9,10,11,12,13,14,15,17,18,19,20,21,22,23,24,25,34,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,83,88,96].
Table 5.
Principal ongoing or planned randomized trials on CSP-based CRT. Reprinted with permission from Ref. [97].
| Study | Comparison | Design | Inclusion Criteria | Sample | Primary Outcome | Mean FU (Months) |
Secondary Outcomes |
|---|---|---|---|---|---|---|---|
| His-ALTERNATIVE-II NCT05814263 |
HBP/LBBAP vs. BiVP | randomized parallel |
HF, LVEF ≤ 35%, QRS > 130 ms, typical LBBB | 40 | CRT response by change in LVESV | 6 | LVEF, QoL, 6MWD, NT-proBNP, QRS duration, device complications |
| Left Bundle CRT NCT05434962 |
LBBAP vs. BiVP | randomized parallel |
HF, LVEF ≤ 35%, QRS > 130 ms, typical LBBB | 176 | CRT response by change in LVESV or clinical composite score | 12 | LVEF, QoL, 6MWD, death, HF hospitalization, ventricular arrhythmias, device complications |
| Left vs. Left NCT05650658 |
HBP/LBBAP vs. BiVP | randomized parallel |
HF, LVEF ≤ 50%, QRS > 130 ms or anticipated VP > 40% | 2136 | Combined all-casue mortality or HF hospitalization | 66 | QoL, NYHA, 6MWD, death, CV death, hospitalization, CV hospitalization |
| CONSYST-CRT NCT05187611 |
HBP/LBBAP vs. BiVP | randomized parallel |
QRS > 130 ms, LVEF < 35–40% | 130 | Combined all-cause mortality, cardiac transplant, HFH, LVEF improvement < 5 points | 6 | LVEF, LVESV, HFH, mortality, QRS duration, NYHA, correction of septal flash |
| PhysioSync-HF NCT05572736 |
HBP/LBBAP vs. BiVP | randomized parallel |
HF, LVEF ≤ 35%, QRS ≥ 130 ms, typical LBBB | 304 | Non-inferiority of clinical benefit | 12 | Composite (all-cause death, HFH, urgent HF visit), cost-efectiveness, QoL, 6MWD, NYHA, NT-proBNP, QRS duration |
| CSP-SYNC NCT05155865 |
HBP/LBBAP vs. BiVP | randomized parallel |
LVEF ≤ 35%, QRS ≥ 130 ms, typical LBBB, NYHA II–III | 60 | LV volume, LVEF, NYHA, NT-proBNP, 6MWD, QoL | 6 | myocardial work redistribution, QRS duration, procedure complications, arrhythmias |
| RECOVER-HF NCT05769036 |
LBBP vs. BiVP | randomized parallel |
QRS > 130 ms, LVEF < 35–40%, NYHA ≥ II | 60 | Combined all-cause mortality or HFH | 24 | all-cause mortality, CV mortality, HFH, CRT-D shocks, LVEF, QoL, NYHA |
10. Conclusions
HBP and LBBAP are new physiologic pacing modalities that are able to provide effective CRT. Initial observational studies have shown that both techniques are safe and feasible and, in comparison to conventional BiVP-CRT, may be associated with further LVEF improvement and a significant reduction in HF hospitalizations in patients with CRT indications, but these results should be taken cautiously until randomized data become available. Ongoing randomized controlled studies should elucidate if CSP-based CRT is non-inferior or even superior to conventional BiVP-CRT.
Abbreviations
| AV | atrioventricular |
| BiVP | biventricular pacing |
| CS | coronary sinus |
| CSP | conduction system pacing |
| CRT | cardiac resynchronization therapy |
| HBP | His bundle pacing |
| HF | heart failure |
| HOT-CRT | His-optimized cardiac resynchronization therapy |
| IVCD | intraventricular conduction disturbance |
| LBB | left bundle branch |
| LBBAP | left bundle branch area pacing |
| LBBP | left bundle branch pacing |
| LBBB | left bundle branch block |
| LOT-CRT | left bundle branch-optimized cardiac resynchronization therapy |
| LV | left ventricle |
| LVAT | left ventricular activation time |
| LVEF | left ventricular ejection fraction |
| LVESV | left ventricular end-systolic volume |
| LVSP | left ventricular septal pacing (LVSP) |
| NS-HBP | non-selective HB pacing |
| NYHA | New York Heart Association functional class |
| PICM | pacemaker induced cardiomyopathy |
| QoL | quality of life |
| RBBB | right bundle branch block |
| RV | right ventricle |
| S-HBP | selective HB pacing |
| VV | interventricular interval |
Author Contributions
Conceptualization, Ó.C.; methodology, Ó.C., J.O. and M.I.; software, Ó.C., J.N.-N., P.J. and H.D.A.; validation, Ó.C., J.O., M.I., J.N. and L.M.-D.; formal analysis, Ó.C. and J.N.-N.; investigation, Ó.C., J.N.-N., P.J. and H.D.A.; resources, Ó.C.; data curation, Ó.C., J.N.-N. and P.J.; writing—original draft preparation, Ó.C.; writing—review and editing, Ó.C., J.O., M.I. and J.N.; visualization, Ó.C.; supervision, L.M.-D.; project administration, Ó.C. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
Cano has received consultant fees from Biotronik, Boston Scientific, Medtronic, and Microport. All other authors have nothing to disclose.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Glikson M., Nielsen J.C., Kronborg M.B., Michowitz Y., Auricchio A., Barbash I.M., Barrabés J.A., Boriani G., Braunschweig F., Brignole M., et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364. Erratum in Eur. Heart J. 2022, 43, 1651. [DOI] [PubMed] [Google Scholar]
- 2.Cazeau S., Ritter P., Bakdach S., Lazarus A., Limousin M., Henao L., Mundler O., Daubert J.C., Mugica J. Four chamber pacing in dilated cardiomyopathy. Pt 2Pacing Clin. Electrophysiol. 1994;11:1974–1979. doi: 10.1111/j.1540-8159.1994.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 3.Cazeau S., Ritter P., Lazarus A., Gras D., Backdach H., Mundler O., Mugica J. Multisite pacing for end-stage heart failure: Early experience. Pt 2Pacing Clin. Electrophysiol. 1996;11:1748–1757. doi: 10.1111/j.1540-8159.1996.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 4.Leclercq C., Cazeau S., Le Breton H., Ritter P., Mabo P., Gras D., Pavin D., Lazarus A., Daubert J.C. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J. Am. Coll. Cardiol. 1998;32:1825–1831. doi: 10.1016/S0735-1097(98)00492-6. [DOI] [PubMed] [Google Scholar]
- 5.Blanc J.J., Etienne Y., Gilard M., Mansourati J., Munier S., Boschat J., Benditt D.G., Lurie K.G. Evaluation of different ventricular pacing sites in patients with severe heart failure: Results of an acute hemodynamic study. Circulation. 1997;96:3273–3277. doi: 10.1161/01.CIR.96.10.3273. [DOI] [PubMed] [Google Scholar]
- 6.Butter C., Auricchio A., Stellbrink C., Fleck E., Ding J., Yu Y., Huvelle E., Spinelli J., Pacing Therapy for Chronic Heart Failure II Study Group Effect of resynchronization therapy stimulation site on the systolic function of heart failure patients. Circulation. 2001;104:3026–3029. doi: 10.1161/hc5001.102229. [DOI] [PubMed] [Google Scholar]
- 7.Cazeau S., Leclercq C., Lavergne T., Walker S., Varma C., Linde C., Garrigue S., Kappenberger L., Haywood G.A., Santini M., et al. Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N. Engl. J. Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 8.Linde C., Leclercq C., Rex S., Garrigue S., Lavergne T., Cazeau S., McKenna W., Fitzgerald M., Deharo J.C., Alonso C., et al. Long-term benefits of biventricular pacing in congestive heart failure: Results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J. Am. Coll. Cardiol. 2002;40:111–118. doi: 10.1016/S0735-1097(02)01932-0. [DOI] [PubMed] [Google Scholar]
- 9.Auricchio A., Stellbrink C., Sack S., Block M., Vogt J., Bakker P., Huth C., Schöndube F., Wolfhard U., Böcker D., et al. Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J. Am. Coll. Cardiol. 2002;39:2026–2033. doi: 10.1016/S0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 10.Abraham W.T., Fisher W.G., Smith A.L., Delurgio D.B., Leon A.R., Loh E., Kocovic D.Z., Packer M., Clavell A.L., Hayes D.L., et al. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N. Engl. J. Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 11.Young J.B., Abraham W.T., Smith A.L., Leon A.R., Lieberman R., Wilkoff B., Canby R.C., Schroeder J.S., Liem L.B., Hall S., et al. Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: The MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 12.Higgins S.L., Hummel J.D., Niazi I.K., Giudici M.C., Worley S.J., Saxon L.A., Boehmer J.P., Higginbotham M.B., De Marco T., Foster E., et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J. Am. Coll. Cardiol. 2003;42:1454–1459. doi: 10.1016/S0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 13.Abraham W.T., Young J.B., León A.R., Adler S., Bank A.J., Hall S.A., Lieberman R., Liem L.B., O’connell J.B., Schroeder J.S., et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–2868. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 14.Bristow M.R., Saxon L.A., Boehmer J., Krueger S., Kass D.A., De Marco T., Carson P., DiCarlo L., DeMets D., White B.G., et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 15.Cleland J.G., Daubert J.-C., Erdmann E., Freemantle N., Gras D., Kappenberger L., Tavazzi L., Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 16.Cleland J.G., Daubert J.C., Erdmann E., Freemantle N., Gras D., Kappenberger L., Tavazzi L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase] Eur. Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 17.Kindermann M., Hennen B., Jung J., Geisel J., Böhm M., Fröhlig G. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: The Homburg Biventricular Pacing Evaluation (HOBIPACE) J. Am. Coll. Cardiol. 2006;47:1927–1937. doi: 10.1016/j.jacc.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 18.Beshai J.F., Grimm R.A., Nagueh S.F., Baker J.H., Beau S.L., Greenberg S.M., Pires L.A., Tchou P.J., RethinQ Study Investigators Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N. Engl. J. Med. 2007;357:2461–2471. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 19.Chung E.S., Leon A.R., Tavazzi L., Sun J.-P., Nihoyannopoulos P., Merlino J., Abraham W.T., Ghio S., Leclercq C., Bax J.J., et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 20.Linde C., Gold M.R., Abraham W.T., Sutton M.S.J., Ghio S., Cerkvenik J., Daubert C., REsynchronization reVErses Remodeling in Systolic Left vEntricular Dysfunction Study Group Long-term impact of cardiac resynchronization therapy in mild heart failure: 5-year results from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study. Eur. Heart J. 2013;34:2592–2599. doi: 10.1093/eurheartj/eht160. [DOI] [PubMed] [Google Scholar]
- 21.Moss A.J., Hall W.J., Cannom D.S., Klein H., Brown M.W., Daubert J.P., Estes N.A.M., III, Foster E., Greenberg H., Higgins S.L., et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 22.Tang A.S., Wells G.A., Talajic M., Arnold M.O., Sheldon R., Connolly S., Hohnloser S.H., Nichol G., Birnie D.H., Sapp J.L., et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N. Engl. J. Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 23.Curtis A.B., Worley S.J., Adamson P.B., Chung E.S., Niazi I., Sherfesee L., Shinn T., Sutton M.S.J., Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators Biventricular pacing for atrioventricular block and systolic dysfunction. N. Engl. J. Med. 2013;368:1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 24.Ruschitzka F., Abraham W.T., Singh J.P., Bax J.J., Borer J.S., Brugada J., Dickstein K., Ford I., Gorcsan J., III, Gras D., et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N. Engl. J. Med. 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687. [DOI] [PubMed] [Google Scholar]
- 25.Thibault B., Harel F., Ducharme A., White M., Ellenbogen K.A., Frasure-Smith N., Roy D., Philippon F., Dorian P., Talajic M., et al. Cardiac resynchronization therapy in patients with heart failure and a QRS complex <120 milliseconds: The Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH) trial. Circulation. 2013;127:873–881. doi: 10.1161/circulationaha.112.001239. [DOI] [PubMed] [Google Scholar]
- 26.Gamble J.H.P., Herring N., Ginks M., Rajappan K., Bashir Y., Betts T.R. Procedural Success of Left Ventricular Lead Placement for Cardiac Resynchronization Therapy: A Meta-Analysis. JACC Clin. Electrophysiol. 2016;2:69–77. doi: 10.1016/j.jacep.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Moubarak G., Bouzeman A., Ollitrault J., Anselme F., Cazeau S. Phrenic nerve stimulation in cardiac resynchronization therapy. J. Interv. Card. Electrophysiol. 2014;41:15–21. doi: 10.1007/s10840-014-9917-8. [DOI] [PubMed] [Google Scholar]
- 28.Biffi M., Exner D.V., Crossley G.H., Ramza B., Coutu B., Tomassoni G., Kranig W., Li S., Kristiansen N., Voss F. Occurrence of phrenic nerve stimulation in cardiac resynchronization therapy patients: The role of left ventricular lead type and placement site. Europace. 2013;15:77–82. doi: 10.1093/europace/eus237. [DOI] [PubMed] [Google Scholar]
- 29.Auricchio A., Heggermont W.A. Technology Advances to Improve Response to Cardiac Resynchronization Therapy: What Clinicians Should Know. Rev. Esp. Cardiol. 2018;71:477–484. doi: 10.1016/j.recesp.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Abdelrahman M., Subzposh F.A., Beer D., Durr B., Naperkowski A., Sun H., Oren J.W., Dandamudi G., Vijayaraman P. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J. Am. Coll. Cardiol. 2018;71:2319–2330. doi: 10.1016/j.jacc.2018.02.048. [DOI] [PubMed] [Google Scholar]
- 31.Zanon F., Abdelrahman M., Marcantoni L., Naperkowski A., Subzposh F.A., Pastore G., Baracca E., Boaretto G., Raffagnato P., Tiribello A., et al. Long term performance and safety of His bundle pacing: A multicenter experience. J. Cardiovasc. Electrophysiol. 2019;30:1594–1601. doi: 10.1111/jce.14063. [DOI] [PubMed] [Google Scholar]
- 32.Keene D., Arnold A.D., Jastrzębski M., Burri H., Zweibel S., Crespo E., Chandrasekaran B., Bassi S., Joghetaei N., Swift M., et al. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: Insights from a large international observational study. J. Cardiovasc. Electrophysiol. 2019;30:1984–1993. doi: 10.1111/jce.14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shan P., Su L., Zhou X., Wu S., Xu L., Xiao F., Zhou X., Ellenbogen K.A., Huang W. Beneficial effects of upgrading to His bundle pacing in chronically paced patients with left ventricular ejection fraction <50. Heart Rhythm. 2018;15:405–412. doi: 10.1016/j.hrthm.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Vijayaraman P., Herweg B., Dandamudi G., Mittal S., Bhatt A.G., Marcantoni L., Naperkowski A., Sharma P.S., Zanon F. Outcomes of His-bundle pacing upgrade after long-term right ventricular pacing and/or pacing-induced cardiomyopathy: Insights into disease progression. Heart Rhythm. 2019;16:1554–1561. doi: 10.1016/j.hrthm.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Huang W., Chen X., Su L., Wu S., Xia X., Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16:1791–1796. doi: 10.1016/j.hrthm.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 36.Vijayaraman P., Subzposh F.A., Naperkowski A., Panikkath R., John K., Mascarenhas V., Bauch T.D., Huang W. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019;16:1774–1782. doi: 10.1016/j.hrthm.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Su L., Wang S., Wu S., Xu L., Huang Z., Chen X., Zheng R., Jiang L., Ellenbogen K.A., Whinnett Z.I., et al. Long-Term Safety and Feasibility of Left Bundle Branch Pacing in a Large Single-Center Study. Circ. Arrhythm. Electrophysiol. 2021;14:e009261. doi: 10.1161/CIRCEP.120.009261. [DOI] [PubMed] [Google Scholar]
- 38.Sharma P.S., Patel N.R., Ravi V., Zalavadia D.V., Dommaraju S., Garg V., Larsen T.R., Naperkowski A.M., Wasserlauf J., Krishnan K., et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: Results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. 2022;19:3–11. doi: 10.1016/j.hrthm.2021.08.033. Erratum in Heart Rhythm. 2023, 20, 1100. [DOI] [PubMed] [Google Scholar]
- 39.Vecera J., Penicka M., Eriksen M., Russell K., Bartunek J., Vanderheyden M., Smiseth O.A. Wasted septal work in left ventricular dyssynchrony: A novel principle to predict response to cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging. 2016;17:624–632. doi: 10.1093/ehjci/jew019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spragg D.D., Leclercq C., Loghmani M., Faris O.P., Tunin R.S., DiSilvestre D., McVeigh E.R., Tomaselli G.F., Kass D.A. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929–932. doi: 10.1161/01.CIR.0000088782.99568.CA. [DOI] [PubMed] [Google Scholar]
- 41.Tan N.Y., Witt C.M., Oh J.K., Cha Y.M. Left Bundle Branch Block: Current and Future Perspectives. Circ. Arrhythm. Electrophysiol. 2020;13:e008239. doi: 10.1161/CIRCEP.119.008239. [DOI] [PubMed] [Google Scholar]
- 42.Sweeney M.O., Hellkamp A.S., Ellenbogen K.A., Greenspon A.J., Freedman R.A., Lee K.L., Lamas G.A., MOde Selection Trial Investigators Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107:2932–2937. doi: 10.1161/01.CIR.0000072769.17295.B1. [DOI] [PubMed] [Google Scholar]
- 43.Wilkoff B.L., Cook J.R., Epstein A.E., Greene H.L., Hallstrom A.P., Hsia H., Kutalek S.P., Sharma A., Dual Chamber and VVI Implantable Defibrillator Trial Investigators Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115–3123. doi: 10.1001/jama.288.24.3115. [DOI] [PubMed] [Google Scholar]
- 44.Kirk J.A., Kass D.A. Cellular and Molecular Aspects of Dyssynchrony and Resynchronization. Card. Electrophysiol. Clin. 2015;7:585–597. doi: 10.1016/j.ccep.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyên U.C., Verzaal N.J., van Nieuwenhoven F.A., Vernooy K., Prinzen F.W. Pathobiology of cardiac dyssynchrony and resynchronization therapy. Europace. 2018;20:1898–1909. doi: 10.1093/europace/euy035. [DOI] [PubMed] [Google Scholar]
- 46.Merchant F.M., Mittal S. Pacing induced cardiomyopathy. J. Cardiovasc. Electrophysiol. 2020;31:286–292. doi: 10.1111/jce.14277. [DOI] [PubMed] [Google Scholar]
- 47.Khurshid S., Obeng-Gyimah E., Supple G.E., Schaller R., Lin D., Owens A.T., Epstein A.E., Dixit S., Marchlinski F.E., Frankel D.S. Reversal of Pacing-Induced Cardiomyopathy Following Cardiac Resynchronization Therapy. JACC Clin. Electrophysiol. 2018;4:168–177. doi: 10.1016/j.jacep.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Lu W., Lin J., Dai Y., Chen K., Zhang S. The therapeutic effects of upgrade to cardiac resynchronization therapy in pacing-induced cardiomyopathy or chronic right ventricular pacing patients: A meta-analysis. Heart Fail. Rev. 2022;27:507–516. doi: 10.1007/s10741-021-10091-z. [DOI] [PubMed] [Google Scholar]
- 49.Deshmukh P., Casavant D.A., Romanyshyn M., Anderson K. Permanent, direct His-bundle pacing: A novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation. 2000;101:869–877. doi: 10.1161/01.CIR.101.8.869. [DOI] [PubMed] [Google Scholar]
- 50.Malagù M., Vitali F., Massafra R.F., Cardelli L.S., Pavasini R., Guardigli G., Rapezzi C., Bertini M. Three-Dimensional Electro-Anatomical Mapping and Myocardial Work Performance during Spontaneous Rhythm, His Bundle Pacing and Right Ventricular Pacing: The EMPATHY Study. J. Cardiovasc. Dev. Dis. 2022;9:377. doi: 10.3390/jcdd9110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang W., Su L., Wu S., Xu L., Xiao F., Zhou X., Ellenbogen K.A. A Novel Pacing Strategy With Low and Stable Output: Pacing the Left Bundle Branch Immediately beyond the Conduction Block. Can. J. Cardiol. 2017;33:1736.e1–1736.e3. doi: 10.1016/j.cjca.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 52.Arnold A.D., Shun-Shin M.J., Keene D., Howard J.P., Sohaib S.M.A., Wright I.J., Cole G.D., Qureshi N.A., Lefroy D.C., Koa-Wing M., et al. His Resynchronization Versus Biventricular Pacing in Patients with Heart Failure and Left Bundle Branch Block. J. Am. Coll. Cardiol. 2018;72:3112–3122. doi: 10.1016/j.jacc.2018.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sussenbek O., Rademakers L., Waldauf P., Jurak P., Smisek R., Stros P., Poviser L., Vesela J., Plesinger F., Halamek J., et al. Left bundle branch area pacing results in more physiological ventricular activation than biventricular pacing in patients with left bundle branch block heart failure. Eur. Heart J. Suppl. 2023;25((Suppl. E)):E17–E24. doi: 10.1093/eurheartjsupp/suad109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vijayaraman P., Dandamudi G., Zanon F., Sharma P.S., Tung R., Huang W., Koneru J., Tada H., Ellenbogen K.A., Lustgarten D.L. Permanent His bundle pacing: Recommendations from a Multicenter His Bundle Pacing Collaborative Working Group for standardization of definitions, implant measurements, and follow-up. Heart Rhythm. 2018;15:460–468. doi: 10.1016/j.hrthm.2017.10.039. [DOI] [PubMed] [Google Scholar]
- 55.Burri H., Jastrzebski M., Cano Ó., Čurila K., de Pooter J., Huang W., Israel C., Joza J., Romero J., Vernooy K., et al. EHRA clinical consensus statement on conduction system pacing implantation: Executive summary. Endorsed by the Asia-Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS) and Latin-American Heart Rhythm Society (LAHRS) Europace. 2023;25:1237–1248. doi: 10.1093/europace/euad044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barba-Pichardo R., Manovel Sánchez A., Fernández-Gómez J.M., Moriña-Vázquez P., Venegas-Gamero J., Herrera-Carranza M. Ventricular resynchronization therapy by direct His-bundle pacing using an internal cardioverter defibrillator. Europace. 2013;15:83–88. doi: 10.1093/europace/eus228. [DOI] [PubMed] [Google Scholar]
- 57.Lustgarten D.L., Crespo E.M., Arkhipova-Jenkins I., Lobel R., Winget J., Koehler J., Liberman E., Sheldon T. His-bundle pacing versus biventricular pacing in cardiac resynchronization therapy patients: A crossover design comparison. Heart Rhythm. 2015;12:1548–1557. doi: 10.1016/j.hrthm.2015.03.048. [DOI] [PubMed] [Google Scholar]
- 58.Sharma P.S., Dandamudi G., Herweg B., Wilson D., Singh R., Naperkowski A., Koneru J.N., Ellenbogen K.A., Vijayaraman P. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: A multicenter experience. Heart Rhythm. 2018;15:413–420. doi: 10.1016/j.hrthm.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Huang W., Su L., Wu S., Xu L., Xiao F., Zhou X., Mao G., Vijayaraman P., Ellenbogen K.A. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart. 2019;105:137–143. doi: 10.1136/heartjnl-2018-313415. [DOI] [PubMed] [Google Scholar]
- 60.Moriña-Vázquez P., Moraleda-Salas M.T., Manovel-Sánchez A.J., Fernández-Gómez J.M., Arce-Léon Á., Venegas-Gamero J., Barba-Pichardo R. Early improvement of left ventricular ejection fraction by cardiac resynchronization through His bundle pacing in patients with heart failure. Europace. 2020;22:125–132. doi: 10.1093/europace/euz296. [DOI] [PubMed] [Google Scholar]
- 61.Upadhyay G.A., Vijayaraman P., Nayak H.M., Verma N., Dandamudi G., Sharma P.S., Saleem M., Mandrola J., Genovese D., Oren J.W., et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: A secondary analysis of the His-SYNC Pilot Trial. Heart Rhythm. 2019;16:1797–1807. doi: 10.1016/j.hrthm.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 62.Vinther M., Risum N., Svendsen J.H., Møgelvang R., Philbert B.T. A Randomized Trial of His Pacing Versus Biventricular Pacing in Symptomatic HF Patients with Left Bundle Branch Block (His-Alternative) JACC Clin. Electrophysiol. 2021;7:1422–1432. doi: 10.1016/j.jacep.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 63.Huang W., Wang S., Su L., Fu G., Su Y., Chen K., Zou J., Han H., Wu S., Sheng X., et al. His-bundle pacing vs. biventricular pacing following atrioventricular nodal ablation in patients with atrial fibrillation and reduced ejection fraction: A multicenter, randomized, crossover study—The ALTERNATIVE-AF trial. Heart Rhythm. 2022;19:1948–1955. doi: 10.1016/j.hrthm.2022.07.009. [DOI] [PubMed] [Google Scholar]
- 64.Whinnett Z.I., Shun-Shin M.J., Tanner M., Foley P., Chandrasekaran B., Moore P., Adhya S., Qureshi N., Muthumala A., Lane R., et al. Effects of haemodynamically atrio-ventricular optimized His bundle pacing on heart failure symptoms and exercise capacity: The His Optimized Pacing Evaluated for Heart Failure (HOPE-HF) randomized, double-blind, cross-over trial. Eur. J. Heart Fail. 2023;25:274–283. doi: 10.1002/ejhf.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li X., Qiu C., Xie R., Ma W., Wang Z., Li H., Wang H., Hua W., Zhang S., Yao Y., et al. Left bundle branch area pacing delivery of cardiac resynchronization therapy and comparison with biventricular pacing. ESC Heart Fail. 2020;7:1711–1722. doi: 10.1002/ehf2.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vijayaraman P., Ponnusamy S., Cano Ó., Sharma P.S., Naperkowski A., Subsposh F.A., Moskal P., Bednarek A., Forno A.R.D., Young W., et al. Left Bundle Branch Area Pacing for Cardiac Resynchronization Therapy: Results from the International LBBAP Collaborative Study Group. JACC Clin. Electrophysiol. 2021;7:135–147. doi: 10.1016/j.jacep.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Jastrzębski M., Kiełbasa G., Cano O., Curila K., Heckman L., De Pooter J., Chovanec M., Rademakers L., Huybrechts W., Grieco D., et al. Left bundle branch area pacing outcomes: The multicentre European MELOS study. Eur. Heart J. 2022;43:4161–4173. doi: 10.1093/eurheartj/ehac445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen X., Ye Y., Wang Z., Jin Q., Qiu Z., Wang J., Qin S., Bai J., Wang W., Liang Y., et al. Cardiac resynchronization therapy via left bundle branch pacing vs. optimized biventricular pacing with adaptive algorithm in heart failure with left bundle branch block: A prospective, multi-centre, observational study. Europace. 2022;24:807–816. doi: 10.1093/europace/euab249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y., Zhu H., Hou X., Wang Z., Zou F., Qian Z., Wei Y., Wang X., Zhang L., Li X., et al. Randomized Trial of Left Bundle Branch vs. Biventricular Pacing for Cardiac Resynchronization Therapy. J. Am. Coll. Cardiol. 2022;80:1205–1216. doi: 10.1016/j.jacc.2022.07.019. [DOI] [PubMed] [Google Scholar]
- 70.Pujol-Lopez M., Jiménez-Arjona R., Garre P., Guasch E., Borràs R., Doltra A., Ferró E., García-Ribas C., Niebla M., Carro E., et al. Conduction System Pacing vs. Biventricular Pacing in Heart Failure and Wide QRS Patients: LEVEL-AT Trial. JACC Clin. Electrophysiol. 2022;8:1431–1445. doi: 10.1016/j.jacep.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Vijayaraman P., Zalavadia D., Haseeb A., Dye C., Madan N., Skeete J.R., Vipparthy S.C., Young W., Ravi V., Rajakumar C., et al. Clinical outcomes of conduction system pacing compared to biventricular pacing in patients requiring cardiac resynchronization therapy. Heart Rhythm. 2022;19:1263–1271. doi: 10.1016/j.hrthm.2022.04.023. [DOI] [PubMed] [Google Scholar]
- 72.Ezzeddine F.M., Pistiolis S.M., Pujol-Lopez M., Lavelle M., Wan E.Y., Patton K.K., Robinson M., Lador A., Tamirisa K., Karim S., et al. Outcomes of conduction system pacing for cardiac resynchronization therapy in patients with heart failure: A multicenter experience. Heart Rhythm. 2023;20:863–871. doi: 10.1016/j.hrthm.2023.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diaz J.C., Sauer W.H., Duque M., Koplan B.A., Braunstein E.D., Marín J.E., Aristizabal J., Niño C.D., Bastidas O., Martinez J.M., et al. Left Bundle Branch Area Pacing versus Biventricular Pacing as Initial Strategy for Cardiac Resynchronization. Pt 2JACC Clin. Electrophysiol. 2023;8:1568–1581. doi: 10.1016/j.jacep.2023.04.015. [DOI] [PubMed] [Google Scholar]
- 74.Vijayaraman P., Sharma P.S., Cano Ó., Ponnusamy S.S., Herweg B., Zanon F., Jastrzebski M., Zou J., Chelu M.G., Vernooy K., et al. Comparison of Left Bundle Branch Area Pacing and Biventricular Pacing in Candidates for Resynchronization Therapy. J. Am. Coll. Cardiol. 2023;82:228–241. doi: 10.1016/j.jacc.2023.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Mariani M.V., Piro A., Forleo G.B., Della Rocca D.G., Natale A., Miraldi F., Vizza C.D., Lavalle C. Clinical, procedural and lead outcomes associated with different pacing techniques: A network meta-analysis. Int. J. Cardiol. 2023;377:52–59. doi: 10.1016/j.ijcard.2023.01.081. [DOI] [PubMed] [Google Scholar]
- 76.Ali N., Arnold A.D., Miyazawa A.A., Keene D., Chow J.J., Little I., Peters N.S., Kanagaratnam P., Qureshi N., Ng F.S., et al. Comparison of methods for delivering cardiac resynchronization therapy: An acute electrical and haemodynamic within-patient comparison of left bundle branch area, His bundle, and biventricular pacing. Europace. 2023;25:1060–1067. doi: 10.1093/europace/euac245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hua J., Wang C., Kong Q., Zhang Y., Wang Q., Xiong Z., Hu J., Li J., Chen Q., Hong K. Comparative effects of left bundle branch area pacing, His bundle pacing, biventricular pacing in patients requiring cardiac resynchronization therapy: A network meta-analysis. Clin. Cardiol. 2022;45:214–223. doi: 10.1002/clc.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Upadhyay G.A., Cherian T., Shatz D.Y., Beaser A.D., Aziz Z., Ozcan C., Broman M.T., Nayak H.M., Tung R. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns. Circulation. 2019;139:1876–1888. doi: 10.1161/CIRCULATIONAHA.118.038648. [DOI] [PubMed] [Google Scholar]
- 79.Surawicz B., Childers R., Deal B.J., Gettes L.S. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram, part III: Intraventricular conduction disturbances: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e235–e240. doi: 10.1161/circulationaha.108.191095. [DOI] [PubMed] [Google Scholar]
- 80.Vijayaraman P., Herweg B., Ellenbogen K.A., Gajek J. His-Optimized Cardiac Resynchronization Therapy to Maximize Electrical Resynchronization: A Feasibility Study. Circ. Arrhythm. Electrophysiol. 2019;12:e006934. doi: 10.1161/CIRCEP.118.006934. [DOI] [PubMed] [Google Scholar]
- 81.Zweerink A., Zubarev S., Bakelants E., Potyagaylo D., Stettler C., Chmelevsky M., Lozeron E.D., Hachulla A.L., Vallée J.P., Burri H. His-Optimized Cardiac Resynchronization Therapy with Ventricular Fusion Pacing for Electrical Resynchronization in Heart Failure. JACC Clin. Electrophysiol. 2021;7:881–892. doi: 10.1016/j.jacep.2020.11.029. [DOI] [PubMed] [Google Scholar]
- 82.Deshmukh A., Sattur S., Bechtol T., Heckman L.I.B., Prinzen F.W., Deshmukh P. Sequential His bundle and left ventricular pacing for cardiac resynchronization. J. Cardiovasc. Electrophysiol. 2020;31:2448–2454. doi: 10.1111/jce.14674. [DOI] [PubMed] [Google Scholar]
- 83.Jastrzębski M., Moskal P., Huybrechts W., Curila K., Sreekumar P., Rademakers L.M., Ponnusamy S.S., Herweg B., Sharma P.S., Bednarek A., et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): Results from an international LBBAP collaborative study group. Heart Rhythm. 2022;19:13–21. doi: 10.1016/j.hrthm.2021.07.057. [DOI] [PubMed] [Google Scholar]
- 84.Feng X.F., Yang L.C., Zhao Y., Yu Y.C., Liu B., Li Y.G. Effects of adaptive left bundle branch-optimized cardiac resynchronization therapy: A single centre experience. BMC Cardiovasc. Disord. 2022;22:360. doi: 10.1186/s12872-022-02742-2. Erratum in BMC Cardiovasc. Disord. 2022, 22, 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zareba W., Klein H., Cygankiewicz I., Hall W.J., McNitt S., Brown M., Cannom D., Daubert J.P., Eldar M., Gold M.R., et al. Effectiveness of Cardiac Resynchronization Therapy by QRS Morphology in the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 86.Baldasseroni S., Opasich C., Gorini M., Lucci D., Marchionni N., Marini M., Campana C., Perini G., Deorsola A., Masotti G., et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: A report from the Italian network on congestive heart failure. Am. Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 87.Sharma P.S., Naperkowski A., Bauch T.D., Chan J.Y.S., Arnold A.D., Whinnett Z.I., Ellenbogen K.A., Vijayaraman P. Permanent His Bundle Pacing for Cardiac Resynchronization Therapy in Patients with Heart Failure and Right Bundle Branch Block. Circ. Arrhythm. Electrophysiol. 2018;11:e006613. doi: 10.1161/CIRCEP.118.006613. [DOI] [PubMed] [Google Scholar]
- 88.Vijayaraman P., Cano O., Ponnusamy S.S., Molina-Lerma M., Chan J.Y.S., Padala S.K., Sharma P.S., Whinnett Z.I., Herweg B., Upadhyay G.A., et al. Left bundle branch area pacing in patients with heart failure and right bundle branch block: Results from International LBBAP Collaborative-Study Group. Heart Rhythm. 2022;3:358–367. doi: 10.1016/j.hroo.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koniari I., Gerakaris A., Kounis N., Velissaris D., Rao A., Ainslie M., Adlan A., Plotas P., Ikonomidis I., Mplani V., et al. Outcomes of Atrioventricular Node Ablation and Pacing in Patients with Heart Failure and Atrial Fibrillation: From Cardiac Resynchronization Therapy to His Bundle Pacing. J. Cardiovasc. Dev. Dis. 2023;10:272. doi: 10.3390/jcdd10070272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang W., Su L., Wu S., Xu L., Xiao F., Zhou X., Ellenbogen K.A. Benefits of Permanent His Bundle Pacing Combined with Atrioventricular Node Ablation in Atrial Fibrillation Patients with Heart Failure with Both Preserved and Reduced Left Ventricular Ejection Fraction. J. Am. Heart Assoc. 2017;6:e005309. doi: 10.1161/JAHA.116.005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Su L., Cai M., Wu S., Wang S., Xu T., Vijayaraman P., Huang W. Long-term performance and risk factors analysis after permanent His-bundle pacing and atrioventricular node ablation in patients with atrial fibrillation and heart failure. Europace. 2020;22((Suppl. 2)):ii19–ii26. doi: 10.1093/europace/euaa306. [DOI] [PubMed] [Google Scholar]
- 92.Ivanovski M., Mrak M., Mežnar A.Z., Žižek D. Biventricular versus Conduction System Pacing after Atrioventricular Node Ablation in Heart Failure Patients with Atrial Fibrillation. J. Cardiovasc. Dev. Dis. 2022;9:209. doi: 10.3390/jcdd9070209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rijks J.H.J., Lankveld T., Manusama R., Broers B., Stipdonk A.M.W.V., Chaldoupi S.M., Bekke R.M.A.T., Schotten U., Linz D., Luermans J.G.L.M., et al. Left Bundle Branch Area Pacing and Atrioventricular Node Ablation in a Single-Procedure Approach for Elderly Patients with Symptomatic Atrial Fibrillation. J. Clin. Med. 2023;12:4028. doi: 10.3390/jcm12124028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vijayaraman P., Herweg B., Verma A., Sharma P.S., Batul S.A., Ponnusamy S.S., Schaller R.D., Cano O., Molina-Lerma M., Curila K., et al. Rescue left bundle branch area pacing in coronary venous lead failure or nonresponse to biventricular pacing: Results from International LBBAP Collaborative Study Group. Heart Rhythm. 2022;19:1272–1280. doi: 10.1016/j.hrthm.2022.04.024. [DOI] [PubMed] [Google Scholar]
- 95.Chung M.K., Patton K.K., Lau C.-P., Forno A.R.D., Al-Khatib S.M., Arora V., Birgersdotter-Green U.M., Cha Y.-M., Chung E.H., Cronin E.M., et al. 2023 HRS/APHRS/LAHRS guideline on cardiac physiologic pacing for the avoidance and mitigation of heart failure. Heart Rhythm. 2023;20:e17–e91. doi: 10.1016/j.hrthm.2023.03.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vijayaraman P., Pokharel P., Subzposh F.A., Oren J.W., Storm R.H., Batul S.A., Beer D.A., Hughes G., Leri G., Manganiello M., et al. His-Purkinje Conduction System Pacing Optimized Trial of Cardiac Resynchronization Therapy (HOT-CRT) versus Biventricular Pacing. JACC Clin Electrophysiol. 2023 doi: 10.1016/j.jacep.2023.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Cano O., Jover P., Vijayaraman P. An evidence-based update on physiological pacing. Curr. Treat. Options Cardio. Med. 2023;25:415–439. doi: 10.1007/s11936-023-01003-5. [DOI] [Google Scholar]