Abstract
Tocilizumab prevents clinical worsening of chronic antibody-mediated rejection (CAMR) of kidney transplant recipients. Optimization of this treatment is necessary. We identified the determinants of early tocilizumab exposure (within the first three months) and investigated the relationship between early plasma tocilizumab exposure and graft function. Patients with CAMR who started treatment with tocilizumab were retrospectively included. Demographic, clinical, and biological determinants of the tocilizumab trough concentration (Cmin) were studied using a linear mixed effect model, and the association between early exposure to tocilizumab (expressed as the sum of Cmin over the three first months (M) of treatment (ΣCmin)) and the urinary albumin-to-creatinine ratio (ACR) determined at M3 and M6 were investigated. Urinary tocilizumab was also measured in seven additional patients. Seventeen patients with 51 tocilizumab Cmin determinations were included. In the multivariate analysis, the ACR and time after tocilizumab initiation were independently associated with the tocilizumab Cmin. The ΣCmin was significantly lower (p = 0.014) for patients with an ACR > 30 mg/mmol at M3 and M6 than for patients with an ACR < 30 mg/mmol. Tocilizumab was detected in urine in only 1/7 patients. This study is the first to suggest that early exposure to tocilizumab may be associated with macroalbuminuria within the first six months in CAMR patients.
Keywords: tocilizumab, pharmacokinetics, antibody-mediated rejection, kidney transplant
1. Introduction
Chronic antibody-mediated rejection (CAMR) is a major complication of kidney transplantation, leading to degradation of renal function and, ultimately, loss of the graft. This complication is observed in up to 5% of first kidney transplant recipients [1,2]. Treatment of CAMR remains a challenge as there is no current consensus on its management, and the treatment options are limited beyond optimization of immunosuppression [3]. Intravenous administration of immunoglobulins, apheresis, and pulse of corticosteroids are a part of the treatment strategies in association or not with biologics disrupting B-cells (rituximab), complement (eculizumab) or interleukin 6 (IL-6) pathways (tocilizumab TCZ) [4]. Indeed, some recent studies suggested that targeting IL-6 pathway in particular could be a promising pharmacological strategy in the management of CAMR, given the key role of IL-6 in the regulation of systemic inflammation, the development and maturation of T cells and B cells leading to the synthesis of donor-specific antibody (DSA) [5,6,7,8,9].
TCZ is a humanized immunoglobulin G (IgG) 1 monoclonal antibody that competitively inhibits the IL-6 signaling pathway by binding to both its soluble and membrane-bound receptors [10]. TCZ is approved for treatment of rheumatic diseases (rheumatoid arthritis and idiopathic juvenile arthritis), in which it normalizes C reactive protein (CRP) levels and erythrocyte sedimentation rates within two weeks [11]. Recent studies suggested that TCZ could also be a promising therapeutic for the salvage treatment of CAMR [12], as six months of TCZ treatment reduced microvascular graft inflammation [13] and stabilized renal function [12,13,14,15,16,17,18,19,20]. In patients with CAMR, TCZ is administrated intravenously, monthly, at a dose of 8 mg/kg (with a maximum dose of 800 mg), in combination with corticosteroids and mycophenolate mofetil or an anticalcineurin, or belatacept. TCZ presents highly variable plasma concentrations in patients with rheumatic diseases [21,22] and kidney-transplant candidate patients undergoing desensitization [23]. Several studies even suggested a link between the TCZ concentration and clinical efficacy [11,24,25] in patients with rheumatic diseases. However, the pharmacokinetics of TCZ have never been studied for patients with kidney CAMR. Moreover, as for numerous other monoclonal therapeutic antibodies, it can take up to five to eight weeks to reach the pharmacokinetic steady state due to its long elimination half-life. Given the clinical severity of CAMR and the high cost of TCZ, the study of a potential association between early exposure to TCZ and kidney function is therefore of major importance.
The aims of this study were to describe the variability of TCZ early exposure, i.e., within the first three months (M) of treatment, identify the determinants of this variability, and investigate the relationship between early plasma TCZ exposure and graft function.
2. Materials and Methods
2.1. Study Design
We performed a retrospective monocentric cohort study that was approved by the Grenoble University Hospital review board (registration RnIPH 2022, protocol TOCIREJET; CNIL number: 2205066 v 0). This study was conducted according to the ethical guidelines of the Declaration of Helsinki [26]. As we previously showed that TCZ concentrations are highly variable among kidney-transplant candidates [23], the TCZ trough concentrations (Cmin) of all patients treated with TCZ were monitored during their routine care.
The inclusion criteria were being a renal-transplant patient who started TCZ treatment (intravenous 8 mg/kg every 4 weeks) as first-line salvage therapy for CAMR in the Nephrology Unit of the Grenoble University Hospital between December 2020 and May 2022, with TCZ plasma Cmin available at M1, M2, and M3 after the initiation of treatment. CAMR was defined according to last Banff classification [27] as the presence of chronic transplant glomerulopathy (cg score > 0) with severe peritubular capillaries (ptc score), basement membrane multilayering either with or without C4d deposition in peritubular capillaries, and the presence of anti-HLA DSA. In case of the association with at least moderate microvascular inflammation (g + ptc ≥ 2), lesions were defined as CAMR. The exclusion criteria were previous TCZ treatment or treatment for any indication other than CAMR.
The following data were retrospectively collected from the medical records at M1, M2, and M3: sex, age, weight, body mass index (BMI), treatment administration (dose, TCZ initiation date, time since the first dose, immunosuppressive co-treatment), and biological data (TCZ Cmin, serum creatinine, aspartate aminotransferase [ASAT], alanine aminotransferase [ALAT], gamma-glutamyl-transferase [GGT], total protein, and CRP levels, CKD-EPI glomerular filtration rate [GFR], and the urinary albumin-to-creatinine ratio [ACR]). The ACR was also collected at M0 (the day of TCZ initiation treatment) and at M6 (6 months after TCZ initiation treatment).
The biological parameters were measured on samples taken the same day as the samples used for the determination of the TCZ concentration. A patient was considered to be macroalbuminuric when his/her ACR was > 30 mg/mmol [28,29]. Liver function was considered to be impaired when transaminases were greater than three times the upper limit of normal. Finally, the urinary excretion of TCZ was evaluated in a separate cohort of seven patients (see Supplementary Materials Table S1).
2.2. Measurement of TCZ Concentration and Exposure
TCZ Cmin were determined at months M1, M2, and M3 after TCZ initiation using a validated liquid chromatography-tandem mass spectrometry method, as previously described [30]. The imprecision of this method was < 10%, with a bias < 15% over the validated range of 1.0–200.0 mg/L.
Early exposure to TCZ is expressed as the sum of the three Cmin (ΣCmin) over the first three months of treatment for a given patient.
2.3. Statistical Analysis
Continuous data are expressed as medians (10th–90th percentile) and categorical variables as percentages (numbers). The relationship between the TCZ Cmin (dependent variable) and other variables (age, gender, time after first administration, dose, weight, BMI, serum creatinine, GFR, urinary ACR, total serum protein, and co-treatment with tacrolimus, mycophenolic acid, or belatacept) was tested using linear mixed-effect models with the patient and number of injections as random factors (to account for the multiplicity of TCZ Cmin values obtained for the same patient at different times post injections). Multivariate linear mixed-effect analysis including all factors and covariates with a p-value < 0.15 in univariate analyses was conducted.
A Friedman test was used to compare the ACR between months M0, M2, M1, M3, and M6. The association between early TCZ exposure (ΣCmin) and the ACR determined at M3 and M6 was investigated using the Mann–Whitney test and Spearman t test.
The Shapiro–Wilk test was used to assess the normality of the distribution of continuous variables, and Levene’s test was used to assess the homogeneity of the variances. Data were log-transformed to satisfy the conditions of application of linear models when not normally distributed. All statistical tests were performed with an alpha threshold of 0.05. Statistical tests were performed using Jamovi® (version 1.6, Sydney, Australia).
3. Results
3.1. Baseline Characteristics
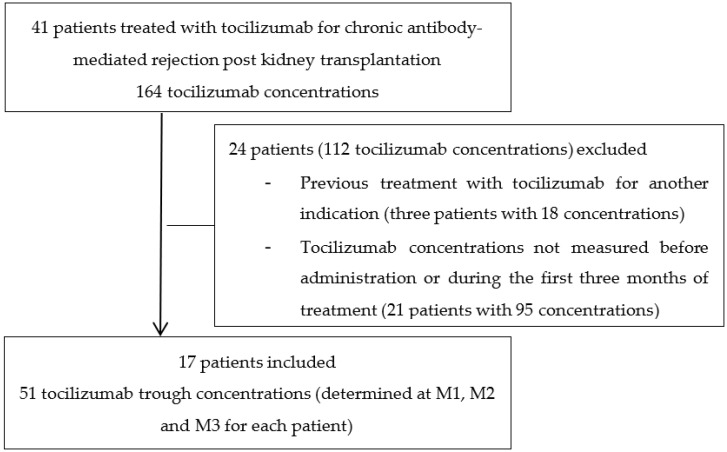
Seventeen kidney transplant patients treated with TCZ for CAMR were included, (see flow chart in Figure 1). Ten (58.8%) were men. Their median (10th–90th percentile) age, weight, and BMI were 52 (37–73) years, 75 (53–85) kg and 23.3 (20.4–29.3) kg/m2, respectively. All patients (100%) were treated with corticosteroids, fourteen (82.4%) received tacrolimus, fifteen (88.2%) received mycophenolic acid, and four (23.5%) received belatacept. The biological characteristics of the patients at M1, M2 and M3 are presented in Table 1. Regarding the histological data, the median g+cpt score before TCZ was 4 (3–5), indicating CAMR. Median cg score was 2 (1–3).
Figure 1.
Flow chart of this study.
Table 1.
Characteristics of tocilizumab therapeutic drug monitoring and laboratory parameters throughout the follow up (months (M) M1, M2 and M3).
| M1 (n = 17) | M2 (n = 17) | M3 (n = 17) | |
|---|---|---|---|
| Tocilizumab pharmacological data | |||
| Trough concentration (mg/L) | 17.4 (6.7–24.6) CV = 38.6% |
18.7 (11.0–31.2) CV = 41.1% |
20.3 (8.8–39.2) CV = 45.0% |
| Dose (mg) | 600 (426–694) | 600 (434–669) | 600 (392–683) |
| Laboratory parameters | |||
| Creatinine (µmol/L) | 151 (112–190) | 150 (113–190) | 147 (108–196) |
| GFR (mL/mn/1.73 m2) | 44 (30–55) | 41 (27–61) | 40 (29–60) |
| ACR (mg/mmol) | 9.3 (1.1–160) | 10.2 (0.9–149) | 8.3 (0.9–100) |
| Total proteins (g/L) | 68 (64–71) | 69 (60–72) | 67 (62–72) |
| Hepatic function altered a % (n) | 6.3 (1) | 6.3 (1) | 6.3 (1) |
| C reactive protein (mg/L) | <4 (<4–<4) | <4 (<4–<4) | <4 (<4–<4) |
Data are presented as medians (10th–90th percentile) or percentages (n); GFR, CKD-EPI glomerular filtration rate; ACR, urinary albumin-to-creatinine ratio; CV, coefficient of variation. a Liver function is considered impaired if transaminases are greater than three times the upper limit of normal.
3.2. Variability of TCZ trough Concentrations and Early Exposure (ΣCmin)
The TCZ Cmin at M1, M2, and M3 are described in Table 1. The median (10th–90th percentile) of all TCZ Cmin was 19.7 mg/L (7.7–36.6) and the coefficient of variation was 42.2%. The median (10th–90th percentile) of ΣCmin was 57.6 mg/L (26.2–100.0) and the coefficient of variation was 38.3%.
3.3. Determinants of the TCZ trough Concentration
In the univariate analysis, time from treatment initiation, co-treatment by tacrolimus, and the ACR were associated with the TCZ Cmin (Table 2). In the multivariate analysis, only the ACR and time from TCZ initiation remained independently associated with the TCZ Cmin.
Table 2.
Univariate and multivariate linear mixed-effect regression analyses for the identification of the determinants of log tocilizumab trough concentrations (n = 51) during longitudinal therapeutic drug monitoring.
| Variable | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Estimate | 95% Confidence Interval | p-Value | Estimate | 95% Confidence Interval | p-Value | |
| log(Age) | −0.34 | −1.5–0.82 | 0.578 | |||
| Gender (male–female) | 0.16 | −0.10–0.41 | 0.253 | |||
| log(Time after TCZ initiation) | 0.40 | 0.18–0.61 | 0.001 | 0.42 | 0.17–0.66 | 0.002 |
| log(Dose) | 0.96 | −0.094–2.0 | 0.087 | 0.88 | −0.0044–1.8 | 0.067 |
| Weight | 0.0053 | −0.0023–0.013 | 0.188 | |||
| log(BMI) | 0.57 | −1.1–2.2 | 0.507 | |||
| Creatinine | 0.00030 | −0.0036–0.0030 | 0.857 | |||
| GFR | 0.0014 | −0.0072–0.0099 | 0.758 | |||
| log(ACR) | −0.13 | −0.24–−0.020 | 0.037 | −0.11 | −0.21–−0.0085 | 0.050 |
| Total protein | −0.0098 | −0.029–0.0092 | 0.316 | |||
| Tacrolimus (yes–no) | 0.33 | 0.033–0.64 | 0.046 | 0.22 | −0.30–0.47 | 0.107 |
| Mycophenolic acid (yes–no) | −0.084 | −0.49–0.32 | 0.690 | |||
| Belatacept (yes–no) | 0.057 | −0.25–0.37 | 0.725 | |||
TCZ, tocilizumab; BMI, body mass index; GFR, glomerular filtration rate; ACR, urinary albumin-to-creatinine ratio. Significant values in bold.
3.4. Association between Early TCZ Plasma Exposure and Kidney Function
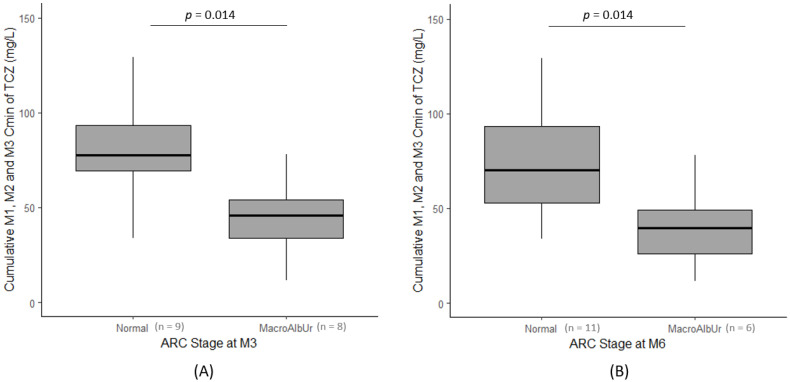
The ΣCmin were significantly lower (p = 0.014) for patients with ACR > 30 mg/mmol at M3 (median ΣCmin of 46.0 mg/L [21.9–63.8]) than for patients with ACR < 30 mg/mmol (median ΣCmin of 77.7 mg/L [45.8–106.0]) (Figure 2A).
Figure 2.
Cumulative M1, M2, and M3 Cmin of TCZ in patients with an ACR < 30 mg/mmol (normal) or > 30 mg/mmol (macroalbuminuria (MacroAlbUr)) at M3 (A) and M6 (B). Boxplots represent the medians and 25th–75th percentile.
At M6, the ΣCmin were also significantly lower (p = 0.014) for patients with ACR>30 mg/mmol (median ΣCmin of 39.9 mg/L [19.0–63.5]) than for patients with ACR < 30 mg/mmol (median ΣCmin of 70.2 mg/L [48.8–100.0]) (Figure 2B). Conversely, the ΣCmin were not associated with the glomerular filtration rate (p = 0.891).
Figure S1 presents the temporal evolution of the ACR before the TCZ administration (M0) and at M1, M2, M3, and M6 after the initiation of the TCZ treatment. All patients who were macroalbuminuric at M0 (two missing data points) kept the ACR > 30 mg/mmol at M1, M2, M3, and M6 (except one patient with an ACR = 21.1 mg/mmol at M6) post TCZ treatment initiation. Except one, all patients who had an ACR < 30 mg/mmol at M0 kept a normal ACR at M1, M2, M3, and M6. The ACR values were not significantly different between months (p = 0.430).
As urinary loss of the monoclonal antibody rituximab was previously described in patients with kidney disease, such as those with the nephrotic syndrome [31,32], we investigated whether urinary excretion of TCZ could contribute to the variability of the TCZ plasma concentrations. We measured the TCZ urinary concentrations in a separate cohort of seven additional kidney transplant patients with CAMR who were treated with TCZ. The characteristics of this separate cohort are presented in the Supplementary Materials (Table S1). For 6/7 patients, the urinary concentration of TCZ was lower than 1 mg/L, whereas their urinary ACRs ranged from 10 to 343.9 mg/mmol. The only patient who had detectable TCZ in urine (2.9 mg/L) also presented with the highest ACR of the study population (ACR = 948 mg/mmol).
4. Discussion
This study is the first to describe the pharmacokinetics of TCZ in kidney transplant patients with CAMR. The TCZ Cmin showed high interindividual variability among the CAMR patients, as recently reported in a separate cohort of kidney-transplant candidates undergoing desensitization [23]. These data highlight the need to identify the determinants of the variability of the TCZ Cmin to individually adjust the dose of TCZ.
Our results showed that time since TCZ initiation was an independent determinant of the TCZ Cmin in the multivariate analysis. The impact of time since TCZ initiation on the TCZ Cmin is consistent with the long elimination half-life of TCZ, which requires up to three to four months of treatment to reach the pharmacokinetic steady state equilibrium. The ACR was also an independent determinant of TCZ Cmin. Moreover, early TCZ Cmin measured within the first three months were higher for patients with an ACR < 30 mg/mmol than for those with an ACR > 30 mg/mmol.
Conversely, the TCZ Cmin were not associated with the glomerular filtration rate. These data were expected since, in the absence of nephropathy, the clearance of monoclonal antibodies involves target-mediated drug disposition and non-specific proteolytic elimination but not glomerular filtration, given their high molecular weight.
However, the urinary excretion of monoclonal antibodies has been reported in certain particular clinical settings, including nephropathy. For example, patients with membranous nephropathy, such as nephrotic syndrome, showed urinary loss of rituximab and insufficient drug exposure [31,32], requiring higher doses or more frequent administrations [33,34]. Urinary excretion of rituximab appeared to be dependent on the severity of proteinuria, as urinary loss of rituximab was observed in high proteinuria (ACR of 16,469 mg albumin/g creatinine) in a case report of two patients with nephrotic syndrome [35]. Interestingly, rituximab excretion in urine appeared to be associated with IgG urinary excretion, suggesting that urinary monoclonal antibody excretion may occur when the glomerular filter loses selectivity [35]. Although CAMR is a different clinical setting of kidney disease, we wished to further explore whether urinary loss of TCZ could contribute to the variability of the TCZ Cmin. In a separate cohort of kidney transplant patients with CAMR, urinary TCZ concentrations were below the limit of quantification (1 mg/L) for 6/7 patients, for whom the ACR ranged from 10 to 343.9 mg/mmol. The only patient who had detectable TCZ in urine (2.9 mg/L) also had the highest ACR of this study’s population: three-fold higher than that of other patients (see Supplementary Materials). Such low urinary excretion (in comparison to plasma concentrations) with such a high urinary ACR suggests that the urinary excretion of TCZ did not contribute to the variability of TCZ plasma Cmin observed in our cohort of 17 patients in whom the ACR ranged from 0.3 to 666.7 mg/mmol. Urinary excretion of monoclonal antibodies may occur in patients with extremely severe proteinuria, as reported for TCZ in the present study and as previously reported for rituximab [35], but this result must to be confirmed in larger cohorts.
Patients with chronic kidney disease and albuminuria present elevated biomarkers of inflammation [36]. In the Framingham Offspring Cohort, higher TNF-α, IL-6, and TNF receptor 2 levels were associated with the ACR [37]. The elimination of TCZ depends on binding to its target and therefore increases when the number of IL-6 receptor increases [38]. In our study, inflammation (accessed by CRP levels) could not be studied as a determinant of early TCZ exposure because all patients had undetectable CRP. This result was expected because CRP production rapidly decreases with TCZ treatment [11]. Further pharmacokinetic/pharmacodynamic (PK/PD) studies investigating the impact of albuminuria on TCZ clearance are required to investigate the underlying mechanisms associated with reduced TCZ plasma concentration in patients with macroalbuminuria, and to understand whether target-mediated drug disposition could contribute to the increased clearance of TCZ in patients with macroalbuminuria, as shown in the present study.
The description of the determinants of the variability of TCZ exposure is the first necessary step to consider the possibility of future personalization of the dose of TCZ. Additional clinical data linking TCZ plasma exposure and clinical efficacy and safety in kidney transplant patients with CAMR are required to determine the target therapeutic concentration range and then personalize the TCZ dosage according to the determinants of its variability. However, since the ACR presented weak variability throughout TCZ treatment courses (present study), our observations suggested that dose adjustment could be made from the first dose of TCZ according to the presence or the absence of a macroalbumuria, and not only according to the body weight of the patient.
The retrospective and monocentric design of our study and the limited sample size of our cohort were the main limitations of the present paper. However, our data, although preliminary, are the first to show that early exposure to TCZ may be associated with macroalbuminuria in the CAMR patients. In addition to the body weight, albuminuria could be a parameter to take into account to individualize the TCZ dosage. Further prospective and multicentric studies on larger cohorts should be performed to confirm our results and determine a threshold for early TCZ exposure associated with a clinical response in terms of efficacy and safety.
Acknowledgments
We thank Cécile Girard for their technical expertise.
Abbreviations
| ALAT | alanine aminotransferase |
| ASAT | aspartate aminotransferase |
| ACR | urinary albumin-to-creatinine ratio |
| BMI | body mass index |
| BSA | body surface area |
| CAMR | chronic antibody-mediated rejection |
| Cmin | trough concentration |
| ΣCmin | sum of the three Cmin measurements over the three first months of treatment |
| CV | coefficient of variation |
| DSA | donor-specific antibody |
| GFR | glomerular filtration rate |
| IgG | immunoglobulin G |
| IL-6 | interleukin 6 |
| M | month |
| TCZ | tocilizumab |
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm12227141/s1, Table S1: Characteristics of seven patients with simultaneous tocilizumab measurement in the blood and urine. Figure S1: Temporal evolution of the urinary albumin-to-creatinine ratio before tocilizumab administration (M0) and at M1, M2, M3 and M6 after tocilizumab administration.
Author Contributions
Conceptualization, F.S.-L. and M.J.; patient recruitment, T.J., J.N., D.L. and L.R.; data collection, M.J. and C.A.; data analysis, M.J., C.A., F.S.-L. and E.G.-V.; writing, F.S.-L., E.G.-V., M.J. and C.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Grenoble University Hospital Review Board (registration RnIPH 2022, protocol TOCIREJET; CNIL number: 2205066 v 0, approved on 8 September 2022).
Informed Consent Statement
Participants were all informed and did not object. Written consent for participation was not required for this study, in accordance with national legislation and institutional requirements.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Montgomery R.A., Loupy A., Segev D.L. Antibody-mediated rejection: New approaches in prevention and management. Am. J. Transplant. 2018;18((Suppl. S3)):3–17. doi: 10.1111/ajt.14584. [DOI] [PubMed] [Google Scholar]
- 2.Hart A., Singh D., Brown S.J., Wang J.H., Kasiske B.L. Incidence, risk factors, treatment, and consequences of antibody-mediated kidney transplant rejection: A systematic review. Clin. Transplant. 2021;35:e14320. doi: 10.1111/ctr.14320. [DOI] [PubMed] [Google Scholar]
- 3.Chehade H., Pascual M. The Challenge of Acute Antibody-Mediated Rejection in Kidney Transplantation. Transplantation. 2016;100:264. doi: 10.1097/TP.0000000000000959. [DOI] [PubMed] [Google Scholar]
- 4.Rostaing L.P.E., Böhmig G.A., Gibbons B., Taqi M.M. Post-Transplant Surveillance and Management of Chronic Active Antibody-Mediated Rejection in Renal Transplant Patients in Europe. Transpl. Int. 2023;36:11381. doi: 10.3389/ti.2023.11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabezas L., Jouve T., Malvezzi P., Janbon B., Giovannini D., Rostaing L., Noble J. Tocilizumab and Active Antibody-Mediated Rejection in Kidney Transplantation: A Literature Review. Front. Immunol. 2022;13:839380. doi: 10.3389/fimmu.2022.839380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R. Anti-Interleukin 6 Therapeutics for Chronic Antibody-Mediated Rejection In Kidney Transplant Recipients. Exp. Clin. Transplant. 2022;20:709–716. doi: 10.6002/ect.2021.0254. [DOI] [PubMed] [Google Scholar]
- 7.Pearl M., Weng P.L., Chen L., Dokras A., Pizzo H., Garrison J., Butler C., Zhang J., Reed E.F., Kim I.K., et al. Long term tolerability and clinical outcomes associated with tocilizumab in the treatment of refractory antibody mediated rejection (AMR) in pediatric renal transplant recipients. Clin. Transplant. 2022;36:e14734. doi: 10.1111/ctr.14734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller C.L., Madsen J.C. IL-6 Directed Therapy in Transplantation. Curr. Transplant. Rep. 2021;8:191–204. doi: 10.1007/s40472-021-00331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Vugt L.K., Schagen M.R., de Weerd A., Reinders M.E., de Winter B.C., Hesselink D.A. Investigational drugs for the treatment of kidney transplant rejection. Expert Opin. Investig. Drugs. 2022;31:1087–1100. doi: 10.1080/13543784.2022.2130751. [DOI] [PubMed] [Google Scholar]
- 10.McElvaney O.J., Curley G.F., Rose-John S., McElvaney N.G. Interleukin-6: Obstacles to targeting a complex cytokine in critical illness. Lancet Respir. Med. 2021;9:643–654. doi: 10.1016/S2213-2600(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastida C., Soy D., Ruiz-Esquide V., Sanmartí R., Huitema A.D.R. Exposure-response modeling of tocilizumab in rheumatoid arthritis using continuous composite measures and their individual components. Br. J. Clin. Pharmacol. 2019;85:1710–1718. doi: 10.1111/bcp.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J., Aubert O., Vo A., Loupy A., Haas M., Puliyanda D., Kim I., Louie S., Kang A., Peng A., et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am. J. Transplant. 2017;17:2381–2389. doi: 10.1111/ajt.14228. [DOI] [PubMed] [Google Scholar]
- 13.Lavacca A., Presta R., Gai C., Mella A., Gallo E., Camussi G., Abbasciano I., Barreca A., Caorsi C., Fop F., et al. Early effects of first-line treatment with anti-interleukin-6 receptor antibody tocilizumab for chronic active antibody-mediated rejection in kidney transplantation. Clin. Transplant. 2020;34:e13908. doi: 10.1111/ctr.13908. [DOI] [PubMed] [Google Scholar]
- 14.Massat M., Congy-Jolivet N., Hebral A.-L., Esposito L., Marion O., Delas A., Colombat M., Faguer S., Kamar N., Del Bello A., et al. Do anti-IL-6R blockers have a beneficial effect in the treatment of antibody-mediated rejection resistant to standard therapy after kidney transplantation? Am. J. Transplant. 2021;21:1641–1649. doi: 10.1111/ajt.16391. [DOI] [PubMed] [Google Scholar]
- 15.Shin B.-H., Everly M.J., Zhang H., Choi J., Vo A., Zhang X., Huang E., Jordan S.C., Toyoda M. Impact of Tocilizumab (Anti-IL-6R) Treatment on Immunoglobulins and Anti-HLA Antibodies in Kidney Transplant Patients With Chronic Antibody-mediated Rejection. Transplantation. 2020;104:856–863. doi: 10.1097/TP.0000000000002895. [DOI] [PubMed] [Google Scholar]
- 16.Chandran S., Leung J., Hu C., Laszik Z.G., Tang Q., Vincenti F.G. Interleukin-6 blockade with tocilizumab increases Tregs and reduces T effector cytokines in renal graft inflammation: A randomized controlled trial. Am. J. Transplant. 2021;21:2543–2554. doi: 10.1111/ajt.16459. [DOI] [PubMed] [Google Scholar]
- 17.Pottebaum A.A., Venkatachalam K., Liu C., Brennan D.C., Murad H., Malone A.F., Alhamad T. Efficacy and Safety of Tocilizumab in the Treatment of Acute Active Antibody-mediated Rejection in Kidney Transplant Recipients. Transplant. Direct. 2020;6:e543. doi: 10.1097/TXD.0000000000000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noble J., Giovannini D., Laamech R., Imerzoukene F., Janbon B., Marchesi L., Malvezzi P., Jouve T., Rostaing L. Tocilizumab in the Treatment of Chronic Antibody-Mediated Rejection Post Kidney Transplantation: Clinical and Histological Monitoring. Front. Med. 2021;8:790547. doi: 10.3389/fmed.2021.790547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khairallah P., Robbins-Juarez S., Patel S., Shah V., Toma K., Fernandez H., Dube G.K., King K., Mohan S., Husain S.A., et al. Tocilizumab for the treatment of chronic antibody mediated rejection in kidney transplant recipients. Clin. Transplant. 2023;37:e14853. doi: 10.1111/ctr.14853. [DOI] [PubMed] [Google Scholar]
- 20.Boonpheng B., De Castro I.C.C., Ng Y.-H., Blosser C., Bakthavatsalam R., Gimferrer I., Smith K., Leca N. Tocilizumab for treatment of chronic active antibody-mediated rejection in kidney transplant recipients. Clin. Transplant. 2023;37:e14936. doi: 10.1111/ctr.14936. [DOI] [PubMed] [Google Scholar]
- 21.Truffot A., Gautier-Veyret E., Baillet A., Jourdil J.-F., Stanke-Labesque F., Gottenberg J.-E. Variability of rituximab and tocilizumab trough concentrations in patients with rheumatoid arthritis. Fundam. Clin. Pharmacol. 2021;35:1090–1099. doi: 10.1111/fcp.12662. [DOI] [PubMed] [Google Scholar]
- 22.Abdallah H., Hsu J.C., Lu P., Fettner S., Zhang X., Douglass W., Bao M., Rowell L., Burmester G.R., Kivitz A. Pharmacokinetic and Pharmacodynamic Analysis of Subcutaneous Tocilizumab in Patients with Rheumatoid Arthritis from 2 Randomized, Controlled Trials: SUMMACTA and BREVACTA. J. Clin. Pharmacol. 2017;57:459–468. doi: 10.1002/jcph.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truffot A., Jouve T., Noble J., Bardy B., Malvezzi P., Rostaing L., Stanke-Labesque F., Gautier-Veyret E. Tocilizumab Trough Levels Variability in Kidney-Transplant Candidates Undergoing Desensitization. J. Clin. Med. 2021;11:91. doi: 10.3390/jcm11010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arad U., Elkayam O. Association of Serum Tocilizumab Trough Concentrations with Clinical Disease Activity Index Scores in Adult Patients with Rheumatoid Arthritis. J. Rheumatol. 2019;46:1577–1581. doi: 10.3899/jrheum.181431. [DOI] [PubMed] [Google Scholar]
- 25.Kneepkens E.L., van den Oever I., Plasencia C.H., Pascual-Salcedo D., de Vries A., Hart M., Nurmohamed M.T., Balsa A., Rispens T., Wolbink G. Serum tocilizumab trough concentration can be used to monitor systemic IL-6 receptor blockade in patients with rheumatoid arthritis: A prospective observational cohort study. Scand. J. Rheumatol. 2017;46:87–94. doi: 10.1080/03009742.2016.1183039. [DOI] [PubMed] [Google Scholar]
- 26.World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 27.Loupy A., Haas M., Roufosse C., Naesens M., Adam B., Afrouzian M., Akalin E., Alachkar N., Bagnasco S., Becker J.U., et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 2020;20:2318–2331. doi: 10.1111/ajt.15898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin A., Stevens P.E., Bilous R.W., Coresh J., Francisco A.L.M.D., Jong P.E.D., Griffith K.E., Hemmelgarn B.R., Iseki K., Lamb E.J., et al. Kidney disease: Improving global outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013;3:1–150. doi: 10.1038/kisup.2012.73. [DOI] [Google Scholar]
- 29.Haute Autorité de Santé Evaluation du Rapport Albuminurie/Créatininurie dans le Diagnostic de la Maladie Rénale Chronique Chez L’adulte. 2011. [(accessed on 8 June 2023)]. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2011-12/rapport__albuminurie_creatininurie_2011-12-27_14-57-31_440.pdf.
- 30.Willeman T., Jourdil J.-F., Gautier-Veyret E., Bonaz B., Stanke-Labesque F. A multiplex liquid chromatography tandem mass spectrometry method for the quantification of seven therapeutic monoclonal antibodies: Application for adalimumab therapeutic drug monitoring in patients with Crohn’s disease. Anal. Chim. Acta. 2019;1067:63–70. doi: 10.1016/j.aca.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 31.Hartinger J.M., Kratky V., Hruskova Z., Slanar O., Tesar V. Implications of rituximab pharmacokinetic and pharmacodynamic alterations in various immune-mediated glomerulopathies and potential anti-CD20 therapy alternatives. Front. Immunol. 2022;13:1024068. doi: 10.3389/fimmu.2022.1024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Del Vecchio L., Allinovi M., Rocco P., Brando B. Rituximab Therapy for Adults with Nephrotic Syndromes: Standard Schedules or B Cell-Targeted Therapy? J. Clin. Med. 2021;10:5847. doi: 10.3390/jcm10245847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y., Shen Q., Dong M., Xiong Y., Xu H., Li Z. Population Pharmacokinetics of Rituximab in Pediatric Patients with Frequent-Relapsing or Steroid-Dependent Nephrotic Syndrome. Front. Pharmacol. 2021;12:725665. doi: 10.3389/fphar.2021.725665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogueri U., Cheungapasitporn W., Bourne D., Fervenza F.C., Joy M.S. Rituximab Exhibits Altered Pharmacokinetics in Patients with Membranous Nephropathy. Ann. Pharmacother. 2019;53:357–363. doi: 10.1177/1060028018803587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stahl K., Duong M., Schwarz A., Wagner A.D., Haller H., Schiffer M., Jacobs R. Kinetics of Rituximab Excretion into Urine and Peritoneal Fluid in Two Patients with Nephrotic Syndrome. Case Rep. Nephrol. 2017;2017:1372859. doi: 10.1155/2017/1372859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta J., Mitra N., Kanetsky P.A., Devaney J., Wing M.R., Reilly M., Shah V.O., Balakrishnan V.S., Guzman N.J., Girndt M., et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. CJASN. 2012;7:1938–1946. doi: 10.2215/CJN.03500412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upadhyay A., Larson M.G., Guo C.-Y., Vasan R.S., Lipinska I., O’Donnell C.J., Kathiresan S., Meigs J.B., Keaney J.F., Rong J., et al. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol. Dial. Transplant. 2011;26:920–926. doi: 10.1093/ndt/gfq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dostalek M., Gardner I., Gurbaxani B.M., Rose R.H., Chetty M. Pharmacokinetics, Pharmacodynamics and Physiologically-Based Pharmacokinetic Modelling of Monoclonal Antibodies. Clin. Pharmacokinet. 2013;52:83–124. doi: 10.1007/s40262-012-0027-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.