Abstract
Nitroglycerin (glycerol trinitrate [GTN]), an explosive and vasodilatory compound, was metabolized by mixed microbial cultures from aeration tank sludge previously exposed to GTN. Aerobic enrichment cultures removed GTN rapidly in the absence of a supplemental carbon source. Complete denitration of GTN, provided as the sole C and N source, was observed in aerobic batch cultures and proceeded stepwise via the dinitrate and mononitrate isomers, with successive steps occurring at lower rates. The denitration of all glycerol nitrate esters was found to be concomitant, and 1,2-glycerol dinitrate (1,2-GDN) and 2-glycerol mononitrate (2-GMN) were the primary GDN and GMN isomers observed. Denitration of GTN resulted in release of primarily nitrite-N, indicating a reductive denitration mechanism. Biomass growth at the expense of GTN was verified by optical density and plate count measurements. The kinetics of GTN biotransformation were 10-fold faster than reported for complete GTN denitration under anaerobic conditions. A maximum specific growth rate of 0.048 ± 0.005 h−1 (mean ± standard deviation) was estimated for the mixed culture at 25°C. Evidence of GTN toxicity was observed at GTN concentrations above 0.3 mM. To our knowledge, this is the first report of complete denitration of GTN used as a primary growth substrate by a bacterial culture under aerobic conditions.
Nitroglycerin (glycerol trinitrate [GTN]) is manufactured for use as an explosive in double-base gun and rocket propellants and as a pharmaceutical vasodilator. It is commonly found in the waste streams and soils of munitions manufacturing facilities and pharmaceutical plants. Concerns about toxicity and explosion hazards have led to increased efforts to develop safe and cost-effective methods for treating GTN-laden waste streams. Historically, the destruction of energetic materials and explosive mixtures has been accomplished through open-air burning, detonation, or incineration techniques. As more stringent environmental regulations are enacted at the state and federal levels, these techniques are no longer considered viable. Physicochemical methods of GTN destruction involve adsorption on activated carbon followed by reduction with inorganic chemicals (e.g., Na2SO3) or by alkaline hydrolysis, yielding glycerol and nitrite or nitrate. However, these techniques suffer from high operational costs, the presence of excess reactants that remain dissolved in the effluent, and the necessity for secondary treatment to remove nitrogenous products. Preference would therefore be given to environmentally friendly biological treatment methods, provided that a robust GTN biotransformation technology that ensures complete transformation (i.e., complete denitration without accumulation of glycerol dinitrates [GDNs] or glycerol mononitrates [GMNs]) and economic practicality could be developed. Complete denitration is preferred since GDNs and GMNs are more soluble than GTN itself and in some instances are more toxic (6).
Early studies of GTN biotransformation (21) used activated sludge, in batch and continuous bioreactors, supplemented with excess primary carbon sources. The authors postulated a stepwise GTN biotransformation pathway via the dinitrate and mononitrate glycerol esters, with successive steps proceeding more slowly. It is questionable whether complete GTN denitration was observed, since residual dinitrate and mononitrate isomers were found in spent medium from both the batch and continuous bioreactors and conflicting results on the presence of glycerol nitrates in effluent samples were reported (21). The authors observed little or no reduction in GTN concentration in controls without supplemental carbon, suggesting that GTN biotransformation was a cometabolic process. In a later study, high destruction efficiencies of GTN were reported in a sequencing batch reactor used to treat munitions wastewater from a ball powder production facility (11). It is unclear if complete denitration of GTN was achieved, since no attempts were made to measure concentrations of GDNs or GMNs. Cometabolism was again suggested to be the mechanism of GTN biotransformation, since GTN-acclimated cultures were incapable of utilizing GTN as the sole carbon source in bench-scale reactors (11).
White and Snape (22) and Blehert et al. (2) recently reported on the ability of pure bacterial cultures to utilize GTN as the sole N source. An Agrobacterium radiobacter strain denitrated GTN with the formation of both GDN isomers and subsequent conversion to 1-GMN and 2-GMN; the strain was not able to denitrate the GMN isomers, resulting in GMN accumulation (22). A purified GTN reductase of A. radiobacter was NADH dependent and mediated the reductive scission of GTN to GDN only (20). NADPH-dependent GTN reductases isolated from Pseudomonas putida and Pseudomonas fluorescens strains mediated sequential reduction of GTN to GDNs and GMNs, but GMNs were not effectively denitrated (2).
Fungal transformation of GTN, by Geotrichum candidum and Phanerochaete chrysosporium, has been reported (4, 5, 13, 14). G. candidum was able to denitrate GTN completely to 1-GMN and 2-GMN (4), while P. chrysosporium was found to denitrate GTN only to 1,2-GDN and 2-GMN (13, 14). Evidence of a complete GTN denitration pathway was not observed with either culture (4, 5, 13, 14).
Meng et al. (10) observed complete denitration of GTN by cell extracts of Bacillus thuringiensis/cereus. Although complete denitration was accomplished, continuous addition of cell extracts was necessary. Incomplete denitration and toxicity of GTN were observed in resting cell studies of the same isolates (10). Christodoulatos et al. (3) reported the complete mineralization of GTN as the sole carbon source by mixed bacterial cultures under strict anaerobiosis. Specific removal rates of nitrate esters were low but increased substantially upon the addition of glucose as a cosubstrate.
A thermodynamic evaluation of biochemical GTN denitration was performed by Smets et al. (19), assuming a sequential denitration pathway of GTN via the dinitrate and mononitrate isomers in which each denitration step is reductive, mediated by a glutathione S-transferase. Various terminal electron acceptors were considered. The authors concluded that mineralization of GTN as the sole carbon and energy source by whole bacterial cells under both aerobic and anoxic conditions was thermodynamically feasible; they suggested that the failures of research groups to obtain enrichment cultures by utilizing GTN as the sole carbon source may have been caused by various limitations in experimental approaches (19).
The objectives of the present work were to (i) investigate whether bacterial cultures could be enriched for utilization of GTN as the sole carbon (C) and nitrogen (N) source under aerobic conditions, (ii) examine whether these cultures could mediate complete GTN denitration, and (iii) provide a preliminary assessment of GTN denitration kinetics. To maximize the chances of developing GTN-mineralizing enrichment cultures, an inoculum was chosen from a locale with historic and continued exposure to GTN.
MATERIALS AND METHODS
Materials.
GTN was obtained as a 5% solution in ethanol from Zeneca Specialties (Wilmington, Del.). Ethanol was evaporated under a stream of air to obtain small aliquots of neat GTN for preparation of filter-sterilized (0.45-μm pore size, Nylon Magna-R; MSI, Westboro, Mass.) aqueous GTN stock solutions (1.0 g/liter). Stock solutions contained minor impurities of 1,2-GDN (<1% of solute weight) and 1,3-GDN (<0.5%), while GMN concentrations were not detected. Analytical reference standards of GTN, 1,2-GDN, 1,3-GDN, 1-GMN, and 2-GMN in acetonitrile (purity, >98%) were obtained from Radian Corporation (Austin, Tex.).
Culture media.
Mineral media for all experiments consisted of the following (per liter): KH2PO4, 340 mg; K2HPO4, 440 mg; MgSO4 · 7H2O, 53 mg; CuSO4, 0.11 mg; MnSO4 · 7H2O, 0.24 mg; ZnSO4 · 7H2O, 0.68 mg; CaCl2 · 2H2O, 28 mg; CoCl2 · 6H2O, 0.17 mg; NaMoO4 · 2H2O, 0.17 mg; H3BO3, 0.04 mg; FeCl3 · 6H2O, 8.1 mg. The pH of this solution was 7.0. Glycerol (Aldrich Chemical Company, Milwaukee, Wis.) and nutrient broth (Difco Laboratories, Detroit, Mich.) were added as required immediately prior to autoclaving. GTN was added to cooled media from filter-sterilized stock solutions.
Enrichment for GTN denitration activity.
Batch enrichment cultures were initiated in 500-ml shake flasks with inocula of mixed liquor from a wastewater treatment facility receiving waste streams containing GTN (Olin Corporation, St. Marks, Fla.). Dilutions of the mixed liquor were made in mineral medium to a final concentration of approximately 1,200 mg (dry weight) per liter, and GTN was added initially at 0.13 mM as the sole C and N source. Control flasks, with autoclaved inocula and without any inocula, were included to quantify abiotic transformations and removal of GTN by sorption to biomass and glassware. All flasks were incubated on an orbital shaker at 150 rpm and room temperature (approximately 25°C). Samples were periodically withdrawn with a glass syringe and centrifuged at 10,000 × g in polypropylene microcentrifuge tubes for 10 min. After centrifugation, the supernatant was transferred to 2.0-ml Teflon-lined screw cap vials and immediately analyzed for glycerol nitrates by high-performance liquid chromatography (HPLC) or stored in the dark at 4°C prior to analysis. Flasks were periodically spiked to increasing concentrations of GTN.
Microbial growth assays.
Test tube cultures were initiated to confirm the ability of the cultures to use GTN as the sole C and N source. Autoclaved mineral medium (10 ml) supplemented with GTN (0.44 mM) as the sole source of C and N was added to sterile test tubes tightly stoppered with Teflon-lined screw caps. Control tubes without GTN were also included. Triplicate sets of tubes were inoculated with culture suspensions (0.1% [vol/vol]) from the batch enrichment culture. Test tubes were incubated horizontally on an orbital shaker at 25°C and 150 rpm. Biomass growth was monitored by optical density at 660 nm (OD660) measurements (Spec 20D+; Milton Roy, Rochester, N.Y.). Culture supernatants did not contain metabolites absorbing at 660 nm.
Substrate utilization assays.
A culture suspension from test tube enrichments taken in late exponential growth phase (as determined by OD660) was used as the inoculum (1% [vol/vol]) for initial substrate depletion studies. Inocula for subsequent substrate depletion assays were prepared by harvesting the cells at the end of an experiment by centrifugation at 10,000 × g for 10 min and resuspension in 0.85% NaCl. Experiments were performed in 500-ml shake flasks containing 200 ml of autoclaved mineral medium supplemented with GTN as the sole C and N source. Control flasks were amended with 0.1% (wt/vol) NaN3 to inhibit biological activity (24). All flasks were incubated in an orbital shaking water bath at 25°C and 150 rpm. Samples were withdrawn for OD660 measurements. Additional samples were removed and centrifuged at 10,000 × g in polypropylene microcentrifuge tubes for 5 min. After centrifugation, fractions of the supernatant were analyzed for nitrite while remaining fractions were transferred to 2-ml Teflon-lined screw cap vials and immediately analyzed for glycerol nitrates by HPLC or stored in the dark at 4°C prior to analysis. Nitrate analysis was performed on stored samples after termination of an experiment.
Viable cells at the beginnings and ends of shake flask experiments were enumerated as CFU by duplicate spread plating of serially diluted samples on tryptic soy agar diluted to one-fourth of the original nutrient concentration (Difco Laboratories). Plates were incubated at room temperature for approximately 1 week prior to colony counting.
Estimation of kinetic parameters.
Maximum specific growth rate coefficients, μmax, were estimated for test tube cultures by linear regression of log transformed OD660-versus-time profiles.
HPLC analysis of nitrate esters.
Nitrate esters were analyzed by isocratic HPLC (Varian Instruments, Palo Alto, Calif.). Complete separation of all glycerol nitrates was accomplished with a 5-μm Hypersil ODS column (25 cm by 4.6 mm) (Keystone Scientific, Bellefonte, Pa.) and an initial mobile phase of 5:95 (vol/vol) acetonitrile-water. After 10 min of elution, the mobile-phase composition was switched to 40:60 (vol/vol) acetonitrile-water. For samples in which the quantification of GMNs was not necessary, a 5-μm Spherisorb ODS-2 column (25 cm by 4.6 mm) (LDC/Milton Roy, Riviera Beach, Fla.) and a constant mobile-phase composition of 40:60 (vol/vol) acetonitrile-water were used. The mobile-phase flow rate for both elution systems was 1.0 ml/min, and detection was by UV absorbance at 214 nm.
Nitrite and nitrate analysis.
Nitrite analysis was performed by a modification of the sulfanilamide method (7). Sulfanilamide and N-(1-naphthyl)ethylenediamine reagents were prepared in nitrite-free deionized water as 1% (wt/vol) sulfanilamide in 10% (vol/vol) concentrated HCl and 0.04% (wt/vol) N-(1-naphthyl)ethylenediamine, respectively. Sample and reagent volumes were 4.0 and 0.2 ml, respectively. Interference from mineral medium components was minimal. The detection limit for nitrite was 0.01 mg of NO2-N/liter. Nitrate analysis was performed on a Dionex Series 4000i ion chromatograph equipped with a conductivity meter and an IONPAC (4-mm) AS12A anion column (Dionex, Sunnyvale, Calif.) operated with a mobile phase of 2.7 mM Na2CO3–0.3 mM NaHCO3. The detection limit for nitrate was 0.04 mg of NO3-N/liter.
Partitioning and losses of GTN.
Experiments were performed to evaluate the nonbiological loss of GTN resulting from typical sampling, centrifugation, and storage practices. A stock solution of GTN (0.48 mM) was sampled directly for analysis by using an HPLC injection syringe. Aliquots of the stock were subjected to successive 5-min centrifugation steps in polypropylene centrifuge tubes. Resulting supernatant fractions were stored in HPLC vials overnight at 4°C prior to analysis. All analyses were performed in triplicate, and losses were quantified by comparison of GTN concentrations of stock and centrifuged samples.
RESULTS
Enrichment for GTN denitration activity.
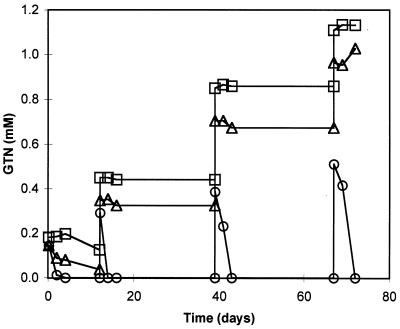
Sequential GTN spikes were administered to the enrichment and control flasks (Fig. 1). Rapid removal of GTN up to 0.5 mM was evident in the enrichment flask, while removal in the abiotic control flasks was negligible and sequential spikes caused a step increase in GTN concentrations. GDN isomers (not shown) accumulated transiently to maximum total concentrations of 0.02 mM, with 1,2-GDN being the dominant isomer observed. Confirmation of GMN removal was not possible with the HPLC method used at that time.
FIG. 1.
GTN removal in aerobic enrichment cultures. Flasks were sequentially spiked with GTN at nominal concentrations of 0.13, 0.26, 0.40, and 0.53 mM. Shown are GTN profiles in biotic flasks (○), abiotic control flasks with autoclaved inocula (▵), and abiotic control flasks without autoclaved inocula (□).
Inclusion of control flasks with autoclaved inoculum and without any inoculum allowed us to estimate a partition coefficient for GTN on biomass, PGTN (liters kg−1 biomass dry weight). The observed sorption of GTN to biomass for sequential GTN spikes was 0.016 ± 0.006 (mean ± standard deviation), 0.020 ± 0.002, 0.031 ± 0.004, and 0.027 ± 0.007 mg of GTN/mg of biomass dry weight. Using the entire data set, a log PGTN of 2.37 ± 0.30 was estimated, assuming that the initial biomass concentration of 1,200 mg (dry weight) per liter remained constant and that the difference in GTN concentration between the control flasks with autoclaved inoculum and without any inoculum (Fig. 1) was due to GTN sorption to biomass.
Microbial growth assays.
After 4 months of growth of the enrichment culture, aliquots were diluted (0.1% [vol/vol]) in test tubes in mineral medium with GTN (0.44 mM) as the sole C and N source to examine sustained growth on GTN as the primary growth substrate. Although samples were not withdrawn for HPLC analysis, an increase in OD660 values demonstrated biomass growth, which implied that some of the GTN was completely denitrated to yield assimilable carbon compounds for biomass synthesis (data not shown). Control test tubes without GTN addition did not show an OD660 increase, indicating that growth in experimental test tubes was due to GTN utilization rather than to CO2 and N2 fixation. From the OD660 profiles, the maximum specific growth rate was estimated at 0.048 ± 0.005 h−1.
Substrate utilization assays.
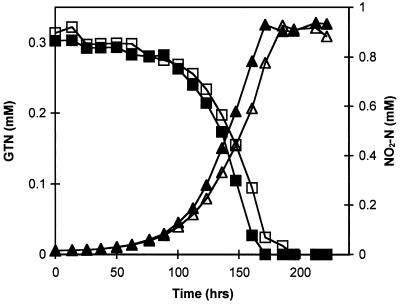
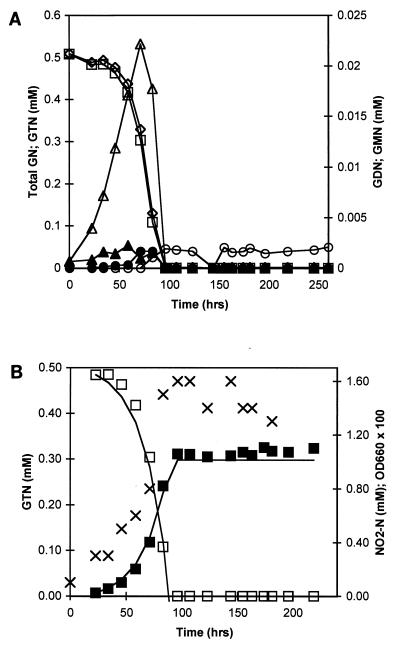
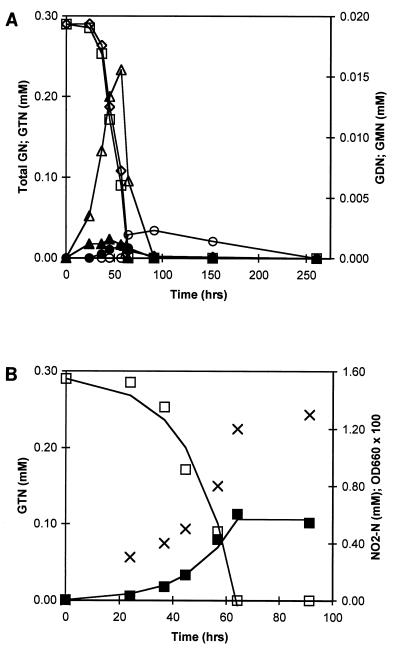
The utilization of GTN as the sole C and N source was investigated in detail in three separate batch experiments (E1 to E3), with initial GTN concentrations of 0.3 mM (E1, E3) and 0.5 mM (E2). No GTN removal was measured in the NaN3-supplemented flasks. Experiment E1 was initiated with an inoculum derived from test tube culturing, while experiments E2 and E3 were initiated with inocula derived from experiments E1 and E2, respectively. GTN removal was concomitant with substantial NO2− release (Fig. 2). GTN removal proceeded simultaneously with the accumulation and removal of each GDN and GMN isomer, with 1,2-GDN and 2-GMN as the dominant observed isomers (Fig. 3A and 4A). Removal of GDN and GMN began before complete GTN depletion. Complete mineralization of GTN was observed in experiments E1 and E3. In experiment E2, 2-GMN persisted at low concentrations (0.002 mM) until the termination of the experiment, 6 days after the disappearance of all other GTN transformation intermediates (Fig. 3). During experiments E1 through E3, 100, 72, and 75%, respectively, of the total nitrogen release expected from complete GTN denitration appeared as nitrite or nitrate, with 69 to 100% as nitrite and 0 to 7% as nitrate. Cell densities at the onset and termination of experiment E2 were 8.1 × 105 and 1.8 × 107 CFU/ml, respectively; during experiment E3, the corresponding values were 4.0 × 105 and 1.5 × 107 CFU/ml. Based on plate count enumerations, cell yields were estimated at (3.4 ± 0.2) × 1010 and (5.0 ± 0.5) × 1010 CFU per mmol of GTN removed for experiments E2 and E3, respectively. Visual inspection of plates from experiments E2 and E3 revealed the presence of six distinct colony morphologies and included both gram-negative and gram-positive bacteria.
FIG. 2.
Denitration of GTN and coupled NO2− release during experiment E1 with an initial GTN concentration of 0.3 mM. Shown are experimental profiles of GTN (□, ■) and nitrite-N (▵, ▴) in duplicate experiments.
FIG. 3.
Partial denitration of GTN at an initial concentration of 0.5 mM during experiment E2. (A) Experimental profiles of total glycerol nitrates (◊), GTN (□), 1,2-GDN (▵), 1,3-GDN (▴), 1-GMN (•), and 2-GMN (○) in test flask. (B) Experimental profiles of GTN (□), nitrite-N (■), and OD660 (×) in test flask. Continuous lines in panel B are best fits to the GTN and nitrite-N profiles obtained with a zero-order substrate depletion model coupled with cell growth through the experimental yield value (3.4 × 1010 CFU mmol of GTN−1).
FIG. 4.
Complete denitration of GTN at an initial concentration of 0.3 mM during experiment E3. (A) Experimental profiles of total glycerol nitrates (◊), GTN (□), 1,2-GDN (▵), 1,3-GDN (▴), 1-GMN (•), and 2-GMN (○) in test flask. (B) Experimental profiles of GTN (□), nitrite-N (■), and OD660 (×) in test flask. Continuous lines in panel B are best fits to the GTN and nitrite-N profiles obtained with a zero-order substrate depletion model coupled with cell growth through the experimental yield value (5.0 × 1010 CFU mmol of GTN−1).
Partitioning and losses of GTN.
An experimentally determined log KOW of 2.26 ± 0.06 for GTN was in agreement with earlier reports (9). In spite of the obvious hydrophobicity of GTN, sorptive losses during typical sampling, centrifugation, and storage practices were found to be insignificant (P = 0.05).
DISCUSSION
Growth on GTN, provided as the sole carbon, nitrogen, and energy source, was observed in mixed microbial enrichment cultures under aerobic conditions. Biotransformation of GTN resulted in the accumulation and subsequent removal of all glycerol dinitrate and mononitrate esters. The fact that GDNs and GMNs accumulated is consistent with earlier studies and suggests that successive denitration steps proceed at lower rates (2, 3, 10, 21). Recently, however, we reported GDN denitration as the rate-limiting step in the complete mineralization of GTN by a mixed culture in an aerobically operated sequencing batch reactor (18). In the present experiments, isomeric GDNs and GMNs coexisted with GTN during periods when a decrease in the total glycerol nitrate concentration was observed (Fig. 3A and 4A), suggesting the simultaneous denitration of all glycerol nitrate esters. The results of White et al. (23) indicated that GDN denitration was not concomitant with GTN denitration and that probably more than one enzyme was responsible for GTN denitration to GMN by whole cells, because the isolated GTN reductase did not denitrate GDN (20). Similarly, Meng et al. (10) noted that GTN and GDN denitration were not concomitant for the examined crude cell extracts, while the reductases isolated by Blehert et al. (2) catalyzed concomitant denitration of GTN and GDN. Whether the concomitant denitration of GTN, GDN, and GMN observed in our study is due to the action of one or more than one enzyme, located in one or more than one member of the mixed culture, is not yet known. Most researchers have observed incomplete GTN denitration (2, 10, 20, 23). The complete denitration of GTN is desirable for detoxification because GDNs and GMNs retain toxic properties (6). In addition, higher volatilities and aqueous solubilities make GDNs and GMNs more mobile than GTN, facts which must be considered during in situ bioremediation of GTN-contaminated soils.
The recalcitrance of nitrate esters to biotransformation has been observed by many researchers (1, 10, 12, 23). Resistance of these compounds to microbial attack is likely the result of their xenobiotic nature and toxicity. The tendency of GTN to partition into organic matter, confirmed by our experimental log KOW value of 2.26 ± 0.06, may explain its reported toxicity (4, 10, 18). Heipieper et al. (8) noted that microbial cells experience extreme inhibition or death when exposed to a medium containing organic compounds with log KOW values between 1 and 5. This is caused by the partitioning of such compounds into the lipid bilayer of the microbial cell membrane, where they may cause leakage resulting in disruption of the membrane potential, loss of proteins and lipids, and ultimate cell death (16). Thus, it is expected that GTN inhibits microbial activity through membrane solvent toxicity. The denitration of GTN into isomeric GDNs (log KOW unknown) and isomeric GMNs (log KOW = 1.46 [9]) may further contribute to cellular toxicity. Moreover, the metabolism of nitrate esters has been characterized as a reductive process, often resulting in the formation of nitrite (22). It is well established that nitrite is toxic to cells at high concentrations through a variety of mechanisms (15).
Complete mineralization of GTN observed during E3, for which the inoculum was derived from E2, suggests that 2-GMN persistence in E2 was not due to the loss of a specific metabolizing organism(s) and that the capacity to denitrate 2-GMN was recoverable. We postulate that toxicity resulting from high initial GTN concentrations and/or high concentrations of nitrite accumulated during E2 inhibited the enzymatic mechanism(s) responsible for 2-GMN denitration. Average cell yield estimates from plate count estimates were 30% smaller for E2 than for E3, further corroborating the toxicity observed during E2.
Christodoulatos et al. (3) recently reported a pseudo-first-order GTN degradation rate coefficient of 6.25 × 10−3 hr−1 in anaerobic batch experiments that were inoculated with approximately 0.5 g (dry weight) of mixed microbial cultures per liter in the absence of supplemental carbon sources. With the reported anaerobic kinetic parameter, the time predicted to achieve 99% removal of initial GTN concentrations of 0.3 mM is 740 h, while the experimental time to attain 100% removal of 0.3 mM GTN in our study was less than 65 h (Fig. 4). GTN biotransformation rates are therefore much higher in aerobic than in anaerobic cultures. Furthermore, GTN depletion profiles during aerobic experiments matched the total glycerol nitrate profiles closely (Fig. 3A and 4A). Therefore, the required times presented for GTN removal are similar to those required to achieve GTN mineralization. In contrast, reported GTN removal by anaerobic cultures did not correspond with any immediate loss in total glycerol nitrates, and additional time would be required to achieve GTN mineralization (3).
Exponential GTN depletion profiles (Fig. 2 to 4), indicative of cell growth coupled with zero-order substrate removal kinetics (17), were observed. The high substrate affinity displayed by the mixed culture is consistent with the high affinity for GTN expressed by purified GTN reductases (2, 20).
Due to the low initial substrate concentrations (0.33 to 0.5 mM GTN), biomass increases during batch experiments were very small and typical assays for measuring biomass density did not offer the required sensitivity. As a result, viable cell counts were determined to confirm cell growth supported by GTN denitration. The cell number increases during experiments E2 and E3 reflected approximately five cell doublings each. Although cell counts were made on a nonselective growth medium, the contribution of heterotrophic strains present in the original sludge inoculum was estimated to be less than 102 and 1 cell/ml at the start of E2 and E3, respectively. The ability of GTN to support biomass growth as the sole carbon and energy source is important, since it implies the continuing ability of cultures to treat GTN wastes in the absence of, or upon the depletion of, external carbon sources.
During GTN mineralization, 72% (E2), 75% (E3), and 100% (E1) of the GTN-nitrogen was detected as nitrite-N or nitrate-N, with nitrate-N contributing only a minor fraction of the released nitrogen (Fig. 2 to 4). During all assays, more than 66% of the GTN-nitrogen was released, indicating that more than two of the three nitrate ester bonds in GTN were cleaved, corroborating denitration of the GMNs. The lower nitrite-N and nitrate-N concentrations detected during E2 and E3 versus E1 suggest that nitrite and nitrate may further be removed by denitrification. The accumulation of nitrite during GTN mineralization suggests a reductive rather than a hydrolytic denitration mechanism. Reductive denitration is consistent with published accounts of denitration of nitrate esters by bacterial (1, 2, 20, 23) and fungal (13, 14) cultures. Meng et al. (10), working with bacterial cultures, postulated the hydrolytic cleavage of nitrate esters followed by reduction of nitrate to nitrite, although that finding was disrupted afterwards (23). The data gathered in this study do not support the occurrence of such a two-step enzymatic process.
In conclusion, we enriched for microbial cultures that were able to grow on GTN as the sole carbon, nitrogen, and energy source under aerobic conditions. Growth supported by complete GTN denitration was evidenced by an increase in culture OD and viable cell density and the sequential formation and disappearance of GDN and GMN isomers. GTN denitration was reductive and proceeded stepwise via the dinitrate and mononitrate isomers. The significance of microbial inhibition resulting from GTN solvent toxicity is currently under investigation in our laboratory. In addition, we are identifying and characterizing the component bacteria from the mixed culture and are examining whether individual isolates can mineralize GTN or whether cocultivation is required and whether individual isolates are capable of denitrification.
REFERENCES
- 1.Binks P R, French C E, Nicklin S, Bruce N C. Degradation of pentaerythritol tetranitrate by Enterobacter cloacae PB2. Appl Environ Microbiol. 1996;62:1214–1219. doi: 10.1128/aem.62.4.1214-1219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blehert D S, Knoke K L, Fox B G, Chambliss G H. Regioselectivity of nitroglycerin denitration by flavoprotein nitroester reductases purified from two Pseudomonas species. J Bacteriol. 1997;179:6912–6920. doi: 10.1128/jb.179.22.6912-6920.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christodoulatos C, Bhaumik S, Brodman B W. Anaerobic biodegration of nitroglycerin. Water Res. 1997;31:1462–1470. [Google Scholar]
- 4.Ducrocq C, Servy C, Lenfant M. Bioconversion of glyceryl trinitrate into mononitrates by Geotrichum candidum. FEMS Microbiol Lett. 1989;65:219–222. doi: 10.1016/0378-1097(89)90394-7. [DOI] [PubMed] [Google Scholar]
- 5.Ducrocq C, Servy C, Lenfant M. Formation of glyceryl 2-mononitrate by regioselective conversion of glyceryl trinitrate: efficiency of the filamentous fungus Phanerochaete chrysosporium. Biotechnol Appl Biochem. 1990;12:325–330. [PubMed] [Google Scholar]
- 6.Ellis I H V, Hodgson J R, Hwang S W, Halpap L M, Helton D O. Disposition and metabolism and Ames test of additional compounds. Progress report 6, no. NTIS: PC AO3/MF-AO1. Kansas City, Mo: Midwest Research Institute; 1978. [Google Scholar]
- 7.Gerhardt P, Murray R G E, Wood W A, Krieg N R, editors. Methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1994. [Google Scholar]
- 8.Heipieper H J, Weber F J, Sikkema J, Keweloh H, de Bont J A M. Mechanism of resistance of whole cells to toxic organic solvents. Trends Biotechnol. 1994;12:409–415. [Google Scholar]
- 9.Leo A, Hansch C, Elkins D. Partition coefficients and their uses. Chem Rev. 1971;71:525–616. [Google Scholar]
- 10.Meng M, Sun W-Q, Geelhaar L A, Kumar G, Patel A R, Payne G F, Speedie M K, Stacy J R. Denitration of glycerol trinitrate by resting cells and cell extracts of Bacillus thuringiensis/cereus and Enterobacter agglomerans. Appl Environ Microbiol. 1995;61:2548–2553. doi: 10.1128/aem.61.7.2548-2553.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pesari H, Grasso D. Biodegradation of an inhibitory nongrowth substrate (nitroglycerin) in batch reactors. Biotechnol Bioeng. 1993;41:79–87. doi: 10.1002/bit.260410111. [DOI] [PubMed] [Google Scholar]
- 12.Ramos J L, Haïdour A, Duque E, Piñar G, Calvo V, Oliva J-M. Metabolism of nitrate esters by a consortium of two bacteria. Nat Biotechnol. 1996;14:320–322. doi: 10.1038/nbt0396-320. [DOI] [PubMed] [Google Scholar]
- 13.Servent D, Ducrocq C, Henry Y, Guissani A, Lenfant M. Nitroglycerin metabolism by Phanerochaete chrysosporium: evidence for nitric oxide and nitrite formation. Biochim Biophys Acta. 1991;1074:320–325. doi: 10.1016/0304-4165(91)90170-l. [DOI] [PubMed] [Google Scholar]
- 14.Servent D, Ducrocq C, Henry Y, Servy C, Lenfant M. Multiple enzymatic pathways involved in the metabolism of glycerol trinitrate by Phanerochaete chrysosporium. Biotechnol Appl Biochem. 1992;15:257–266. [PubMed] [Google Scholar]
- 15.Sijbesma W F H, Almeida J S, Reis M A M, Santos H. Uncoupling effect of nitrite during denitrification by Pseudomonas fluorescens: an in vivo 31P-NMR study. Biotechnol Bioeng. 1996;52:176–182. doi: 10.1002/(SICI)1097-0290(19961005)52:1<176::AID-BIT18>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 16.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simkins S, Alexander M. Models for mineralization kinetics with variables of substrate concentration and population density. Appl Environ Microbiol. 1984;47:1299–1306. doi: 10.1128/aem.47.6.1299-1306.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smets B F, Accashian J V, Dizigan R A. Aerobic biodegradation of nitroglycerin in a sequencing batch reactor, AC#9734002. Presented at the 70th Annual WEF Conference and Exposition. 1997. Chicago, Ill. [Google Scholar]
- 19.Smets B F, Vinopal R T, Grasso D, Strevett K A, Kim B-J. Nitroglycerin biodegradation: theoretical thermodynamic considerations. J Energet Mater. 1995;13:385–398. [Google Scholar]
- 20.Snape J R, Walkley N A, Morby A P, Nicklin S, White G F. Purification, properties, and sequence of glycerol trinitrate reductase from Agrobacterium radiobacter. J Bacteriol. 1997;179:7796–7802. doi: 10.1128/jb.179.24.7796-7802.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wendt T M, Cornell J H, Kaplan A M. Microbial degradation of glycerol nitrates. Appl Environ Microbiol. 1978;36:693–699. doi: 10.1128/aem.36.5.693-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White G F, Snape J R. Microbial cleavage of nitrate esters: defusing the environment. J Gen Microbiol. 1993;139:1947–1957. doi: 10.1099/00221287-139-9-1947. [DOI] [PubMed] [Google Scholar]
- 23.White G F, Snape J R, Nicklin S. Biodegradation of glycerol trinitrate and pentaerythrol tetranitrate by Agrobacterium radiobacter. Appl Environ Microbiol. 1996;62:637–642. doi: 10.1128/aem.62.2.637-642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf D C, Dao T H, Scott H D, Lavy T L. Influence of sterilization methods on selected soil microbiological, physical, and chemical properties. J Environ Qual. 1989;18:39–44. [Google Scholar]