Abstract
This meta-analysis of observational studies aimed at estimating the overall prevalence of overdiagnosis and overtreatment in subjects with a clinical diagnosis of Chronic Obstructive Pulmonary Disease (COPD). MedLine, Scopus, Embase and Cochrane databases were searched, and random-effect meta-analyses of proportions were stratified by spirometry criteria (Global Initiative for COPD (GOLD) or Lower Limit of Normal (LLN)), and setting (hospital or primary care). Forty-two studies were included. Combining the data from 39 datasets, including a total of 23,765 subjects, the pooled prevalence of COPD overdiagnosis, according to the GOLD definition, was 42.0% (95% Confidence Interval (CI): 37.3–46.8%). The pooled prevalence according to the LLN definition was 48.2% (40.6–55.9%). The overdiagnosis rate was higher in primary care than in hospital settings. Fourteen studies, including a total of 8183 individuals, were included in the meta-analysis estimating the prevalence of COPD overtreatment. The pooled rates of overtreatment according to GOLD and LLN definitions were 57.1% (40.9–72.6%) and 36.3% (17.8–57.2%), respectively. When spirometry is not used, a large proportion of patients are erroneously diagnosed with COPD. Approximately half of them are also incorrectly treated, with potential adverse effects and a massive inefficiency of resources allocation. Strategies to increase the compliance to current guidelines on COPD diagnosis are urgently needed.
Keywords: chronic obstructive pulmonary disease (COPD), overdiagnosis, overtreatment, meta-analysis
1. Introduction
According to the Global Burden of Disease, in 2019, Chronic Obstructive Pulmonary Diseases (COPDs) were the third most common cause of death across the world, causing over 3.3 million deaths [1]. In addition, the current literature consistently predicts a substantial increase in the future health burden of COPD [2]. Despite clear criteria for the diagnosis of COPD, produced by the Global Initiative for Chronic Obstructive Lung Disease (GOLD), being available for two decades [3,4], underdiagnosis and overdiagnosis are still common, causing, in turn, under- or overtreatment, and determining a suboptimal disease management [5,6,7,8]. One recent meta-analysis quantified the rate of underdiagnosis in primary healthcare [9], and several studies estimated the rate of overdiagnosis [10]. However, the available evidence is highly heterogeneous, and a summary estimate of the magnitude of overdiagnosis is not yet available. We thus carried out a systematic review and meta-analysis to estimate the overall prevalence of overdiagnosis in subjects with a clinical diagnosis of COPD, both in primary care and hospital settings.
2. Materials and Methods
2.1. Search Strategy and Data Extraction
The reporting of this systematic review was guided by the standards of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 Statement [11]. We extracted data from observational studies evaluating the false positive rate of clinical diagnosis (CD) compared to the spirometry confirmation. We searched MedLine, Scopus, Embase and Cochrane databases, up to 30 March 2023, using the following search strategy: “(COPD) AND (Misdiagnosis OR Overdiagnosis OR Overtreatment)” with the filter for years from 1997–2023. The time frame was chosen according to the theorization and introduction of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines [12]. The references of the reviews and retrieved articles were also screened for additional pertinent papers. Only English-language studies were included. The extended version of the string is available in Table S1.
Each included article was independently evaluated by two reviewers (MF, and MR), who extracted the main study characteristics (first author, publication year, country, study design, population, setting, mean age of the CD-COPD patients, COPD definition, number of false positives, number of patients with CD and overtreated patients among overdiagnosed). Each of the reviewers extracted the data of the same set of articles by using an extraction table. Disagreements were discussed with and solved by a third reviewer (LM).
2.2. Data Analysis
According to the International GOLD guidelines [4] on the management of COPD, all clinical diagnoses must be confirmed by spirometry testing showing the airway’s irreversible obstructions. The primary outcome was the rate of overdiagnosis, defined as the number of subjects with a clinical diagnosis of COPD that was not confirmed after spirometry, divided by all the subjects with a clinical diagnosis of COPD (either confirmed or not after spirometry).
COPD clinical diagnosis was defined by the presence of one of the following [10]:
-
-
Clinical diagnosis by a physician during the study or in recorded administrative data;
-
-
History of medication coherent to COPD diagnosis;
-
-
Physician ignoring a negative result of the spirometry.
A clinical diagnosis of COPD was considered appropriate when confirmed by spirometry using GOLD [4] and/or LLN (Lower Limit of Normal) [13] criteria. According to the GOLD definition, a diagnosis of COPD is confirmed when the spirometry shows a post-bronchodilator FEV1/FVC < 0.7 [4]. According to LLN criterion, a COPD diagnosis is confirmed when the spirometry shows a FEV1/FVC ratio that falls outside of two standard deviations of a reference population [13].
The secondary outcome was the rate of overtreatment among overdiagnosed subjects. It was defined as the number of subjects undergoing at least one COPD therapy, divided by all the overdiagnosed subjects. We used random-effect meta-analyses of proportions to combine data and obtain summary estimates of each outcome. The effect sizes (% of overdiagnosis or overtreatment, ES) and 95% Confidence Interval (CI) of each individual study were displayed using forest plots, in which studies’ ESs are graphically represented by dots, and their CIs are expressed as horizontal bars. All analyses were stratified by setting of care—hospital/healthcare center and primary care/general population—and were carried out using Stata, version 15.0 (Stata Corp., College Station, TX, USA, 2022).
2.3. Quality Assessment
The internal quality of each included report was assessed using the checklist recommended by The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [14], composed of 22 items to evaluate the quality of observational study reports. STROBE does not provide ways to define a score allowing to rate the quality of the study. To investigate the potential impact of study quality in stratified meta-analyses, studies were classified as of “poor quality” if their overall score ranged from 0 to 14, of “intermediate quality” when the scores ranged from 15 to 25, of “good quality” when the scores were higher than 26 [15].
3. Results
3.1. Characteristics of the Included Studies
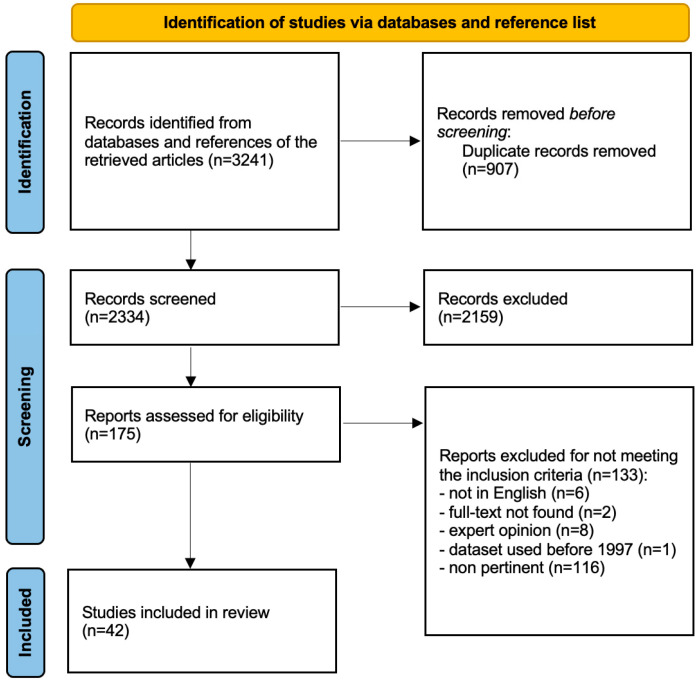
The initial search identified 3241 articles; 907 articles were removed because duplicates, and 2159 were excluded at the title/abstract screening stage. The remaining 175 full-text articles were assessed for eligibility, and 42 papers met the criteria for final inclusion [8,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], (Figure 1).
Figure 1.
PRISMA flowchart. From: Page MJ, McKenzie JE, Bossuyt PM, et al. “The PRISMA 2020 statement: an updated guideline for reporting systematic reviews” [11].
The main characteristics of the included studies are reported in Table S2: these were published from 2005 to 2022; 20 were carried out in Europe [8,16,17,19,22,23,26,28,31,35,36,37,38,42,45,47,48,50,51,55], twelve in America [18,20,24,25,27,29,30,33,41,44,49,52], four in Oceania [34,40,54,56], two in Asia [21,39] and four were multicentric [32,43,46,53]. Almost all studies included had a cross-sectional design [8,16,17,18,19,20,21,22,23,25,27,28,29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56], and the cross-sectional data were extracted from three cohort studies [24,26,30]. In the 42 included papers, we were able to extract 33 datasets that recruited the participants from the general population or primary care patients [8,17,18,21,23,24,25,26,27,28,29,31,32,33,34,35,36,38,39,40,41,42,45,46,47,48,50,51,52,53,54,55,56], six that were performed in hospitals [19,20,30,37,44,49], and three datasets that recruited participants from both settings [16,22,43]. Thirty-two datasets used GOLD criteria to define COPD [8,16,17,18,21,23,24,25,26,27,28,29,30,31,33,34,35,36,38,39,41,42,45,47,48,50,51,52,53,54,55,56], three used LLN definition [19,20,32], and seven datasets adopted both definitions [22,37,40,43,44,46,49].
3.2. Quality Assessment
As reported in Table S3, according to the STROBE checklist, nine studies were classified as of “good quality”, and the remaining 33 as of “intermediate quality”. The most frequent issues pertained the description of potential biases in the Methods (incomplete in all but two studies), the explanation of the statistical analyses (unsatisfactory in all but eight studies), and the indication of the study design with commonly terms in the title or abstract (incomplete in all but eleven studies).
3.3. Overdiagnosis
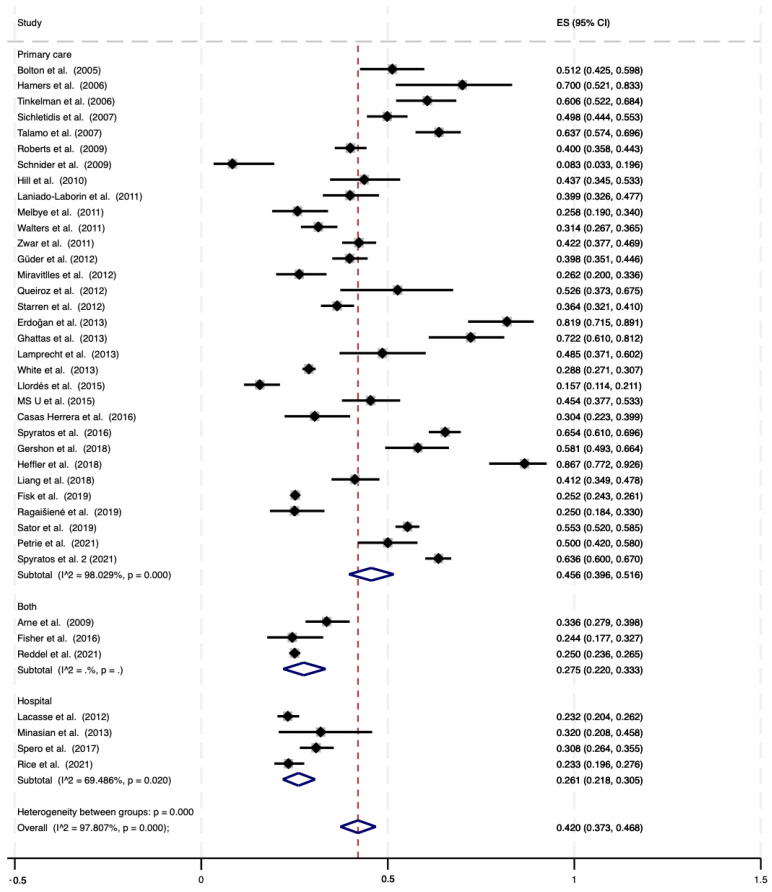
Thirty-nine datasets, including a total of 23,765 subjects, were included in the meta-analysis estimating the prevalence of COPD overdiagnosis according to GOLD definition (Table 1). Overall, the pooled prevalence was 42.0% (95% Confidence Interval (CI): 37.3–46.8% Figure 2), with a large heterogeneity among the individual studies. Five datasets showed a prevalence lower than 25%, while thirteen studies reported values higher than 50%. The summary prevalence of COPD overdiagnosis was significantly higher in the 32 primary care studies (45.6%; 95% CI: 39.6–51.6%) rather than in the four studies that included patients in hospital setting (26.1%; 95% CI: 21.8–30.5%). Among the latter four studies, three included only inpatients [30,44,49] and one included only outpatients [37]. The summary rate of overdiagnosis of the three studies including only inpatients was 25.5% (95% CI: 21.1–30.2%; Table 1).
Table 1.
Pooled rates of COPD overdiagnosis and overtreatment.
| Outcome: Overdiagnosis | Study Ref. | N. Datasets (n/N) a |
Pooled Rates % (95% CI) |
I2, % |
|---|---|---|---|---|
| COPD Definition: GOLD | ||||
| Overall sample | 39 (7710/23,765) | 42.0 (37.3–46.8) | 97.8% | |
| Primary care/ general population setting |
[8,17,18,21,23,24,25,26,27,28,29,31,33,34,35,36,38,39,40,41,42,45,46,47,48,50,51,52,53,54,55,56] | 32 (6356/18,450) | 45.6 (39.6–51.6) | 98.0% |
| Hospital/ healthcare center setting |
[30,37,44,49] | 4 (421/1666) | 26.1 (21.8–30.5) | 69.5% |
| Hospital/inpatients | [30,44,49] | 3 (405/1616) | 25.5(21.1–30.2) | -- |
| Hospital/outpatients | [37] | 1 (16/50) | 32.0 (19.5–46.7) | -- |
| Both settings | [16,22,43] | 3 (933/3649) | 27.5 (22.0–33.3) | -- |
| COPD Definition: LLN | ||||
| Overall sample | 10 (5917/12,455) | 48.2 (40.6–55.9) | 98.3% | |
| Primary care/ general population setting |
[32,40,46] | 3 (1619/2611) | 55.9 (46.1–65.5) | -- |
| Hospital/ healthcare center setting |
[19,20,37,44,49] | 5 (3070/6521) | 43.8 (34.4–53.4) | 95.1% |
| Both settings | [22,43] | 2 (1228/3323) | 36.9 (35.2–38.5) | -- |
| Outcome: Overtreatment | Study ref. |
N. datasets
(n/N) b |
Pooled rates
% (95% CI) |
I2, % |
| COPD Definition: GOLD | [8,23,25,26,39,50,51,53,54,55,56] | 11 (2807/4842) | 57.1 (40.9–72.6) | 99.1% |
| COPD Definition: LLN | [19,20,46] | 3 (1570/3341) | 36.3 (17.8–57.2) | 98.6% |
a Number of patients overdiagnosed among number of CD-COPD patients. b Number of patients overtreated among total number of patients overdiagnosed. CD-COPD: Clinically Diagnosed-Chronic Obstructive Pulmonary Disease. GOLD: Global Initiative for Chronic Obstructive Lung Disease. LLN: Lower limit of normal. CI: Confidence Interval. I2: level of heterogeneity.
Figure 2.
Proportion meta-analysis of overdiagnosis among subjects with clinical diagnosis of COPD, according to GOLD definition [8,16,17,18,21,22,23,24,25,26,27,28,29,30,31,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56]. ES = Effect Size (% of overdiagnosis); CI: Confidence Interval; COPD: Clinically Diagnosed Chronic Obstructive Pulmonary Disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; I2: level of heterogeneity.
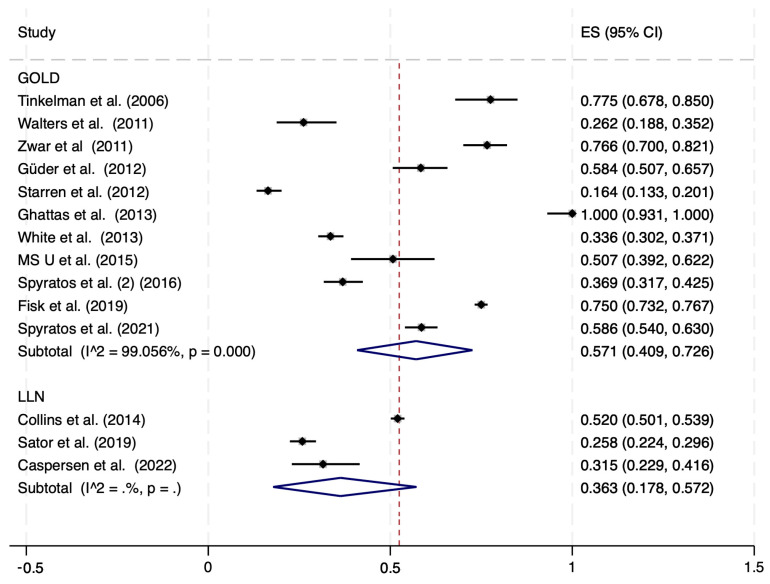
Ten studies quantified the proportion of COPD overdiagnosis according to LLN definition and included a total of 12,455 subjects (Table 1). Overall, the pooled prevalence was 48.2% (95% CI: 40.6–55.9%) (Figure 3), with the two studies including the primary care setting reporting values higher than 60%, and the five hospital-based studies showing a summary prevalence of 43.8% (95% CI: 34.4–53.4%—Figure 3). When the analyses were restricted to the seven studies that evaluated COPD prevalence using both GOLD and LLN criteria, on the same population, the pooled prevalence of COPD according to GOLD and LLN criteria were, respectively, 34.0% (95% CI: 23.0–46.0%) and 47.5% (95% CI: 36.5–58.5%) (Figure S1).
Figure 3.
Proportion meta-analysis of overdiagnosis among subjects with clinical diagnosis of COPD, according to LLN definition [19,20,22,32,37,40,43,44,46,49]. ES = Effect Size (% of overdiagnosis); CI: Confidence Interval; COPD: Clinically Diagnosed Chronic Obstructive Pulmonary Disease; LLN: Lower limit of normal; I2: level of heterogeneity.
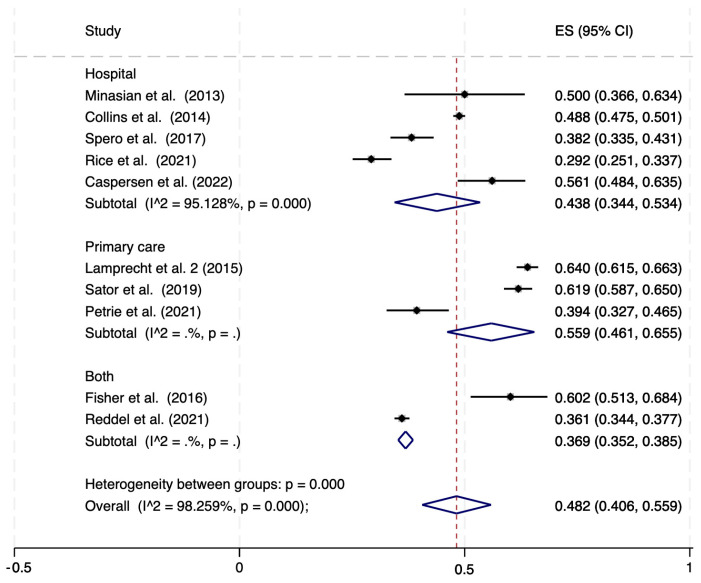
3.4. Overtreatment
Eleven studies, including a total of 4842 individuals, were included in the meta-analysis estimating the prevalence of COPD overtreatment according to GOLD definition (Table S2). Four studies reported a proportion of overtreated subjects, among those that were overdiagnosed, equal or larger than 75% (Figure 4), but the overall estimated prevalence was 57.1% (95% CI: 40.9–72.6%). When the results of the three studies (3341 subjects) that evaluated the proportion of overtreatment according to LLN definition were pooled, the summary prevalence was 36.3% (95% CI: 17.8–57.2%; Figure 4).
Figure 4.
Proportion meta-analysis of overtreatment among overdiagnosed individuals, according to GOLD and LLN definition [8,19,20,23,25,26,39,46,50,51,53,54,55,56]. ES = Effect Size (% of overtreatment); CI: Confidence Interval; COPD: Clinically Diagnosed Chronic Obstructive Pulmonary Disease; GOLD: Global Initiative for Chronic Obstructive Lung Disease; LLN: Lower limit of normal; I2: level of heterogeneity.
4. Discussion
The main findings of this meta-analysis, which included the data of more than 24,000 subjects with a clinical diagnosis of COPD from 35 different countries, are the following: (a) when spirometry was not used, at least four patients out of ten received an erroneous diagnosis of COPD, with rates substantially higher in primary care; (b) the prevalence of overdiagnosis did not decrease over time, and was still higher than 50% in three recent studies [19,46,50]; (c) more than half of the overdiagnosed subjects received an inappropriate COPD treatment; (d) on the same population of subjects, the prevalence of COPD overdiagnosis was consistently lower when GOLD rather than LLN criteria were used.
The false positive prevalence may be attributed to the physician’s incapacity of distinguishing COPD to other clinical conditions due to the similar symptomatology with other diseases [57,58] and the under-use of spirometry [28]. Patients with overlapping COPD symptoms, like cough, breathlessness and dyspnea, may be empirically labeled by the physician as “GOLD 0”, leading to possible diagnostic confusion [59,60]. Conditions like asthma, obesity, cardiac pathologies, restrictive patterns, and aging may be the condition underneath the refereed symptoms [61,62]. Further studies are necessary to explore the prevalence of the underlying condition in the case of false positive CD-COPD. These epidemiological insights may help physicians to make appropriate clinical decisions and policy makers to design better diagnostic-therapeutic pathways.
The use of spirometry testing is highlighted by guidelines to prevent overdiagnosis; still, there are no signs of a substantial improvement in its compliance by the clinicians in recent years. The under-use of spirometry testing may be explained by barriers that span in multiple domains. Ranging from a lack of awareness regarding the importance of assessing lung function [63], to difficulties in accessing spirometry evaluation [64,65], to issues concerning the interpretation of spirometry patterns by primary care physicians [66]. The burden of overdiagnosis is higher in the primary care setting with respect to the hospital. The difference may be driven by the hospital setting having less barriers to guideline implementations [67]. However, further studies are needed to clarify the reason underneath this difference [68]. Considering these challenges, policy makers should welcome all Public Health strategies to improve guideline adherence. Reasonable approaches could be: (a) education of General Practitioners (GPs) to use a well-funded wait-see approach [28]; (b) increase the patient’s awareness about the “too much medicine problem” [69]; (c) creation of spirometry specialized hubs in a coordinate Primary Care network [70]; (d) GPs equipped and trained for the spirometry use [71]; (e) restrictive rules in the drugs prescription [72].
Overall, approximately half of the 40% subjects that were overdiagnosed received an inappropriate COPD treatment, which translates in approximately one patient out of five with a suspect COPD being overtreated. Indeed, overtreatment is unlikely to produce a net benefit for the patients [73], potentially leading to several adverse effects, from cough to pneumonia, and to a delayed diagnosis of the true condition that caused the respiratory symptoms [55]. In addition, as estimated by some global analyses, the costs associated with overtreatment can be massive [74,75,76,77]. The global therapeutics market size of COPD is estimated at $20 billion in 2023 and is projected to reach approximately $33 billion by 2030 [78]. Thus, according to the present findings, every year billions of USD may be potentially wasted on overtreatment for “GOLD 0” patients. Moreover, as White et al. suggested, the overtreatment may not be limited to GOLD 0, but may extend to GOLD 1, 2, 3, and 4 [55].
In this scenario, it may be reassuring that, at least in theory, the solution is relatively straightforward, as an adequate use of spirometry ensures accurate diagnosis and treatment and reduces unnecessary treatment [27,51]. According to Spyratos et al., the resources saved thanks to a proper spirometry-based diagnosis could potentially cover the entire cost of treatment for the underdiagnosed population, thus diverting funds from more urgent and important illnesses needs [8]. Although further studies are needed to more precisely assess the financial upsides of diagnostic adherence to the guidelines, the results of this meta-analysis strongly reinforce the call for strategies that may substantially increase the adherence to current COPD guidelines in all settings.
The American Thoracic Society and the European Respiratory Society recommend the use of age- and sex-specific LLN definition for FEV1/FVC, which may lead to a more precise COPD assessment [79]. However, the meta-analyses stratified by COPD definition showed a higher rate of overdiagnosis when LLN was used. Moreover, in all of the seven studies that estimated the rate of overdiagnosis using both LLN and GOLD definitions, on the same subjects [22,37,40,43,44,46,49], the raw proportion of overdiagnosed subjects was higher when LLN criteria were adopted, and the overall prevalence of overdiagnosis was 34.0% using GOLD definition; 47.5% using LLN. On the other side, however, the proportion of overtreated subjects was higher when GOLD criteria were used (57.1% vs. 36.3% using LLN criteria). While a higher rate of overdiagnosis might be expected when LLN is used, since the GOLD “fixed ratio” approach is known to overestimate COPD in older individuals (given the progressive FEV1/FVC ratio decrease with age) [80], being thus being less efficient at recognizing the errors by the clinicians, the lower proportion of overtreatment that was observed using LLN criteria was unexpected and may be due, at least in part, by the sum of a statistical and an epidemiological issue. Firstly, only three studies were included in the meta-analysis estimating LLN overtreatment, and the summary rate was heavily influenced by the smaller samples, with the arithmetic mean being substantially larger (47.0%) than the weighted one (36.3%). Secondly, and more importantly, while all the studies that evaluated overtreatment using GOLD criteria were performed in primary care (which was associated with higher rates of overtreament), two of the three studies (and 83% of the total sample) that adopted LLN criteria were carried out in the hospital setting, where lower overtreatment rates were observed). Although the above factors may partially explain the difference in overtreatment prevalence that was observed adopting GOLD or LLN definitions, the overall findings provide support to the intense controversy over which criteria to use for the spirometry definition of COPD [81]. Indeed, reaching a consensus on this point is both urgent and essential to proceed with uniform and widely accepted strategies to reduce both overdiagnosis and overtreatment [82].
This meta-analysis has some limitations that must be considered in interpreting the results. First, as with any systematic review, publication bias may have influenced the results. However, this was a meta-analysis of proportions, with no direct comparisons, thus avoiding the typical bias deriving from the lower publication rate of non-significant results [83]. Second, the retrospective studies dealing with hospital data are at high risk of misclassification bias. Notably, however, the results of these studies showed a lower prevalence of COPD overdiagnosis. Third, the number of datasets evaluating overdiagnosis or overtreatment according to LLN definition was limited, although the overall sample was larger than 3000 patients for both outcomes. Fourth, we extracted spirometry-based estimates using the LLN and GOLD criteria, but these have some limitations. Different studies may use distinct tools, spirometers, and protocols, which may contribute to the high heterogeneity in the prevalence rates. Unfortunately, other potential definitions of overdiagnosis, such as normalization after therapy with multiple spirometry follow-ups [35], or use of a pre-bronchodilator, were adopted in very few studies [84,85] and could not be explored with a meta-analysis [10]. Fifth, most meta-analyses showed a high level of heterogeneity. However, of the 42 studies that were included in the meta-analysis to estimate the degree of overdiagnosis, 37 reported a prevalence of overdiagnosis higher than 25%; of the 14 studies included to estimate the overtreatment, eleven reported a prevalence higher than 30%. Thus, although a precise estimate cannot be obtained, it is very unlikely that the mean incidence of overdiagnosis and overtreatment are actually lower than 25% and 30%. Lastly, we only searched for studies written in English, which may have caused a selection bias, since other regions show greater difficulties in adhering to the recommendations and management guidelines for COPD [86], which in turn causes greater overdiagnosis of hospitalized patients and underdiagnosis in relation to the general population.
5. Conclusions
This meta-analysis shows that, when the diagnosis of COPD is exclusively clinical, and no spirometry is used, four out of ten patients are erroneously diagnosed with COPD, and should be further examined to ascertain the true causes of the respiratory symptoms. The proportion of overdiagnosis was substantially higher in the primary setting. In addition, approximately half of the overdiagnosed subjects are erroneously treated for COPD, with potential adverse effects and a massive inefficiency of resources allocation. The prevalence of overdiagnosis did not decrease over time and was higher when LLN rather than GOLD definition was adopted. These findings strongly reinforce the need to increase compliance to current guidelines on the use of spirometry in COPD diagnostic process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12226978/s1, Table S1: Search strategies’ extended version; Table S2: Characteristics and main results of the studies included in the meta-analysis on overdiagnosis; Table S3: Quality assessment of observational studies. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement; Figure S1: Prevalence of overdiagnosed in the same samples, according to GOLD and LLN definition; Figure S2: Proportion meta-analysis of overdiagnosis among Outpatients (OUT) and Inpatients (IN), according to GOLD definition.
Author Contributions
Conceptualization, M.F. and M.R.; methodology, M.F., M.R., A.R. and M.E.F.; software, M.F., M.R. and L.M.; validation, M.E.F. and L.M.; formal analysis, M.F. and M.R.; investigation, A.R. and M.E.F.; data curation, M.F. and M.R.; writing—original draft preparation, M.F., M.R. and L.M.; writing—review and editing, A.R., M.E.F. and L.M.; supervision, L.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and ethical review and approval were not needed due to the use of already published material.
Data Availability Statement
All data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Safiri S., Carson-Chahhoud K., Noori M., Nejadghaderi S.A., Sullman M.J.M., Ahmadian Heris J., Ansarin K., Mansournia M.A., Collins G.S., Kolahi A.A., et al. Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. BMJ. 2022;378:e069679. doi: 10.1136/bmj-2021-069679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khakban A., Sin D.D., FitzGerald J.M., McManus B.M., Ng R., Hollander Z., Sadatsafavi M. The Projected Epidemic of Chronic Obstructive Pulmonary Disease Hospitalizations over the Next 15 Years. A Population-based Perspective. Am. J. Respir. Crit. Care Med. 2017;195:287–291. doi: 10.1164/rccm.201606-1162PP. [DOI] [PubMed] [Google Scholar]
- 3.Doña E., Reinoso-Arija R., Carrasco-Hernandez L., Doménech A., Dorado A., Lopez-Campos J.L. Exploring Current Concepts and Challenges in the Identification and Management of Early-Stage COPD. J. Clin. Med. 2023;12:5293. doi: 10.3390/jcm12165293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GOLD (Global Initiative for Obstructive Lung Disease) Spirometry for Health Care Providers. [(accessed on 6 July 2010)]. Available online: https://goldcopd.org/wp-content/uploads/2016/04/GOLD_Spirometry_2010.pdf.
- 5.Fernandez-Villar A., Soriano J.B., Lopez-Campos J.L. Overdiagnosis of COPD: Precise definitions and proposals for improvement. Br. J. Gen. Pract. 2017;67:183–184. doi: 10.3399/bjgp17X690389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diab N., Gershon A.S., Sin D.D., Tan W.C., Bourbeau J., Boulet L.P., Aaron S.D. Underdiagnosis and Overdiagnosis of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2018;198:1130–1139. doi: 10.1164/rccm.201804-0621CI. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Campos J.L., Tan W., Soriano J.B. Global burden of COPD. Respirology. 2016;21:14–23. doi: 10.1111/resp.12660. [DOI] [PubMed] [Google Scholar]
- 8.Spyratos D., Chloros D., Michalopoulou D., Sichletidis L. Estimating the extent and economic impact of under and overdiagnosis of chronic obstructive pulmonary disease in primary care. Chron. Respir. Dis. 2016;13:240–246. doi: 10.1177/1479972316636989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perret J., Yip S.W.S., Idrose N.S., Hancock K., Abramson M.J., Dharmage S.C., Walters E.H., Waidyatillake N. Undiagnosed and ‘overdiagnosed’ COPD using postbronchodilator spirometry in primary healthcare settings: A systematic review and meta-analysis. BMJ Open Respir. Res. 2023;10:e001478. doi: 10.1136/bmjresp-2022-001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas E.T., Glasziou P., Dobler C.C. Use of the terms “overdiagnosis” and “misdiagnosis” in the COPD literature: A rapid review. Breathe. 2019;15:e8–e19. doi: 10.1183/20734735.0354-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Roisin R. Twenty Years of GOLD (1997–2017). The Origins. [(accessed on 30 March 2019)]. Available online: https://goldcopd.org/wp-content/uploads/2019/03/GOLD-Origins-Final-Version-mar19.pdf.
- 13.Quanjer P.H., Enright P.L., Miller M.R., Stocks J., Ruppel G., Swanney M.P., Crapo R.O., Pedersen O.F., Falaschetti E., Schouten J.P., et al. The need to change the method for defining mild airway obstruction. Eur. Respir. J. 2011;37:720–722. doi: 10.1183/09031936.00135110. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Masini A., Marini S., Gori D., Leoni E., Rochira A., Dallolio L. Evaluation of school-based interventions of active breaks in primary schools: A systematic review and meta-analysis. J. Sci. Med. Sport. 2020;23:377–384. doi: 10.1016/j.jsams.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Arne M., Lisspers K., Stallberg B., Boman G., Hedenstrom H., Janson C., Emtner M. How often is diagnosis of COPD confirmed with spirometry? Respir. Med. 2010;104:550–556. doi: 10.1016/j.rmed.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Bolton C.E., Ionescu A.A., Edwards P.H., Faulkner T.A., Edwards S.M., Shale D.J. Attaining a correct diagnosis of COPD in general practice. Respir. Med. 2005;99:493–500. doi: 10.1016/j.rmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Casas Herrera A., Montes de Oca M., Lopez Varela M.V., Aguirre C., Schiavi E., Jardim J.R., Team P. COPD Underdiagnosis and Misdiagnosis in a High-Risk Primary Care Population in Four Latin American Countries. A Key to Enhance Disease Diagnosis: The PUMA Study. PLoS ONE. 2016;11:e0152266. doi: 10.1371/journal.pone.0152266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caspersen N.F., Soyseth V., Lyngbakken M.N., Berge T., Ariansen I., Tveit A., Rosjo H., Einvik G. Treatable Traits in Misdiagnosed Chronic Obstructive Pulmonary Disease: Data from the Akershus Cardiac Examination 1950 Study. Chronic Obstr. Pulm. Dis. 2022;9:165–180. doi: 10.15326/jcopdf.2021.0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins B.F., Ramenofsky D., Au D.H., Ma J., Uman J.E., Feemster L.C. The association of weight with the detection of airflow obstruction and inhaled treatment among patients with a clinical diagnosis of COPD. Chest. 2014;146:1513–1520. doi: 10.1378/chest.13-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erdoğan A., Uçar E.Y., Araz Ö., Sağlam L., Mirici N.A. Contribution of spirometry to early diagnosis of chronic obstructive pulmonary disease in primary health care centers. Turk. J. Med. Sci. 2013;43:690–694. doi: 10.3906/sag-1207-60. [DOI] [Google Scholar]
- 22.Fisher A.J., Yadegarfar M.E., Collerton J., Small T., Kirkwood T.B., Davies K., Jagger C., Corris P.A. Respiratory health and disease in a U.K. population-based cohort of 85 year olds: The Newcastle 85+ Study. Thorax. 2016;71:255–266. doi: 10.1136/thoraxjnl-2015-207249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisk M., McMillan V., Brown J., Holzhauer-Barrie J., Khan M.S., Baxter N., Roberts C.M. Inaccurate diagnosis of COPD: The Welsh National COPD Audit. Br. J. Gen. Pract. 2019;69:e1–e7. doi: 10.3399/bjgp18X700385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gershon A.S., Thiruchelvam D., Chapman K.R., Aaron S.D., Stanbrook M.B., Bourbeau J., Tan W., To T., Canadian Respiratory Research N. Health Services Burden of Undiagnosed and Overdiagnosed COPD. Chest. 2018;153:1336–1346. doi: 10.1016/j.chest.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Ghattas C., Dai A., Gemmel D.J., Awad M.H. Over diagnosis of chronic obstructive pulmonary disease in an underserved patient population. Int. J. Chron. Obstruct. Pulmon. Dis. 2013;8:545–549. doi: 10.2147/COPD.S45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guder G., Brenner S., Angermann C.E., Ertl G., Held M., Sachs A.P., Lammers J.W., Zanen P., Hoes A.W., Stork S., et al. GOLD or lower limit of normal definition? A comparison with expert-based diagnosis of chronic obstructive pulmonary disease in a prospective cohort-study. Respir. Res. 2012;13:13. doi: 10.1186/1465-9921-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamers R., Bontemps S., van den Akker M., Souza R., Penaforte J., Chavannes N. Chronic obstructive pulmonary disease in Brazilian primary care: Diagnostic competence and case-finding. Prim. Care Respir. J. 2006;15:299–306. doi: 10.1016/j.pcrj.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heffler E., Crimi C., Mancuso S., Campisi R., Puggioni F., Brussino L., Crimi N. Misdiagnosis of asthma and COPD and underuse of spirometry in primary care unselected patients. Respir. Med. 2018;142:48–52. doi: 10.1016/j.rmed.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Hill K., Goldstein R.S., Guyatt G.H., Blouin M., Tan W.C., Davis L.L., Heels-Ansdell D.M., Erak M., Bragaglia P.J., Tamari I.E., et al. Prevalence and underdiagnosis of chronic obstructive pulmonary disease among patients at risk in primary care. CMAJ. 2010;182:673–678. doi: 10.1503/cmaj.091784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacasse Y., Daigle J.M., Martin S., Maltais F. Validity of chronic obstructive pulmonary disease diagnoses in a large administrative database. Can. Respir. J. 2012;19:e5–e9. doi: 10.1155/2012/260374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamprecht B., Mahringer A., Soriano J.B., Kaiser B., Buist A.S., Studnicka M. Is spirometry properly used to diagnose COPD? Results from the BOLD study in Salzburg, Austria: A population-based analytical study. Prim. Care Respir. J. 2013;22:195–200. doi: 10.4104/pcrj.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamprecht B., Soriano J.B., Studnicka M., Kaiser B., Vanfleteren L.E., Gnatiuc L., Burney P., Miravitlles M., García-Rio F., Akbari K., et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148:971–985. doi: 10.1378/chest.14-2535. [DOI] [PubMed] [Google Scholar]
- 33.Laniado-Laborin R., Rendon A., Bauerle O. Chronic obstructive pulmonary disease case finding in Mexico in an at-risk population. Int. J. Tuberc. Lung Dis. 2011;15:818–823. doi: 10.5588/ijtld.10.0546. [DOI] [PubMed] [Google Scholar]
- 34.Liang J., Abramson M.J., Zwar N.A., Russell G.M., Holland A.E., Bonevski B., Mahal A., Phillips K., Eustace P., Paul E., et al. Diagnosing COPD and supporting smoking cessation in general practice: Evidence-practice gaps. Med. J. Aust. 2018;208:29–34. doi: 10.5694/mja17.00664. [DOI] [PubMed] [Google Scholar]
- 35.Llordes M., Jaen A., Almagro P., Heredia J.L., Morera J., Soriano J.B., Miravitlles M. Prevalence, Risk Factors and Diagnostic Accuracy of COPD Among Smokers in Primary Care. COPD. 2015;12:404–412. doi: 10.3109/15412555.2014.974736. [DOI] [PubMed] [Google Scholar]
- 36.Melbye H., Drivenes E., Dalbak L.G., Leinan T., Hoegh-Henrichsen S., Ostrem A. Asthma, chronic obstructive pulmonary disease, or both? Diagnostic labeling and spirometry in primary care patients aged 40 years or more. Int. J. Chron. Obstruct. Pulmon. Dis. 2011;6:597–603. doi: 10.2147/COPD.S25955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minasian A.G., van den Elshout F.J., Dekhuijzen P.N., Vos P.J., Willems F.F., van den Bergh P.J., Heijdra Y.F. COPD in chronic heart failure: Less common than previously thought? Heart Lung. 2013;42:365–371. doi: 10.1016/j.hrtlng.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Miravitlles M., Andreu I., Romero Y., Sitjar S., Altes A., Anton E. Difficulties in differential diagnosis of COPD and asthma in primary care. Br. J. Gen. Pract. 2012;62:e68–e75. doi: 10.3399/bjgp12X625111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U M.S., Leong W.K., Wun Y.T., Tse S.F. A study on COPD in primary care: Comparing correctly and incorrectly diagnosed patients. HK Pract. 2015;37:14–22. [Google Scholar]
- 40.Petrie K., Toelle B.G., Wood-Baker R., Maguire G.P., James A.L., Hunter M., Johns D.P., Marks G.B., George J., Abramson M.J. Undiagnosed and Misdiagnosed Chronic Obstructive Pulmonary Disease: Data from the BOLD Australia Study. Int. J. Chron. Obstruct. Pulmon. Dis. 2021;16:467–475. doi: 10.2147/COPD.S287172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Queiroz M.C., Moreira M.A., Rabahi M.F. Underdiagnosis of COPD at primary health care clinics in the city of Aparecida de Goiania, Brazil. J. Bras. Pneumol. 2012;38:692–699. doi: 10.1590/S1806-37132012000600003. [DOI] [PubMed] [Google Scholar]
- 42.Ragaisiene G., Kibarskyte R., Gauronskaite R., Giedraityte M., Dapsauskaite A., Kasiulevicius V., Danila E. Diagnosing COPD in primary care: What has real life practice got to do with guidelines? Multidiscip. Respir. Med. 2019;14:28. doi: 10.1186/s40248-019-0191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddel H.K., Vestbo J., Agusti A., Anderson G.P., Bansal A.T., Beasley R., Bel E.H., Janson C., Make B., Pavord I.D., et al. Heterogeneity within and between physician-diagnosed asthma and/or COPD: NOVELTY cohort. Eur. Respir. J. 2021;58:2003927. doi: 10.1183/13993003.03927-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rice R.D., Han X., Wang X., Al-Jaghbeer M.J. COPD Overdiagnosis and Its Effect on 30-Day Hospital Readmission Rates. Respir. Care. 2021;66:11–17. doi: 10.4187/respcare.07536. [DOI] [PubMed] [Google Scholar]
- 45.Roberts C.M., Abedi M.K.A., Barry J.S., Williams E., Quantrill S.J. Predictive value of primary care made clinical diagnosis of chronic obstructive pulmonary disease (COPD) with secondary care specialist diagnosis based on spirometry performed in a lung function laboratory. Prim. Health Care Res. Dev. 2009;10:49–53. doi: 10.1017/S1463423608000960. [DOI] [Google Scholar]
- 46.Sator L., Horner A., Studnicka M., Lamprecht B., Kaiser B., McBurnie M.A., Buist A.S., Gnatiuc L., Mannino D.M., Janson C., et al. Overdiagnosis of COPD in Subjects With Unobstructed Spirometry: A BOLD Analysis. Chest. 2019;156:277–288. doi: 10.1016/j.chest.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 47.Schneider A., Gindner L., Tilemann L., Schermer T., Dinant G.J., Meyer F.J., Szecsenyi J. Diagnostic accuracy of spirometry in primary care. BMC Pulm. Med. 2009;9:31. doi: 10.1186/1471-2466-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sichletidis L., Chloros D., Spyratos D., Chatzidimitriou N., Chatziiliadis P., Protopappas N., Charalambidou O., Pelagidou D., Zarvalis E., Patakas D. The validity of the diagnosis of chronic obstructive pulmonary disease in general practice. Prim. Care Respir. J. 2007;16:82–88. doi: 10.3132/pcrj.2007.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spero K., Bayasi G., Beaudry L., Barber K.R., Khorfan F. Overdiagnosis of COPD in hospitalized patients. Int. J. Chron. Obstruct Pulmon Dis. 2017;12:2417–2423. doi: 10.2147/COPD.S139919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spyratos D., Chloros D., Michalopoulou D., Tsiouprou I., Christoglou K., Sichletidis L. Underdiagnosis, false diagnosis and treatment of COPD in a selected population in Northern Greece. Eur. J. Gen. Pract. 2021;27:97–102. doi: 10.1080/13814788.2021.1912729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Starren E.S., Roberts N.J., Tahir M., O’Byrne L., Haffenden R., Patel I.S., Partridge M.R. A centralised respiratory diagnostic service for primary care: A 4-year audit. Prim. Care Respir. J. 2012;21:180–186. doi: 10.4104/pcrj.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talamo C., de Oca M.M., Halbert R., Perez-Padilla R., Jardim J.R., Muino A., Lopez M.V., Valdivia G., Pertuze J., Moreno D., et al. Diagnostic labeling of COPD in five Latin American cities. Chest. 2007;131:60–67. doi: 10.1378/chest.06-1149. [DOI] [PubMed] [Google Scholar]
- 53.Tinkelman D.G., Price D.B., Nordyke R.J., Halbert R.J. Misdiagnosis of COPD and asthma in primary care patients 40 years of age and over. J. Asthma. 2006;43:75–80. doi: 10.1080/02770900500448738. [DOI] [PubMed] [Google Scholar]
- 54.Walters J.A., Walters E.H., Nelson M., Robinson A., Scott J., Turner P., Wood-Baker R. Factors associated with misdiagnosis of COPD in primary care. Prim. Care Respir. J. 2011;20:396–402. doi: 10.4104/pcrj.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White P., Thornton H., Pinnock H., Georgopoulou S., Booth H.P. Overtreatment of COPD with inhaled corticosteroids--implications for safety and costs: Cross-sectional observational study. PLoS ONE. 2013;8:e75221. doi: 10.1371/journal.pone.0075221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zwar N.A., Marks G.B., Hermiz O., Middleton S., Comino E.J., Hasan I., Vagholkar S., Wilson S.F. Predictors of accuracy of diagnosis of chronic obstructive pulmonary disease in general practice. Med. J. Aust. 2011;195:168–171. doi: 10.5694/j.1326-5377.2011.tb03271.x. [DOI] [PubMed] [Google Scholar]
- 57.Ohar J.A., Sadeghnejad A., Meyers D.A., Donohue J.F., Bleecker E.R. Do symptoms predict COPD in smokers? Chest. 2010;137:1345–1353. doi: 10.1378/chest.09-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung K.F. Chronic ‘cough hypersensitivity syndrome’: A more precise label for chronic cough. Pulm. Pharmacol. Ther. 2011;24:267–271. doi: 10.1016/j.pupt.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Han M.K., Agusti A., Celli B.R., Criner G.J., Halpin D.M.G., Roche N., Papi A., Stockley R.A., Wedzicha J., Vogelmeier C.F. From GOLD 0 to Pre-COPD. Am. J. Respir. Crit. Care Med. 2021;203:414–423. doi: 10.1164/rccm.202008-3328PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wouters E.F.M., Breyer M.K., Breyer-Kohansal R., Hartl S. COPD Diagnosis: Time for Disruption. J. Clin. Med. 2021;10:4660. doi: 10.3390/jcm10204660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jain V.V., Allison D.R., Andrews S., Mejia J., Mills P.K., Peterson M.W. Misdiagnosis Among Frequent Exacerbators of Clinically Diagnosed Asthma and COPD in Absence of Confirmation of Airflow Obstruction. Lung. 2015;193:505–512. doi: 10.1007/s00408-015-9734-6. [DOI] [PubMed] [Google Scholar]
- 62.Choi J.Y., Rhee C.K. Diagnosis and Treatment of Early Chronic Obstructive Lung Disease (COPD) J. Clin. Med. 2020;9:3426. doi: 10.3390/jcm9113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roberts N.J., Smith S.F., Partridge M.R. Why is spirometry underused in the diagnosis of the breathless patient: A qualitative study. BMC Pulm. Med. 2011;11:37. doi: 10.1186/1471-2466-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindensmith J., Morrison D., Deveau C., Hernandez P. Overdiagnosis of asthma in the community. Can. Respir. J. 2004;11:111–116. doi: 10.1155/2004/276493. [DOI] [PubMed] [Google Scholar]
- 65.MacNeil J., Loves R.H., Aaron S.D. Addressing the misdiagnosis of asthma in adults: Where does it go wrong? Expert. Rev. Respir. Med. 2016;10:1187–1198. doi: 10.1080/17476348.2016.1242415. [DOI] [PubMed] [Google Scholar]
- 66.Rothnie K.J., Chandan J.S., Goss H.G., Mullerova H., Quint J.K. Validity and interpretation of spirometric recordings to diagnose COPD in UK primary care. Int. J. Chron. Obstruct. Pulmon. Dis. 2017;12:1663–1668. doi: 10.2147/COPD.S133891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Maue S.K., Segal R., Kimberlin C.L., Lipowski E.E. Predicting physician guideline compliance: An assessment of motivators and perceived barriers. Am. J. Manag. Care. 2004;10:383–391. [PubMed] [Google Scholar]
- 68.Cabana M.D., Rand C.S., Powe N.R., Wu A.W., Wilson M.H., Abboud P.A., Rubin H.R. Why don’t physicians follow clinical practice guidelines? A framework for improvement. Jpn. Automob. Manuf. Assoc. 1999;282:1458–1465. doi: 10.1203/00006450-199904020-00719. [DOI] [PubMed] [Google Scholar]
- 69.Busfield J. Assessing the overuse of medicines. Soc. Sci. Med. 2015;131:199–206. doi: 10.1016/j.socscimed.2014.10.061. [DOI] [PubMed] [Google Scholar]
- 70.Rossaki F.M., Hurst J.R., van Gemert F., Kirenga B.J., Williams S., Khoo E.M., Tsiligianni I., Tabyshova A., van Boven J.F. Strategies for the prevention, diagnosis and treatment of COPD in low- and middle- income countries: The importance of primary care. Expert. Rev. Respir. Med. 2021;15:1563–1577. doi: 10.1080/17476348.2021.1985762. [DOI] [PubMed] [Google Scholar]
- 71.Barnes T.A., Fromer L. Spirometry use: Detection of chronic obstructive pulmonary disease in the primary care setting. Clin. Interv. Aging. 2011;6:47–52. doi: 10.2147/CIA.S15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.AIFA (Italian Medicines Agency) Nota 99. [(accessed on 15 February 2022)]; Available online: https://www.aifa.gov.it/en/nota-99.
- 73.Armstrong N. Overdiagnosis and overtreatment as a quality problem: Insights from healthcare improvement research. BMJ Qual. Saf. 2018;27:571–575. doi: 10.1136/bmjqs-2017-007571. [DOI] [PubMed] [Google Scholar]
- 74.Hensher M., Tisdell J., Zimitat C. “Too much medicine”: Insights and explanations from economic theory and research. Soc. Sci. Med. 2017;176:77–84. doi: 10.1016/j.socscimed.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 75.Lewis J.S., Cook C.E., Hoffmann T.C., O’Sullivan P. The Elephant in the Room: Too Much Medicine in Musculoskeletal Practice. J. Orthop. Sports Phys. Ther. 2020;50:1–4. doi: 10.2519/jospt.2020.0601. [DOI] [PubMed] [Google Scholar]
- 76.Moynihan R., Doust J., Henry D. Preventing overdiagnosis: How to stop harming the healthy. BMJ. 2012;344:e3502. doi: 10.1136/bmj.e3502. [DOI] [PubMed] [Google Scholar]
- 77.Berwick D.M., Hackbarth A.D. Eliminating waste in US health care. Jpn. Automob. Manuf. Assoc. 2012;307:1513–1516. doi: 10.1001/jama.2012.362. [DOI] [PubMed] [Google Scholar]
- 78.Research and Markets Global Chronic Obstructive Pulmonary Disease Therapeutics Market by Drug Class, Distribution Channel. [(accessed on 17 July 2022)]. Available online: https://www.researchandmarkets.com/reports/4989588/global-chronic-obstructive-pulmonary-disease.
- 79.Steinacher R., Parissis J.T., Strohmer B., Eichinger J., Rottlaender D., Hoppe U.C., Altenberger J. Comparison between ATS/ERS age- and gender-adjusted criteria and GOLD criteria for the detection of irreversible airway obstruction in chronic heart failure. Clin. Res. Cardiol. 2012;101:637–645. doi: 10.1007/s00392-012-0438-0. [DOI] [PubMed] [Google Scholar]
- 80.Mohamed Hoesein F.A., Zanen P., Sachs A.P., Verheij T.J., Lammers J.W., Broekhuizen B.D. Spirometric thresholds for diagnosing COPD: 0.70 or LLN, pre- or post-dilator values? COPD. 2012;9:338–343. doi: 10.3109/15412555.2012.667851. [DOI] [PubMed] [Google Scholar]
- 81.Hardie J.A., Buist A.S., Vollmer W.M., Ellingsen I., Bakke P.S., Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur. Respir. J. 2002;20:1117–1122. doi: 10.1183/09031936.02.00023202. [DOI] [PubMed] [Google Scholar]
- 82.Ooi K. The Pitfalls of Overtreatment: Why More Care is not Necessarily Beneficial. Asian Bioeth. Rev. 2020;12:399–417. doi: 10.1007/s41649-020-00145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Easterbrook P.J., Berlin J.A., Gopalan R., Matthews D.R. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 84.Strong M., Green A., Goyder E., Miles G., Lee A.C., Basran G., Cooke J. Accuracy of diagnosis and classification of COPD in primary and specialist nurse-led respiratory care in Rotherham, UK: A cross-sectional study. Prim. Care Respir. J. 2014;23:67–73. doi: 10.4104/pcrj.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kurmi O.P., Li L., Smith M., Augustyn M., Chen J., Collins R., Guo Y., Han Y., Qin J., Xu G., et al. Regional variations in the prevalence and misdiagnosis of air flow obstruction in China: Baseline results from a prospective cohort of the China Kadoorie Biobank (CKB) BMJ Open Respir. Res. 2014;1:e000025. doi: 10.1136/bmjresp-2014-000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kelly A.M., Van Meer O., Keijzers G., Motiejunaite J., Jones P., Body R., Craig S., Karamercan M., Klim S., Harjola V.P., et al. AANZDEM and EuroDEM Study Groups. Get with the guidelines: Management of chronic obstructive pulmonary disease in emergency departments in Europe and Australasia is sub-optimal. Intern. Med. J. 2020;50:200–208. doi: 10.1111/imj.14323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon request from the corresponding author.