Abstract
Methanethiol has been strongly associated with desirable Cheddar cheese flavor and can be formed from the degradation of methionine (Met) via a number of microbial enzymes. Methionine γ-lyase is thought to play a major role in the catabolism of Met and generation of methanethiol in several species of bacteria. Other enzymes that have been reported to be capable of producing methanethiol from Met in lactic acid bacteria include cystathionine β-lyase and cystathionine γ-lyase. The objective of this study was to determine the production, stability, and activities of the enzymes involved in methanethiol generation in bacteria associated with cheese making. Lactococci and lactobacilli were observed to contain high levels of enzymes that acted primarily on cystathionine. Enzyme activity was dependent on the concentration of sulfur amino acids in the growth medium. Met aminotransferase activity was detected in all of the lactic acid bacteria tested and α-ketoglutarate was used as the amino group acceptor. In Lactococcus lactis subsp. cremoris S2, Met aminotransferase was repressed with increasing concentrations of Met in the growth medium. While no Met aminotransferase activity was detected in Brevibacterium linens BL2, it possessed high levels of l-methionine γ-lyase that was induced by addition of Met to the growth medium. Met demethiolation activity at pH 5.2 with 4% NaCl was not detected in cell extracts but was detected in whole cells. These data suggest that Met degradation in Cheddar cheese will depend on the organism used in production, the amount of enzyme released during aging, and the amount of Met in the matrix.
The primary classes of compounds that contribute to cheese flavor include amino acids and their degradation products, peptides, carbonyl compounds, and fatty acids. These partition primarily into the aqueous fraction of cheese (3). The volatile fraction of cheese has sulfur-containing compounds such as methanethiol, methional, dimethyl sulfide, dimethyl tetrasulfide, carbonyl sulfide, and hydrogen sulfide (28), and they contribute to the aroma of cheese (7). Methanethiol has been associated with desirable Cheddar-type sulfur notes in good-quality Cheddar cheese (2), and it is also implicated as an influential aroma and flavor compound in many foods, including surface-ripened cheeses that use brevibacteria (17). However, methanethiol, when present alone, does not contribute to typical Cheddar-like flavor notes in cheese (17).
Production of methanethiol is important in cheese, but the Met biosynthetic and catabolic pathways vary among bacteria (23). The mechanisms involved and amounts of methanethiol produced during cheese ripening also vary. In an effort to increase and accelerate the development of typical Cheddar cheese flavor, adjunct bacteria have been used during the manufacture of low- and full-fat cheese. Initial selection of flavor adjunct cultures focused on those bacteria used to accelerate flavor development in full-fat cheese, which are typically lactobacilli because they dominate (107 to 109 CFU/g of cheese during storage at 8°C) the microflora during aging (15). The Lactobacillus genus is considered to be a member of the nonstarter lactic acid bacteria subgroup because it is not added with the starter culture for Cheddar cheese. In addition to lactobacilli, micrococci and pediococci have been used as adjunct bacteria to aid in flavor development (20). Brevibacteria, which are normally found on the surfaces of Limburger and other Trappist-type cheeses, are not traditionally used as flavor adjuncts in Cheddar cheese. One advantage these organisms have over other adjuncts is their profuse production of methanethiol (8). Weimer et al. (30) successfully used Brevibacterium linens as an adjunct to improve the flavor of low-fat Cheddar cheese.
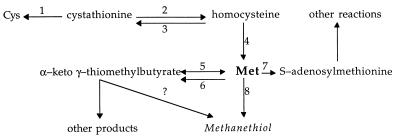
The mechanism for the production of methanethiol in cheese by bacteria can be a result of the direct catabolism of Met or it can arise from inadvertent catalysis by other enzymes (1, 6, 17). The most direct route to methanethiol is the conversion of Met to methanethiol, ammonia, and α-ketobutyrate (Fig. 1). This transformation is catalyzed by inducible Met γ-lyase, a pyridoxal phosphate (PLP)-dependent enzyme (24) which has been purified to homogeneity from Pseudomonas putida (14, 26), Aeromonas spp. (27), and Clostridium sporogenes (16) and partially purified from B. linens (6).
FIG. 1.
Metabolic pathways for Met interconversion. The primary intermediates and enzymes are listed. Enzyme 1 is cystathionine γ-lyase, enzyme 2 is cystathionine β-lyase, enzyme 3 is cystathionine β-synthase, enzyme 4 is homocysteine methyltransferase, enzyme 5 is aromatic aminotransferase (tyrB) or transaminase B (ilvE), enzyme 6 is amino acid oxidase, enzyme 7 is Met adenosyltransferase, and enzyme 8 is Met γ-lyase (adapted from reference 18 and 23).
Pathways leading away from Met are important to consider because this amino acid is central to many other critical metabolic functions (Fig. 1). Utilization of Met for other metabolic functions would lower the pool of Met available for conversion to methanethiol. Methionine adenosyltransferase (S-adenosylmethionine [SAM] synthetase) converts Met into SAM at the expense of one ATP. SAM, one of the major methylating agents in a cell, is also important in the regulation of several of the Met-biosynthetic enzymes (22). Reduced SAM synthetase activity leads to low intracellular levels of SAM, resulting in the induction of the Met-biosynthetic pathway (32).
Another mechanism that directs Met away from methanethiol is the deamination reaction to form α-keto γ-methyl thiobutyrate (KMTB). This conversion can be catalyzed by various aminotransferases (33) or amino acid oxidases (21). These enzymes are common in bacteria and are usually the last step in amino acid synthetic pathways (13). Amino acid oxidase activity is a possible route for KMTB production, and it is a possible route for subsequent methanethiol production in cheese, but this is unlikely because cheese tends to be anaerobic. Evidence for the conversion of KMTB to methanethiol is lacking for bacteria; however, this reaction has been shown to take place enzymatically in fungal species (23).
When the catabolic pathways for Met are considered, the enzymes involved in the biosynthesis of Met must also be included. Although the principal reactions that these enzymes catalyze are involved in the synthesis of Met, they also coincidentally catalyze catabolic reactions that lead to the production of methanethiol and possibly other cheese flavor compounds. For example, cystathionine β-lyase, which primarily catalyzes the conversion of cystathionine to homocysteine, a reaction involved in the synthesis of Met (29), also catalyzes the conversion of Met to methanethiol, ammonia, and α-ketobutyrate but with 100 times less efficiency than that of its conversion to homocysteine in Lactococcus lactis subsp. cremoris S2 (1). This enzyme was purified from lactococci and has been implicated in the generation of methanethiol in Cheddar cheese (1). Cystathionine γ-lyase catalyzes the α,γ elimination of cystathionine to produce cysteine (Cys), α-ketobutyrate, and ammonia (19). A cystathionine γ-lyase purified from L. lactis subsp. cremoris is capable of catalyzing the α,γ elimination of Met to produce methanethiol at an efficiency much lower than that of the primary reaction it catalyzes (4). These enzymes may be present in the cells and liberated when the cells die and lyse during cheese storage, as occurs in Cheddar cheese ripening (10). With these observations in mind, the objective of this study was to determine the conversion pathways of Met to free thiols under laboratory and cheese-like conditions in bacteria used as starter cultures and flavor adjuncts in Cheddar cheese.
MATERIALS AND METHODS
Bacterial strains.
Lactococci and lactobacilli were grown in Elliker’s broth (Difco, Detroit, Mich.) at their respective optimum growth temperatures (Table 1) and frozen in 10% nonfat dry milk containing 30% glycerol. Brevibacteria and micrococci were grown in tryptic soy broth (Difco) with aeration at 25°C and frozen in sterile tryptic soy broth containing 30% glycerol (Table 1). All strains were stored at −70°C until further use. Before each use, frozen stock cultures were thawed and grown in a chemically defined medium (11) at their respective temperatures (Table 1) for two transfers prior to further testing.
TABLE 1.
Bacteria, media, and growth conditions
| Strain | Source | Growth temp (°C) | Aeration (250 rpm) |
|---|---|---|---|
| L. lactis subsp. cremoris S1 | Marschall Productsa | 30 | − |
| L. lactis subsp. cremoris S2 | Laboratory collection | 30 | − |
| L. lactis subsp. lactis S3 | Marschall Products | 30 | − |
| Lactobacillus helveticus CNRZ32 | Laboratory collection | 37 | − |
| Lactobacillus casei LC301 | Laboratory collection | 30 | − |
| Micrococcus sp. strain 21829 | ATCCb | 30 | + |
| Micrococcus luteus 383 | ATCC | 30 | + |
| Micrococcus varians 15306 | ATCC | 30 | + |
| Micrococcus naucinus 15935 | ATCC | 30 | + |
| Brevibacterium linens BL1 | Laboratory collection | 25 | + |
| Brevibacterium linens BL2 | Laboratory collection | 25 | + |
| Brevibacterium linens BL3 | Laboratory collection | 25 | + |
| Brevibacterium linens 8377 | ATCC | 25 | + |
| Brevibacterium linens 9172 | ATCC | 25 | + |
| Brevibacterium linens 9175 | ATCC | 25 | + |
| Brevibacterium linens 19391 | ATCC | 25 | + |
| Brevibacterium flavum 14067 | ATCC | 25 | + |
| Brevibacterium flavum 15940 | ATCC | 25 | + |
| Brevibacterium flavum 15941 | ATCC | 25 | + |
| Brevibacterium flavum 15942 | ATCC | 25 | + |
| Brevibacterium flavum 21127 | ATCC | 25 | + |
| Brevibacterium flavum 21128 | ATCC | 25 | + |
| Brevibacterium flavum 21129 | ATCC | 25 | + |
| Brevibacterium acetylicum 953 | ATCC | 25 | + |
Rhodia Inc., Madison, Wis.
ATCC, American Type Culture Collection.
Whole-cell preparation for enzyme assays.
Cells were harvested by centrifugation (5,000 × g for 10 min at 4°C), washed twice with 0.05 M potassium phosphate buffer (pH 7.2), and adjusted to an optical density at 600 nm of 0.8 in 0.05 M potassium phosphate (pH 7.2) or 0.05 M potassium citrate (pH 5.2). The appropriate enzyme assays were done immediately with these bacterial suspensions.
CE preparation.
Cells grown to mid-log phase in 100 ml of chemically defined medium were harvested by centrifugation (5,000 × g for 10 min at 4°C). The pelleted cells were washed twice with 0.05 M potassium phosphate buffer (pH 7.2) and then lysed by mixing them with glass beads (bead diameter, 106 μm; Sigma Chemical Co., St. Louis, Mo.) at maximum speed on a vortex mixer for 30 s at 4°C. This process was repeated 10 times at 30-s intervals. After centrifugation (8,000 × g for 30 min at 4°C), the supernatant was collected and considered to be the cell extract (CE).
Protein assay.
The total protein content of each CE was determined by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.) according to the manufacturer’s instructions. Bovine serum albumin was used to obtain a standard curve.
MTPC.
The methanethiol-producing capacity (MTPC) of each organism was determined as described by Ferchichi et al. (8). Methanethiol produced from l-Met was reacted with 5,5′-dithio-bis-2-nitrobenzoic acid (DTNB; Sigma Chemical Co.) to produce a yellow aryl mercaptan that can be detected spectrophotometrically at 412 nm. Controls with substrate plus DTNB only and cells plus DTNB only were also included. A standard curve was obtained with different concentrations of ethanethiol. At pH 5.2, 0.05 M potassium citrate buffer was used; however, at pH 7.2, a 0.05 M potassium phosphate buffer was used. Data are reported as means of results from duplicate reactions.
α-Keto acid determination.
Separation of α-keto acids and amino acids was done by micellar electrokinetic capillary chromatography (MECC). The method of Strickland et al. (25) was modified by using 0.1 M sodium borate–0.1 M sodium dodecyl sulfate as the run buffer. Electrophoresis was done at 25°C for 30 min at 12 kV with a 1-s pressure injection into a 57-cm by 75-μm untreated silica capillary on a P/ACE 2100 automated capillary electrophoresis system (Beckman Instruments, Fullerton, Calif.). The polarity was set with the positive pole at the capillary inlet. Sample detection was achieved at 214 nm with the detector range at 0.02 absorbance units, full scale, and with a data collection rate of 2 Hz.
Cystathionine degradation.
Cystathionine β-lyase and cystathionine γ-lyase activities were determined by measuring the amount of free thiol formed in 2 h from cystathionine at 25, 30, or 37°C depending on the organism being tested. Free thiols were measured with DTNB (8). The reaction mix contained 0.05 M potassium phosphate (pH 7.2), 10 mM cystathionine (Sigma Chemical Co.), 20 μM PLP (Boehringer Mannheim, Mannheim, Germany), 5 mM DTNB, and 100 μl of CE.
Aminotransferase activity.
The aminotransferase activity in CE was determined by measuring the amount of KMTB formed in 2 h. The reaction mix contained 0.05 M potassium phosphate (pH 7.2), 10 mM Met, 10 mM α-ketoglutarate, 20 μM PLP, and 100 μl of the CE. KMTB was measured by the MECC method previously mentioned.
KMTB demethiolase or decarboxylase activity.
Decarboxylase activity in the CE was determined with KMTB as the substrate. The reaction mix contained 0.05 M potassium phosphate (pH 7.2), 20 μM PLP, 20 μM thiamine pyrophosphate (Sigma Chemical Co.), 10 mM KMTB, and 100 μl of CE. Samples were tested for degradation of KMTB at regular intervals during a 48-h period by the above-mentioned MECC method.
Nonoptimum enzyme assays.
Each of the enzyme assays was performed with CEs at pH 5.2 (0.05 M potassium citrate) and pH 7.2 (0.05 M potassium phosphate) with and without 5% NaCl as described above.
RESULTS
Methionine degradation.
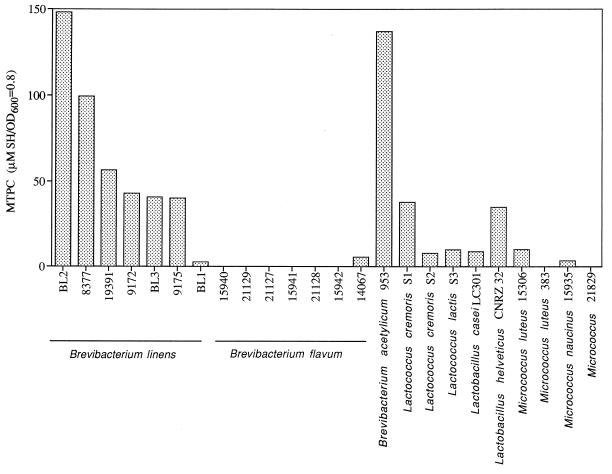
The methanethiol-producing capability of whole cells was characterized under laboratory growth conditions for a broad range of organisms, with Met as the substrate (Fig. 2). B. linens BL2, followed by Brevibacterium acetylicum ATCC 953, had the highest MTPC under optimum assay conditions. The lactic acid bacteria and micrococci tested possessed 0 to 30% of the MTPC of B. linens BL2 (Fig. 2).
FIG. 2.
MTPC of whole cells of selected bacteria grown and assayed under optimum conditions. OD600, optical density at 600 nm.
CEs also varied in their MTPCs from Met and their abilities to produce free thiols from cystathionine under physiological conditions. B. linens BL2 demonstrated the highest MTPC from Met (Table 2). Other organisms tested contained only 2.5 to 10.2% of the MTPC of B. linens BL2 (Table 2). CEs from these organisms were not observed to have MTPC from Met under conditions that mimic aging cheese (pH 5.2, 5% salt) (Table 2).
TABLE 2.
Activities of intracellular enzymes capable of degrading methionine in CE
| Strain | Amt of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Free thiols produced from methionine (μM/mg of protein/h) under conditions:
|
Free thiols produced from cystathionine (μM/mg of protein/h) under conditions:
|
Met ATasea produced (mM/mg of protein/h) under conditions:
|
KMTB degradedb (mM/mg of protein/h) under conditions:
|
|||||
| pH 7.2, 0% NaCl | pH 5.2, 5% NaCl | pH 7.2, 0% NaCl | pH 5.2, 5% NaCl | pH 7.2, 0% NaCl | pH 5.2, 5% NaCl | pH 7.2, 0% NaCl | pH 5.2, 5% NaCl | |
| S1 | 8.6 | 0.0 | 39.5 | 5.5 | 3.6 | 0.0 | 0.0 | 0.0 |
| S2 | 2.5 | 0.0 | 144.1 | 37.2 | 1.9 | 0.0 | 0.0 | 0.0 |
| S3 | 3.3 | 0.0 | 72.1 | 0.6 | 3.3 | 0.0 | 0.0 | 0.0 |
| LH32 | 0.0 | 0.0 | 4.8 | 0.0 | 3.6 | 2.7 | 0.0 | 0.0 |
| LC301 | 0.0 | 0.0 | 17.0 | 10.7 | 0.6 | 0.0 | 0.0 | 0.0 |
| BL1 | 10.2 | 0.0 | 32.2 | 0.0 | 0.5 | 0.0 | 0.0 | 0.0 |
| BL2 | 100.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
Approximations of Met aminotransferase (ATase) activity in CEs determined by monitoring increases in KMTB.
Approximations of reversible l amino acid oxidase activity, decarboxylase activity, and levels of production of methanethiol from KMTB.
Cystathionine degradation.
Lactococci had the highest level of cystathionine-degrading enzymes, followed by lactobacilli. B. linens BL2 was unable to degrade cystathionine under laboratory assay conditions (Table 2). Under cheese-like conditions (pH 5.2 and 5% NaCl), the enzyme activities were reduced by different amounts in the strains tested (Table 2). Lactococci, especially L. lactis subsp. cremoris S2, retained significant residual levels of thiol production ability. Lactobacillus casei LC301 also retained activity.
Aminotransferase activity.
High levels of KMTB production, which allows us to estimate Met aminotransferase activity, were detected in the strains of lactococci tested. Lactobacillus helveticus CNRZ32 and L. lactis subsp. cremoris S1 had the highest Met aminotransferase activity as measured by KMTB production (Table 2). Interestingly, less than half of the activity was lost in Lactobacillus helveticus CNRZ32 when it was assayed under cheese-like conditions. There was no production of KMTB in B. linens BL2, but there was a small amount of production detected in B. linens BL1. Under cheese-like incubation conditions, no KMTB production was detected after 1 h of incubation; however, if the assay mix was incubated for an additional 24 h under cheese-like conditions, a peak that comigrated with KMTB was observed in all the lactic acid bacteria tested but brevibacteria.
KMTB demethiolase or decarboxylase activity.
Catabolism of KMTB was determined by measuring the loss of the added KMTB in the assay mixture by capillary electrophoresis. The peak area of KMTB did not change in any strain tested (Table 2) when the CE was incubated with KMTB in the presence of both PLP and thiamine pyrophosphate for 48 h. Similar observations were made for both physiological and cheese-like conditions.
Influence of methionine and cysteine on MTPC.
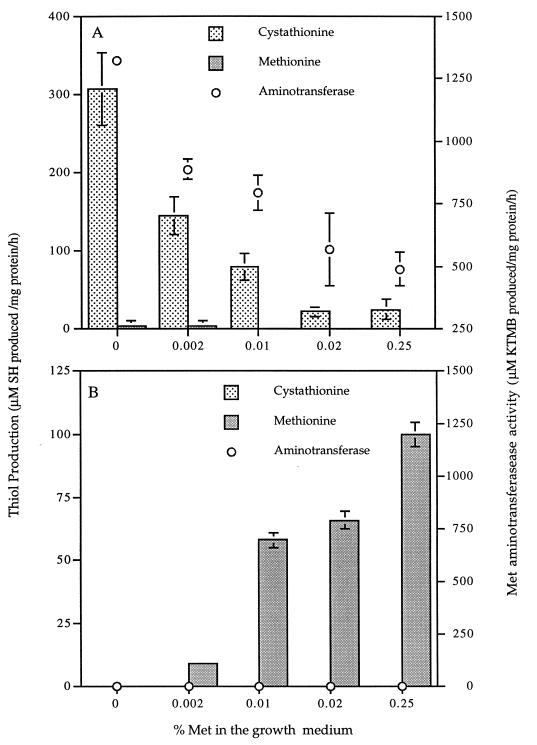
Addition of Met to the growth medium suppressed MTPC in L. lactis subsp. cremoris S2. As the initial concentration of Met in the medium increased, the rate of cystathionine breakdown decreased (Fig. 3A). When Cys was removed from the growth medium, the MTPC from cystathionine increased (Table 3). KMTB production decreased as the level of Met in the growth medium increased (Fig. 3A).
FIG. 3.
Effects of methionine concentration in the growth medium on free thiol production and Met aminotransferase activity in CEs of L. lactis subsp. cremoris S2 (A) and B. linens BL2 (B). Methionine and cystathionine were used as substrates in the thiol production assays.
TABLE 3.
Effect of cysteine concentration in the growth medium on the production of free thiols from cystathionine at a methionine concentration of 0.002% in B. linens BL2 and L. lactis subsp. cremoris S2
| % Cys in growth medium | Amt of free thiols produced (μM/per mg of protein/h) in:
|
|
|---|---|---|
| L. lactis subsp. cremoris S2 | B. linens BL2 | |
| 0.002 | 17 | 13 |
| 0 | 968 | 161 |
When similar experiments were done with B. linens BL2, no cystathionine degradation or KMTB production or degradation was detected (Fig. 3B). Unlike with lactococci, as the initial Met concentration in the growth medium of B. linens BL2 increased, the MTPC from Met increased (Fig. 3B).
DISCUSSION
It is widely hypothesized that lactococci, the bacteria used as starter bacteria, die and lyse during ripening, releasing their intracellular contents into the cheese matrix (10). It is also thought that the released enzymes act on cheese constituents, such as protein and fat, to produce compounds important in Cheddar cheese flavor. One example is the degradation of Met to other sulfur-containing compounds that have been associated with improved Cheddar cheese flavor (28), even though these compounds alone do not contain the essential cheddar-type flavor.
It is assumed that the majority of the intracellular enzymes are still active in the cheese matrix. However, cheese is a harsh environment, with a pH of 5.0 to 5.2 and salt at 3 to 5% in the water phase. Recently, Weimer et al. (30) demonstrated that aminopeptidase (AP) and lipase and esterase (LE) activities in aging Cheddar cheese were low initially and decreased further to a lower but detectable residual level. Further, they compared AP and LE activities in cells grown to the logarithmic phase in the laboratory and observed a decrease in the level of activity similar to the level of residual activity found in cheese under optimum assay conditions. These data indicate that enzyme assays done under cheese-like conditions approximate enzyme activity in cheese and that some intracellular enzymes considered to be important in the ripening process decrease in activity during cheese aging. Results from this investigation were similar to the results obtained with different intracellular enzymes by Weimer et al. (30). Hence, if we take into account observations from this study and the previous observations of Weimer et al., it is reasonable to generalize that the activities of intracellular enzymes decrease as they are released into the cheese matrix and that this reduction can be approximated by assaying an enzyme in the laboratory at pH 5.2 with 3 to 5% NaCl added to the reaction mix.
Additionally, Weimer et al. (30) found that whole cells retained MTPC under cheese-like conditions but observed no MTPC in CE assayed under the same conditions. The present study confirms the observations of Weimer et al. (30) with regard to some of the brevibacteria, lactococci, and lactobacilli. With a broader sampling of bacteria important in cheese making, micrococci were observed to have little or no detectable MTPC (Fig. 1). These data suggest that production of sulfur-containing metabolites from Met by whole cells in cheese is likely but the absolute quantities of sulfur-containing metabolites will vary depending on the bacteria used in cheese production and the relative amount of cell lysis during cheese aging.
To study which enzyme system is responsible for free thiol formation under cheese-like conditions, two organisms were selected based on an evaluation of the trained sensory responses of Weimer et al. (30) and on the observations of Alting et al. (1), Bruinenberg et al. (4), and Collin and Law (6), who define the MTPC of lactococci and brevibacteria as being produced by cystathionine γ-lyase, β-lyase, and methionine γ-lyase, respectively. The demethiolation mechanism in lactobacilli is unknown. The CEs of the bacteria tested had MTPC from Met under optimum assay conditions but did not have measurable MTPC at pH 5.2 with 5% NaCl added (Table 2). However, with cystathionine as the substrate, we observed residual free thiol production which approximated that of cystathionine γ- and β-lyases under cheese-like conditions in all strains tested except Lactobacillus helveticus CNRZ32, B. linens BL1, and B. linens BL2 (Table 2). In lactococci residual free thiol production was 0.01 to 26% of the original activity, and in Lactobacillus casei LC301 it was 63% after 1 h of incubation under cheese-like conditions. These data support the observations of Alting et al. (1), Bruinenberg et al. (4), and Collin and Law (6). In addition, these data suggest that B. linens BL1 contains all three enzymes; however, unlike with lactococci, these enzymes are inactive under cheese-like conditions in this strain. Further, these data indicate that the demethiolation enzymes in lactobacilli are cystathionine γ- or β-lyase; however, the enzymes of Lactobacillus casei LC301 are more stable with acid and salt than the lactococcal enzymes or those of Lactobacillus helveticus CNRZ32.
Cystathionine γ- and β-lyases have recently been purified from L. lactis subsp. cremoris (1, 4). Alting et al. (1) describe cystathionine β-lyase from lactococci as having the additional ability to demethiolate Met to generate methanethiol as a secondary reaction. This inadvertent reaction is 100-fold less efficient than its primary catabolic reaction of transforming cystathionine to homocysteine. They postulate that this enzyme is responsible for methanethiol formation in Cheddar cheese, but conclusive evidence is not available. Alternatively, Bruinenberg et al. (4) propose that cystathionine γ-lyase, which catalyzes the conversion of cystathionine to Cys, is another enzyme responsible for the production of methanethiol in cheese. Again, definitive evidence demonstrating activity of this enzyme in ripening cheese is lacking. Observations made in our study indicate that these enzymes are active under cheese-like conditions on cystathionine (suggesting that they may be active in cheese) but that their relative activities will depend on which strain is used in manufacture. However, this type of activity was not observed for the flavor adjunct bacteria tested except for Lactobacillus casei LC301. These data suggest that these enzymes may be active in ripening cheese but that the activity on Met in the cheese matrix will be at least 100-fold lower than the values reported in Table 2.
If we take into account the inefficient conversion of Met to methanethiol by cystathionine β-lyase (based on 100-fold reduction in activity), the theoretical residual free thiol production of cystathionine β-lyase under cheese-like conditions is between 0.0001 and 0.25% of the activity observed with cystathionine as the substrate. If we assume further decreases in activity, as Weimer et al. observed for AP and LE enzymes in cheese (30), the probable free thiol production in the cheese matrix from Met would be a small fraction of 1%. Therefore, if these enzymes are of importance in cheese flavor development via production of sulfur-containing metabolites from Met, they must be produced in high concentrations before cell lysis in the cheese matrix. This suggests that the addition of other enzymes that behave differently from cystathionine γ-lyase and cystathionine β-lyase may be beneficial in improving Cheddar cheese flavor. Initial observations to support this hypothesis are reported by Weimer et al. (30), who used whole cells of B. linens BL1 and BL2.
Brevibacteria contain methionine γ-lyase, which converts Met directly to methanethiol (6). This enzyme has been purified to homogeneity from several other genera unrelated to cheese manufacture (16, 26, 27) and partially purified from brevibacteria (6). Use of brevibacterium cell lysates to accelerate cheese ripening is partially successful (12), but sensory evaluation judged the flavor change to be undesirable in a short period. Weimer et al. (30) used whole cells of brevibacteria in cheese making and observed an increase in cheese flavor compared to that after the addition of lactobacilli. Presumably, the flavor increase was linked in part to the ability of brevibacteria to produce volatile sulfur compounds. Observations made in our study suggest that B. linens BL1 contains methionine γ-lyase, as well as cystathionine γ- and β-lyases, but that B. linens BL2 has only methionine γ-lyase. B. linens BL2 is auxotrophic for Met, which further supports the supposition that it lacks cystathionine γ- and β-lyases; however, this may not be the only explanation for the auxotrophy. This auxotrophy for Met may also explain why BL2 has the highest MTPC. The lack of enzymes to convert Met to Cys and the lack of aminotransferases to produce KMTB may force the utilization of Met into other pathways, namely, demethiolation. In any case, these enzymes are not active under cheese-like conditions in brevibacteria, suggesting that if brevibacteria lyse in the cheese matrix, the MTPC will decrease.
The metabolic importance of these enzyme systems is well established, and in brevibacteria these activities are known to be controlled by the concentration of Met in the medium (9). In this study it was found that the demethiolation enzyme activities in CEs of L. lactis subsp. cremoris S2 and B. linens BL2 are influenced by the Met and Cys concentrations in the growth medium (Fig. 3; Table 3). Even small increases in Cys concentration decrease free thiol production from cystathionine in L. lactis subsp. cremoris S2 about 57-fold (Table 3), suggesting that cystathionine γ- and β-lyases are also controlled by the Cys concentration in lactococci. Additionally, aminotransferase activity in L. lactis subsp. cremoris S2 decreased with the addition of Met to the growth medium but no influence on Met aminotransferase was observed for B. linens BL2 (Fig. 3). With the addition of 0.002% Met to the medium, the Met aminotransferase activity decreased 43%, and with the addition of 0.02% Met, the activity decreased 63%. Conversely, addition of Met significantly increased MTPC in BL2. These data suggest that the amount of free Met in milk is sufficient both to decrease the concentrations of cystathionine γ- and β-lyases and the Met aminotransferase activities in lactococci by at least 50% and to stimulate MTPC in brevibacteria.
In cheese, free amino acids are at low concentrations, with free Met being present at 0.02 to 3% (5, 31) and Cys being present at 0.4% (31). These levels of Met and Cys are high enough to inhibit the free thiol production of L. lactis subsp. cremoris S2 with cystathionine as the substrate (Fig. 2A), but the same levels are stimulatory in B. linens BL2 with Met as the substrate (Fig. 2B). In consideration of the overall interconversion pathway between Cys and Met, these data suggest that B. linens BL2 will produce more volatile sulfur-containing compounds than will L. lactis subsp. cremoris S2 in cheese, providing the brevibacteria do not lyse. Preliminary data indicate that brevibacteria survive at least 12 months at a constant cell density, suggesting that it is possible to add these bacteria to cheese and increase volatile sulfur compounds beyond the capabilities of lactic acid bacteria. The implication of these data in cheese flavor development is complex, but one possible interpretation is that the MTPC in lactococci will decrease as the cheese ages since the cells lyse as the Met concentration increases above the inhibitory level for intact cells. Alternatively, if cystathionine is present in cheese, which is unknown, the action of cystathionine γ- or β-lyase would yield Cys or homocysteine. Conversely, the MTPC of B. linens BL2 increased with increasing amounts of Met, which might lead to more methanethiol in cheese should the cells remain intact.
Production of KMTB, which allows us to estimate Met aminotransferase activity and which leads away from the direct conversion of Met to methanethiol, was found under optimum conditions for all strains tested except B. linens BL2 (Table 2). However, under cheese-like conditions only L. helveticus CNRZ32 contained measurable activity after 1 h of incubation; with an additional 24 h of incubation, KMTB production was observed for all strains tested except B. linens BL2. These data suggest that the Met aminotransferase activities in lactococci and lactobacilli are present under cheese-like conditions. Additionally, once KMTB was formed, we did not detect further catalysis (Table 2), indicating that if this enzyme is active in cheese, it will remove Met from the amino acid pool and hence decrease methanethiol production.
If this hypothesis is true, one would expect the Cheddar flavor score in an evaluation of trained sensory responses to decrease if the organism contained Met aminotransferase activity under cheese-like conditions. Weimer et al. (30) observed that the addition of L. helveticus CNRZ32 to Cheddar cheese did not increase the rate of Cheddar flavor development as much as B. linens BL2. Considering the formation and degradation of KMTB in these cultures and their influence on cheese flavor, we conclude that Met may be taken from the free amino acid pool in cheese and become unavailable for thiol formation by the bacteria tested, thereby decreasing the cheddar score in the sensory evaluation of cheese made with the addition of lactobacilli as compared to the addition of brevibacteria.
In conclusion, we observed that lactococci and lactobacilli contain cystathionine γ- and β-lyases, which produce free thiols. Brevibacteria contain methionine γ-lyase, which converts Met directly to methanethiol. Based on the influence of pH, salt, and Met and Cys concentrations and on theoretical calculations for the inadvertent catalysis of Met by cystathionine γ- or β-lyase, we suggest that the cystathionine γ- or β-lyase from lysed lactococci makes an insignificant contribution to the production of volatile sulfur compounds from Met in cheese. Finally, taking into account the overall pathway that leads to methanethiol, we conclude that the addition of whole cells of brevibacteria is an alternative to the addition of other lactic acid bacteria to improve Cheddar cheese flavor via the metabolism of Met.
Footnotes
Approved by the director as contribution 6067 of the Utah Agricultural Experiment Station.
REFERENCES
- 1.Alting A C, Engels W J, Schalkwijk S, Exterkate F A. Purification and characterization of cystathionine β-lyase from Lactococcus lactis subsp. cremoris B78 and its possible role in flavor development in cheese. Appl Environ Microbiol. 1995;61:4037–4042. doi: 10.1128/aem.61.11.4037-4042.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aston J W, Dulley J R. Cheddar cheese flavor. Aust J Dairy Technol. 1982;37:59–64. [Google Scholar]
- 3.Aston J W, Creamer L K. Contribution of the components of the water-soluble fraction to the flavor of cheddar cheese. N Z J Dairy Sci Technol. 1986;21:229–248. [Google Scholar]
- 4.Bruinenberg P G, deRoo G, Limsowtin G K. Purification and characterization of cystathionine γ-lyase from Lactococcus lactis subsp. cremoris SK11: possible role in flavor compound formation during cheese maturation. Appl Environ Microbiol. 1997;63:561–566. doi: 10.1128/aem.63.2.561-566.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christensen J E, Johnson M E, Steele J L. Production of Cheddar using a Lactococcus lactis ssp. cremoris SK11 derivative with enhanced aminopeptidase activity. Int Dairy J. 1995;5:367–379. [Google Scholar]
- 6.Collin J C, Law B A. Isolation and characterization of l-methionine-γ-demethiolase from Brevibacterium linens NCDO 739. Sci Aliments. 1989;9:805–812. [Google Scholar]
- 7.Engels W J M, Visser S. Isolation and comparative characterization of components that contribute to the flavor of different types of cheese. Neth Milk Dairy J. 1994;48:127–140. [Google Scholar]
- 8.Ferchichi M, Hemme D, Nardi M, Pamboukdjian N. Production of methanethiol from methionine by Brevibacterium linens CNRZ 918. J Gen Microbiol. 1985;131:715–723. doi: 10.1099/00221287-131-4-715. [DOI] [PubMed] [Google Scholar]
- 9.Ferchichi M, Hemme D, Nardi M. Induction of methanethiol production by Brevibacterium linens CNRZ 918. J Gen Microbiol. 1986;132:3075–3082. doi: 10.1099/00221287-131-4-715. [DOI] [PubMed] [Google Scholar]
- 10.Fox P F, Singh T, McSweeney T K. Biogenesis of flavor compounds in cheese. In: Mailin E L, editor. Chemistry of structure/function relationships in cheese. New York, N.Y: Plenum Press; 1994. pp. 59–98. [Google Scholar]
- 11.Gao S, Oh D H, Broadbent J R, Johnson M E, Weimer B C, Steele J L. Aromatic amino acid catabolism by lactococci. Lait. 1997;77:371–381. [Google Scholar]
- 12.Hayashi K, Revel D F, Law B A. The accelerated ripening of Cheddar cheese with the aminopeptidase of Brevibacterium linens and a commercial neutral proteinase. J Dairy Res. 1990;57:571–577. [Google Scholar]
- 13.Ince J E, Knoles C J. Ethelene formation by cultures of Escherichia coli. Arch Microbiol. 1985;141:209–213. doi: 10.1007/BF00408060. [DOI] [PubMed] [Google Scholar]
- 14.Ito S, Nakamura T, Eguchi Y. Purification of methioninase from Pseudomonas putida. J Biochem. 1979;79:1263–1272. doi: 10.1093/oxfordjournals.jbchem.a131180. [DOI] [PubMed] [Google Scholar]
- 15.Khalid N M, Marth E H. Lactobacilli—their enzymes and role in ripening and spoilage of cheese: a review. J Dairy Sci. 1990;73:2669–2684. [Google Scholar]
- 16.Kreis W, Hession C. Isolation and purification of l-methionine-α-deamino-γ-mercaptomethane-lyase (l-methionase) from Clostridium sporogenes. Cancer Res. 1973;33:1862–1865. [PubMed] [Google Scholar]
- 17.Lindsay C R, Rippe J K. Enzymic generation of methanethiol to assist in the flavor development of Cheddar cheese and other foods. In: Parliment T H, Croteau R, editors. Biogeneration of aromas. Washington, D.C: American Chemical Society; 1986. pp. 286–308. [Google Scholar]
- 18.Moat A, Foster J. Microbial physiology. 3rd ed. New York, N.Y: Wiley-Liss; 1995. p. 457. [Google Scholar]
- 19.Nagasawa T, Kanzaki H, Yamada H. Cystathionine γ-lyase of Streptomyces phaeochromogenes. J Biol Chem. 1984;259:10393–10403. [PubMed] [Google Scholar]
- 20.Peterson B D, Marshall R T. Nonstarter lactobacilli in Cheddar cheese: a review. J Dairy Sci. 1990;73:1395–1410. [Google Scholar]
- 21.Reuiz-Herrera J, Starkey R L. Dissimilation of methionine by Achromobacter starkeyi. J Bacteriol. 1970;104:1286–1293. doi: 10.1128/jb.104.3.1286-1293.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoeman R, Redfield B, Coleman T, Brot N, Weissbach H, Greene R C, Smith A A, Saint-Girons I, Zakin M M, Cohen G N. Regulation of the methionine regulon in Escherichia coli. Bioessays. 1985;3:210–213. doi: 10.1002/bies.950030506. [DOI] [PubMed] [Google Scholar]
- 23.Soda K. Microbial sulfur amino acids: an overview. Methods Enzymol. 1987;143:453–459. doi: 10.1016/0076-6879(87)43080-2. [DOI] [PubMed] [Google Scholar]
- 24.Soda K, Tanaka H, Esaki N. Multifunctional biocatalysis: methionine γ-lyase. Trends Biochem Sci. 1983;8:214–217. [Google Scholar]
- 25.Strickland M, Weimer B C, Broadbent J R. Capillary electrophoresis of cheese. J Chromatogr. 1996;731:305–313. [Google Scholar]
- 26.Tanaka H, Esaki N, Yamamoto T, Soda K. Purification and properties of methionine γ-lyase from Pseudomonas ovalis. FEBS Lett. 1976;66:307–311. doi: 10.1016/0014-5793(76)80528-5. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Esaki N, Soda K. A versatile bacterial enzyme: methionine γ-lyase. Enzyme Microb Technol. 1985;7:530–536. [Google Scholar]
- 28.Urbach G. Contribution of lactic acid bacteria to flavour compound formation in diary products. Int Dairy J. 1995;5:877–903. [Google Scholar]
- 29.Uren J R. Cystathionine β-lyase from Escherichia coli. Methods Enzymol. 1987;143:483–485. doi: 10.1016/0076-6879(87)43086-3. [DOI] [PubMed] [Google Scholar]
- 30.Weimer B, Dias B, Madhavi U, Broadbent J, Brennand C, Jaegi J, Johnson M, Milani F, Steele J, Sisson D V. Influence of NaCl and pH on intracellular enzymes that influence Cheddar cheese ripening. Lait. 1997;77:383–398. [Google Scholar]
- 31.Wood A F, Aston J W, Douglas G K. The determination of free aminoacids in cheese by capillary column gas liquid chromatography. Aust J Dairy Technol. 1985;40:166–169. [Google Scholar]
- 32.Yocum R R, Perkins J B, Howitt C L, Pero J. Cloning and characterization of the metE gene encoding S-adenosylmethionine synthetase from Bacillus subtilis. J Bacteriol. 1996;178:4604–4610. doi: 10.1128/jb.178.15.4604-4610.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yuvon M, Thirouin S, Ruein L, Fromentier D, Gripon J C. An aminotransferase from Lactococcus lactis initiates conversion of amino acids to cheese flavor compounds. Appl Environ Microbiol. 1997;63:415–419. doi: 10.1128/aem.63.2.414-419.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]