Abstract
Salmonella is the leading cause of food-borne zoonotic disease worldwide. Non-typhoidal Salmonella serotypes are the primary etiological agents associated with salmonellosis in poultry. Contaminated poultry eggs and meat products are the major sources of human Salmonella infection. Horizontal and vertical transmission are the primary routes of infection in chickens. The principal virulence genes linked to Salmonella pathogenesis in poultry are located in Salmonella pathogenicity islands 1 and 2 (SPI-1 and SPI-2). Cell-mediated and humoral immune responses are involved in the defense against Salmonella invasion in poultry. Vaccination of chickens and supplementation of feed additives like prebiotics, probiotics, postbiotics, synbiotics, and bacteriophages are currently being used to mitigate the Salmonella load in poultry. Despite the existence of various control measures, there is still a need for a broad, safe, and well-defined strategy that can confer long-term protection from Salmonella in poultry flocks. This review examines the current knowledge on the etiology, transmission, cell wall structure, nomenclature, pathogenesis, immune response, and efficacy of preventative approaches to Salmonella.
Keywords: Salmonella, vaccines, poultry
1. Introduction
Salmonella is the leading cause of foodborne diseases worldwide that infects the gastrointestinal tract and causes diarrhea, nausea, and cramps in humans [1]. The Center for Disease Control and Prevention (CDC) estimates that approximately 1.35 million infections and 420 deaths are reported annually in the United States. Salmonella enterica ser. Enteritidis (S. Enteritidis), and Salmonella enterica ser. Typhimurium (S. Typhimurium), belonging to the non-typhoidal Salmonella group (NTS), is responsible for the majority of human salmonellosis. Globally, non-typhoidal Salmonella is responsible for approximately 93 million cases of gastroenteritis and 155,000 fatalities annually. The severity of human salmonellosis varies depending on factors such as the specific strain causing the infection, health conditions, and host age. It has been reported that the infective dose in a human infant is reported to be 100 bacterial cells, and even fewer cells are required to cause an infection in an immunocompromised individual [2,3,4].
Poultry serves as the main reservoir for various non-typhoidal Salmonella (NTS) serotypes among food-producing animals. Epidemiologically significant NTS serotypes include S. Typhimurium, S. Enteritidis, S. Heidelberg, and S. Newport. In North America and Europe, S. Enteritidis dominates the egg-borne transmission of infection to humans, whereas S. Typhimurium was the primary serovar associated with external egg contamination in Australia [5,6,7]. Between 1998 and 2008, poultry accounted for 17.9% of foodborne illnesses in the United States, with Salmonella ser. Enteritidis and Typhimurium are responsible for 17.4% and 34% of poultry-related foodborne illnesses, respectively [8]. In 2016, a national outbreak of multidrug-resistant S. Heidelberg linked to chicken products produced by a single poultry company was reported in California and Washington, leading to high hospitalization rates and indicating high virulence strains of Salmonella [9].
Society pays significant health costs and economic burdens caused by nontyphoidal Salmonella associated with chickens, estimated at 2.79 billion annually. This concern is growing as the global demand for ready-to-eat food products continues to rise [10]. Given the emergence of multi-drug-resistant bacteria and the associated public health crisis, alternatives to antibiotics are gaining more importance in the poultry sector, as antibiotic residues are known to contaminate consumed meat [11]. Several preharvest and postharvest intervention strategies have been developed to ensure the safety and hygiene of poultry products. Preharvest interventions include farm-level management, including the use of feed additives and biosecurity measures. Post-harvest methods include activities during carcass slaughter and meat processing, including the implementation of Hazard Analysis Critical Control Point (HACCP) plans [4].
Furthermore, with the FDA’s decision to abate the use of antibiotics in animal agriculture and consumer demand for antibiotic-free chicken, alternative control strategies that include vaccination programs are routinely followed by producers to combat pathogenic bacteria. Ensuring the sustainable production of poultry products is critical to meeting the future global demand for poultry products. However, annual Salmonella outbreaks can significantly impact production efficiency and food safety. To eradicate pathogens like Salmonella, which has multiple infectious serotypes, more powerful vaccines that confer cross-protection against multiple serotypes, including emerging serotypes, and induce long-term immunity are needed.
This review article focuses on the current understanding of Salmonella as a pathogen, its pathogenesis, and control strategies against Salmonella in poultry.
2. Etiology and Transmission
Salmonella spp. is a Gram-negative, oxidase-negative, non-spore-forming bacillus member of the Enterobacteriaceae family. Salmonella is a facultative anaerobic intracellular bacillus with a cell length between 2–5 µm. They are non-fastidious, motile, and have peritrichous flagella, except Salmonella enterica serovar Gallinarum and Pullorum. Most Salmonella serovars, except S. Typhimurium, are aerogenic. Salmonella is capable of producing hydrogen sulfide and converting nitrates to nitrites. Most Salmonella typically grows at temperatures ranging from 5–45 °C, with the ideal temperature being between 35–37 °C and at the optimum pH range of 6.5 and 7.5. Some strains can even grow at a pH as low as 3.7. Salmonella is sensitive to salt concentrations ranging from 0.5% to 5% and survives in environments with higher water activity (aw) ranging from 0.96 to 0.99. They can also survive in low-moisture food products for a long duration [12]. The severity of Salmonella infection varies according to many factors, including host age, host immunity, the presence of coinfections, environmental stress, managerial characteristics, and infective dose. Older birds, for instance, tend to be less susceptible to Salmonellosis even with concentrations of 106 CFU/mL of S. Typhimurium [13].
Salmonella is distributed worldwide and is endemic to areas where animal husbandry is practiced. Serovars also vary in their distribution across the world, with ST and SE being prevalent everywhere. Some serovars are host-specific, like Salmonella ser. Abortusovis in sheep, Salmonella ser. Choleraesuis in pigs, and Salmonella ser. Dublin in cattle. Typhoidal Salmonella serovars like S. Typhi and S. Paratyphi are human pathogens transmitted via the fecal-oral route. In contrast, NTS is zoonotic and can infect a wide range of animal reservoirs, including birds, reptiles, dogs, cats, and rodents [14].
The primary reservoir for Salmonella in animals, particularly poultry, is the primary source of food-borne human salmonellosis. Transmission occurs mainly through the consumption of contaminated egg and meat products [12]. During the production cycle, poultry can become infected with Salmonella through various routes, including contact with carrier animals like rodents, cats, and insects. Contaminated poultry feed, litter, water, and aerosol transmission also contribute to the transmission of Salmonella [15].
Salmonella contamination of eggs can occur via two routes, namely horizontal and vertical, particularly by the serovar S. Enteritidis. In vertical or transovarial transmission, the infection occurs directly in the yolk, vitelline membrane, and albumen before the egg is laid. The infection originates in reproductive organs such as the ovary and oviduct with S. Enteritidis. As a result, bacteria enter the egg even before the eggshell is formed in the oviduct [16]. In horizontal or fecal-oral transmission, eggs become contaminated by eggshell penetration from the colonized gastrointestinal tract (GIT). Additionally, contaminated feces transfer the pathogen to eggs during or after oviposition. Feces serve as nutrient reservoirs for Salmonella’s growth, contaminating the environment and potentially infecting the rest of the flock in the same enclosure. The bacterial penetration of the egg is more rapid during the first few minutes post-oviposition, as some cuticles are immature and few pores are open [17]. Outer shell contamination by Salmonella is evident in eggs collected from contaminated nests and hatchery environments. Some studies reported that there is no direct relationship between eggshell thickness and Salmonella Typhimurium penetration, but eggs with high specific gravity shells tend to offer more resistance against S. Enteritidis penetration [16].
Insects can act as vectors for Salmonella on poultry farms. Cockroaches, for example, have the potential to introduce foodborne pathogens like Salmonella into poultry production facilities due to their ability to cross-contaminate and transmit the pathogen to uninfected individuals within their group. Studies have indicated that cockroaches infected with S. Typhimurium can transfer the bacteria to the surface of the table egg [18]. Flies captured in poultry establishments have been shown to harbor Salmonella. The poultry mite (Dermanyssus gallinae) has been implicated as a biological vector of Salmonella Enteritidis and has been reported to carry the bacteria within poultry premises. It is suggested that the primary source of infection could be oral ingestion of crushed contaminated mites by the chicks, as well as the mite’s blood meal [19]. Alphitobius diaperinus, also known as the litter beetle, has been found to transmit Salmonella to poultry in an experimental infection [20]. According to Bastiaan and Aize, rodents such as mice can act as carriers of Salmonella in layer flocks [21]. Feral mice present in poultry farms may serve as a rich source of multiple phenotypes and genotypes of S. Enteritidis [21]. In African countries, S. Kentucky and S. Enteritidis were the major serovars isolated from lizards and rodents inhabiting the poultry houses. It is hypothesized that the feces of lizards and rodents could contaminate the feed and litter, posing a biosecurity threat [22].
Salmonella can colonize the intestines of wild birds, turning them into asymptomatic reservoirs. Hughes et al. reported the isolation of different Salmonella serovars from wild birds, mainly passerine birds, in northern England [23]. S. Typhimurium strain DT160 caused significant mortality in wild birds and gastrointestinal illness in humans in New Zealand in 2000, indicating a zoonotic risk [24]. Wild birds like one buff-necked ibis, red-legged seriema, and eared dove captured near poultry facilities had Salmonella infection. Moreover, S. Typhimurium dominated the serotype isolated from wild pigeons [25]. It is hypothesized these birds play a vital role in the transmission of Salmonella serotypes to poultry and humans during migration, seasonal movements, and feeding [26]. Olga et al. found that 32.3% of the bacterial pathogens identified in the wild bird population in a national park in Ukraine tested positive for S. Enteritidis. These birds migrate to various parts of the world, contributing to the distribution of the pathogen to locations far from its source [27]. Furthermore, a shift in the poultry Salmonella serotypes linked with the spread of clones has been identified as a contributing factor to the increase in human cases of S. Infantis between 2011 and 2013 across Europe [28]. Likewise, S. Heidelberg, with its multiple clones in the US, is one of the top poultry and human serotypes associated with multistate outbreaks [9]. Figure 1 summarizes the overview of the transmission routes and vectors of Salmonella in poultry.
Figure 1.
Overview of the various transmission routes of Salmonella. Created with Biorender.com (accessed on 26 July 2023).
3. Cell Wall and Nomenclature
The cell wall of Salmonella consists of lipids, lipopolysaccharides (LPS), proteins, and lipoproteins [29]. The complex polysaccharide moiety with the Lipid A portion serves as an endotoxin and is responsible for bacterial virulence [29]. The LPS complex comprises three regions: (1) an outer O polysaccharide-specific side chain, (2) a middle core portion, and (3) an inner lipid A. The somatic O antigen is the central component of the endotoxin, composed of various monosaccharides and polysaccharides [29]. The Somatic O antigen is the polysaccharide portion on the bacterial surface that contains multiple [5,6,7,8] monosaccharides. The O-specific side chains determine the immunological specificities of the respective O-antigens [30]. Furthermore, all Salmonella O antigens share five common sugars: heptose, ketodeoxyoctonic acid, D-glucose, D-galactose, and D-glucosamine. It has been hypothesized that all-specific Salmonella polysaccharides have a common core composed of these sugars, with attached chains made of sugars unique to the specific serotype [31]. In summary, variations in the LPS structure exist in the O-polysaccharide chain, and these variations are responsible for the antigenic factors that confer serotype specificity.
Genotypic analysis indicated that Salmonella has about 60 somatic antigens using gene transfer [32]. Based on these antigens and specific antisera, Salmonella can be grouped into different serotypes. In brief, Salmonella has three cell surface antigens: an O antigen (cell-wall somatic), an H antigen (flagellar), and a K antigen (capsular). The flagellar antigen is a thermolabile protein, while the somatic O antigen is thermostable [4]. Some serovars of Salmonella also have a K antigen, which is a heat-sensitive carbohydrate. Among the Enterobacteriaceae, Salmonella is unique for having two distinct H antigens: phase 1 (specific) and phase 2 (non-specific) flagella antigens [31].
Salmonella was initially isolated in 1885 by Theobald Smith and Daniel Elmer Salmon from pigs infected with classical swine fever [33]. Currently, the nomenclature system recommended by the World Health Organization (WHO), namely supplement 2001 (no. 45) to the Kauffmann-White scheme, is followed for research on Salmonella. According to this terminology, the genus Salmonella includes two species, Salmonella enterica and Salmonella bongori, that belong to it based on the differences in 16-sRNA sequence analysis. Historically, the two species are further subdivided into six subspecies based on biochemical and phylogenetic properties and denoted by Roman numerals: enterica (I), salamae (II), arizonae (IIIa), diarizonae (IIIb), houtenae (IV), and indica (VI) [32,34,35,36]. Among the subspecies of Salmonella, S. enterica subsp. enterica is the one most commonly associated with human and animal infections [32].
However, the serovars within the subspecies are named according to the Kauffman and White classification system, which is based on the immunoreactivity of the major Salmonella cell surface antigens. Each Salmonella serovar possesses a unique antigenic combination, and a serovar (or serotype) name is assigned to each individual combination of the O:H1:H2 antigenic formula [37]. The Kauffman-White Scheme was first published in 1934 with 44 serovars. The latest one, released in 2007, has over 2500 serovars [38]. According to this classification system, the Salmonella serovars are formed by different combinations of 46 O antigens and 114 H antigens.
S. enterica serovars are the etiological agents of typhoidal and non-typhoidal Salmonella infection, with the former being host-restricted to humans and the latter zoonotic with a broad homeothermic host range [34]. S. enterica comprises over 2400 serotypes. Among the 100 serovars of epidemiological importance in the S. enterica species, S. Enteritidis and S. Typhimurium are the most prevalent serovars [39].
Salmonella serotypes are characterized and identified using various methods, including phage typing, PCR ribotyping, pulsed-field gel electrophoresis analysis, multi-locus DNA sequencing, and antimicrobial resistance patterns [40,41,42,43,44,45]. Recently, alternative molecular methods like the Check and Trace Salmonella assay (a microarray method using molecular markers) and whole-genome sequencing (WGS)-based serotype prediction tools have been used to characterize Salmonella isolates [46,47]. Compared to traditional serotyping, these methods are claimed to be easy, requiring only a short turn-around time. Traditional serotyping based on phenotypes can be challenging due to the complexity of typing sera, antigen preparation, and antisera required for testing [23,48].
4. Pathogenesis
Salmonella pathogenesis can be divided into several stages, including adhesion and invasion of gut epithelial cells, survival, multiplication within the host cells, and extraintestinal spread. Salmonella, being an enteric pathogen, reaches the intestine via oral ingestion (horizontal transmission) from contaminated environments, feed, and water. Even a very low infective dose of Salmonella Enteritidis, as low as 1–5 bacteria cells, can lead to infection in day-old chicks. The incubation period for Salmonella is usually 7 to 14 days [4]. The bacterium’s ability to withstand a pH of 3.7 in the stomach helps the bacteria pass through the acidic stomach environment [4].
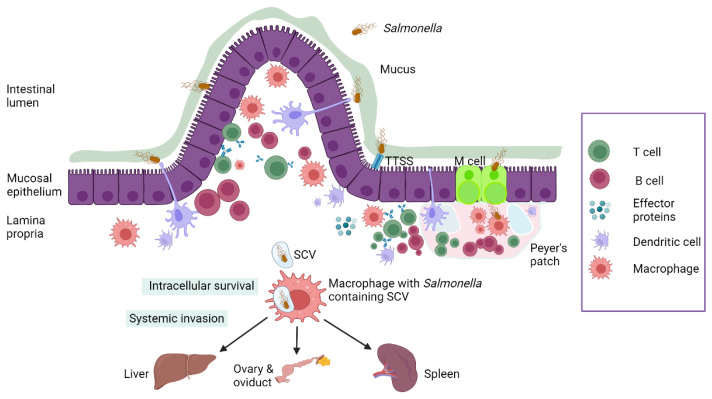
Upon reaching the small intestine, Salmonella invades and adheres to the intestinal epithelial cells using fimbrial adhesins. Salmonella’s entry into the intestinal mucosa is facilitated mainly through M cells located over the Peyer’s patches (mucosal-associated lymphoid tissues). Other routes include internalization by dendritic cells and uptake by enterocytes mediated by effector proteins associated with virulence genes in SPI-1-TTSS [49,50].
M cells, specialized antigen sampling cells of the intestinal epithelium, play a critical role in the uptake and active transportation (transcytosis) of Salmonella to the underlying lymphoid follicles. This uptake of bacterial antigen by M-cells is vital in the development of mucosal and systemic immune responses, as Salmonella antigens are delivered to mononuclear phagocytes like dendritic cells (DCs) and macrophages [51].
Macrophages can internalize Salmonella but are unable to kill them as the bacteria can inhibit the fusion of phagosomes with secondary lysosomes, the mechanism used by macrophages to destroy intracellular pathogens. This enhances the intracellular survivability of the bacteria [52]. Salmonella proliferates inside the macrophages within a structure called the Salmonella-containing vacuole (SCV) and eventually translocates widely to the draining mesenteric lymph nodes, leading to bacteremia and invasion of systemic organs such as the liver, spleen, ovary, and gallbladder [52].
Both young and adult birds are infected by the invasive S. Enteritidis. Young birds tend to develop a systemic disease with high mortality rates, whereas adult birds can remain asymptomatic carriers post-colonization by the bacterial pathogen. The infection dose plays a critical role in developing clinical signs, with clinical salmonellosis being more likely to develop in young birds infected with high doses of S. Enteritidis [53].
Typhoid fever is caused by the bloodstream dissemination of S. enterica serotype Typhi [54]. Non-invasive serotypes of NTS are confined to the gastrointestinal tract, where they induce significant inflammation, including focal and diffuse mononuclear cell infiltration, necrosis of epithelial cells, edema, and eventually enterocolitis [55]. In brief, Salmonella has orchestrated mechanisms to co-evolve with their hosts by altering cellular processes that favor bacterial survival and intracellular proliferation.
Salmonella pathogenic factors are controlled by virulence genes and plasmids, which are located within Salmonella pathogenicity islands (SPIs). SPIs are a group of genes located in specific areas of the bacterial chromosomes that encompass multiple virulence factors, including invasins, adhesins, and toxins. Salmonella contains a total of twenty-three SPIs, with SPI-1 and SPI-2 being the two critical ones. Both of these SPIs encode a molecular apparatus called the type III secretion system (TTSS) or molecular syringe. This TTSS is responsible for injecting effector proteins produced by Salmonella into the host cell, thereby establishing the intracellular survival and propagation of Salmonella inside the host [55].
Salmonella has two types of type III secretion systems, T3SS-1 and T3SS-2, found in SPI-1 and SPI-2, respectively. Briefly, T3SS-1 is responsible for transferring effector proteins needed for bacterial invasion, SCV (Salmonella containing vacuoles) biogenesis, and inflammation. On the other hand, T3SS-2 facilitates the transport of effector proteins that favor SCV maturation, SIF (Salmonella-induced filaments) biogenesis, intracellular survival of the pathogen, and its movement inside Salmonella-containing vacuoles [56].
The effectors of SPI2-T3SS involved in SCV maturation and SIF biogenesis are SifA, SseJ, SopD2, PipB2, SseF, SseG, SpvB, and SteA. These effectors are essential for converting early SCV (endosomes) to late SCV, where bacterial replication occurs. Salmonella-containing vacuoles (SCVs) are specialized structures expressed by TTSS on the SPI-2 used by the bacteria to reside inside epithelial cells and macrophages to escape from killing by macrophages through the inhibition of lysosomal fusion [49].
Salmonella-induced filaments (SIFs) are specialized endosomal tubule projections that extend from the SCV. They are characterized by lysosomal glycoproteins and endocytic markers such as Rab4, Rab9, Rab11, and Rab5, among others. These SIFs inside the host cell cytoplasm form a complex replicative niche. The exact role of SIFs in Salmonella infection is still unknown [57]. It has been reported that the SifA-SKIP-Rab9 complex decreased M6PR (late endosomal/lysosomal markers) recruitment to the SCV membrane and reduced the movement of lysosomal enzymes to the SCV. This reduction in the movement of lysosome enzymes, expanding the SCV population, helps protect intracellular Salmonella from host defense mechanisms [58].
Further, studies have reported that Salmonella Typhimurium infection causes enterocytes to become M-cells, thereby exacerbating inflammation and the immune response associated with enhanced translocation of bacteria across the submucosa. This differentiation of the primed intestinal cells into M cells promotes Salmonella colonization and host invasion. The mechanism is controlled by the type III effector protein SopB via the Wnt/β-catenin signaling pathway [59].
A previous study reported by Yakhya et al. found that the deletion of SPI1 led to a decreased colonization of Salmonella Typhimurium in the cecum and spleen of chickens. They also reported that the deletion of SPI2-T3SS did not significantly affect the cecal colonization by S. Typhimurium. However, the SPI2 mutation reduced the ability of S. Typhimurium’s ability to invade the spleen in one-week-old chicks [60]. In addition, the Michael et al. study revealed that the disruption of spaS, an essential component of the SPI-1 T3SS, had little influence on cecal colonization in day-old chicks. In contrast, the mutant spaS strain was shown to reduce cecal colonization and decrease liver invasion during an experimental S. Typhimurium infection in one-week-old birds [61]. Moreover, the same study illustrates that the deletion of another gene, ssaU, that encodes major components of SPI-2 T3SS did not affect bacterial colonization of the intestine but exhibited a significant reduction in S. Typhimurium dissemination to the liver throughout the study [61]. A recent study with the S. Typhimurium challenge model showed that the deletion of SPI-1 and SPI-2 genes had a negative impact on the mutant strain’s ability to colonize and cause systemic lesions in the cecum and liver in one-day-old chickens [62].
Salmonella spp. has the ability to form biofilms at room temperature on surfaces in poultry environments and food processing plants. These biofilm cells are highly resistant to antimicrobials and contribute to the increased virulence of the bacteria, thereby establishing a chronic infection. The ability of Salmonella to survive in biofilm poses challenges for disinfection procedures in poultry environments [63]. Figure 2 summarizes Salmonella pathogenesis.
Figure 2.
Schematic representation of Salmonella pathogenesis in poultry. Created with Biorender.com (accessed on 26 July 2023).
5. Immune Response to Salmonella
The interaction between humoral and cell-mediated immune systems plays a critical role in clearing Salmonella infection in poultry. The complex immune response against Salmonella depends on multiple factors, including host species, host age, gut microbiota composition, bacterial load, and Salmonella serotype associated with the infection [64,65].
5.1. Innate Immune System
The innate and adaptive immune components are the two main classifications of the avian immune system. The innate immune system serves as the first line of defense against pathogens, and it has several key components, including (1) chemical and physical barriers such as skin, mucosal epithelia, mucus, and antimicrobial molecules; (2) blood proteins such as complement, lectins, and agglutinins; (3) phagocytic effector cells (macrophages, neutrophils, monocytes), dendritic cells, heterophils, and natural killer cells; (4) cytokines; (5) cellular receptors (pathogen recognition receptors) such as Toll-like receptors, NOD-like receptors, and RIG-like receptors; (6) antimicrobial peptides such as defensins and cathelicidins [66].
The avian immune organs can be divided into bone marrow, bursa of Fabricius and thymus, and peripheral lymphoid organs such as the spleen and cecal tonsils [67,68]. MALT (mucosa-associated lymphoid tissue) comprises components of the immune system linked to the gastrointestinal and bronchial mucosa, providing the first line of protection against pathogens that enter the body through these mucosal surfaces. GALT, a major component of MALT, contains an isolated or group of lymphoid follicles disseminated in the lamina propria of the gastrointestinal tract, such as the bursa of Fabricius, cecal tonsils (CT), Meckel’s diverticulum, Peyer’s patches (PP), and intraepithelial lymphocytes. GALT lymphoid structures are the major secondary lymphoid organs since chickens lack other peripheral encapsulated lymph nodes [69,70].
Pathogen-associated molecular patterns (PAMPs) are microbial molecular structures that stimulate the innate and eventually adaptive immune systems. Some examples of PAMPs include nucleic acids such as single-stranded RNA (ssRNA) and double-stranded RNA (dsRNA); proteins such as flagellin; cell wall lipids such as lipopolysaccharide (LPS) and lipoteichoic acids; and carbohydrates like mannans found in fungi [66]. Cellular receptors of the innate immune system that recognize PAMPs in pathogens are known as pathogen recognition receptors (PRRs). PRRs are present in various immune cells, like phagocytes, dendritic cells, heterophils, and barrier epithelial cells. Toll-like receptors, NOD-like receptors, and RIG-like receptors are examples of PRRs. Studies have identified 10 Toll-like receptors in chickens [71]. The binding of PAMP to the specific TLRs results in the production of reactive nitrogen and oxygen intermediates, cytokines, and co-stimulatory molecules, initiating the adaptive immune response [72]. The outer membrane lipopolysaccharide (LPS) of Gram-negative bacteria is the ligand for TLR4, while TLR5 recognizes bacterial flagellin [66].
Okamura et al. reported that when birds were vaccinated with recombinant Salmonella flagellin (rFliC) and challenged with Salmonella Enteritidis, there was a significant reduction in bacterial load in the liver and cecum [73]. A transcriptome analysis study on the cecal mucosal immune system of layer chicks challenged with Salmonella Typhimurium demonstrated upregulation of TLR15, TLR1A, TLR2B, and TLR7 pathways on days 3, 5, and 7 post-infections with S. Typhimurium [74]. There was a measurable variation in TLR expression in multiple regions of the intestinal tract in 2-day-old broiler birds at 24 h post-challenge with S. Enteritidis. The infection upregulated the expression of TLR 1LA from the ceca to the ileum, TLR2A from the duodenum to the ceca, and TLR 15, which is unique to avians. However, there was a three-fold downregulation in the expression of TLR 5 compared to uninfected birds [75]. These findings emphasize the critical role of TLRs in the innate immune response of avians against Salmonella.
It has been reported that heterophils are integral cells of the avian innate immune system. They are granulocytic white blood cells that are equivalent to mammalian neutrophils and act as the first line of defense against pathogens in the avian immune system [76]. The functions of heterophils include phagocytosis of both opsonized and non-opsonized pathogens like Salmonella, degranulation of granular content, and the initiation of oxidative burst (ROS) [76]. Ferro et al. demonstrated upregulation of proinflammatory cytokines IL-6, IL-1, and IL-8 in heterophils of Salmonella Enteritidis-resistant one-day-old chicks compared to infected chicks [77]. Toll-like receptors, including TLR1, TLR 2, TLR 3, TLR 4, TLR 5, TLR 6, TLR 7, and TLR 10 mRNA, are associated with antipathogenic defense pathways in avian heterophils [78], and upregulation of TLR4, TLR15, and TLR 21 in heterophils following Salmonella Enteritidis stimulation [79]. A transcriptome analysis study has shown that the heterophil/ lymphocyte (H/L) ratio is a critical factor that determines the robustness of the immune response in birds [80]. A recent study reported that the number of goblet cells, IL-1, IL-8, and IFN-γ ileal expressions were all inversely linked with the H/L ratio, suggesting that birds with low H/L ratios had improved intestinal immunity and barrier functions [81]. In conclusion, these findings indicate the critical role of heterophils in defense against Salmonella and facilitate its clearance. However, further research is needed to determine whether the H/L ratio could serve as a biomarker to identify chickens resistant to Salmonella.
5.2. Adaptive Immunity
5.2.1. CD4+ T-Cells, CD8+ T-Cells
Adaptive immunity plays a critical role in defense against intracellular pathogens like Salmonella. In brief, T cells are key immune cells in the cell-mediated immune response. Helper T-cells (CD4+ T-cells), cytotoxic/killer T-cells (CD8+ T-cells), and regulatory T-cells (Tregs) are functionally distinct populations of T lymphocytes [66]. CD4+ T cells orchestrate cytokine production and initiate the immune response by activating other immune cells. Their release of IL-2 and IFNγ by CD4 T cells stimulates NK cells, macrophages, and CD8-T cells and aids B cell differentiation into plasma cells. CD8+ T cells, also known as killer/cytotoxic T cells, cause the lysis or apoptotic cell death of intracellular pathogens [66].
Withanage et al. reported that a Th1-mediated decrease in the bacterial load was correlated with an upregulation in the number of CD4+ and CD8+ T cells in the spleen and liver of one-week-old Rhode Island Red chickens infected with S. Typhimurium [82]. Infection with Salmonella Enteritidis in laying hens has been shown to increase the numbers of both CD4+ and CD8+ T cells along with macrophages in the ovaries and oviduct around 7–14 days post-inoculation, which was followed by peak upregulation in B cells. The reduction in the bacterial recovery rate from the ovary and oviduct around the peak lymphocyte period indicated the involvement of cell-mediated immunity [83].
Moreover, Saenz et al. recently reported that an increased CD4+/CD8+ ratio in vaccinated birds challenged with three different Salmonella serovars was linked to a reduction in cecal bacterial load [84]. At one week post-Salmonella Enteritidis inoculation, CD4+ and CD8+ T cells significantly increased in the intestines of vaccinated birds compared to unvaccinated and non-challenged birds [85]. In addition, another study reported an increase in intraepithelial cytotoxic CD8+ T cells in SE-infected birds during multiple time points during the study. However, the number of splenic cytotoxic CD8+ T-cells and splenic helper CD4+ T-cells was comparable between the infected and uninfected birds across the course of infection. Thus, prevention of systemic clearance of pathogens from the spleen alone may be insufficient [86]. Research has shown that at seven days post-infection with SE, the CD11+ MRC1LB+ macrophage level increased in the spleen, where the bacterial load was the highest [86]. The results are supported by a transgenic chicken study that showed increased MHC-II expression levels in MRC1LB+ macrophages. This altogether suggests the importance of increased levels of macrophages and CD4+ and CD8+ immune cells in clearing Salmonella infection in chickens [87].
5.2.2. Regulatory T-Cells
Regulatory T cells, or T-regs, are a subset of Th cells. Chicken T-regs (CD4+ CD25+) develop in the thymus and serve to maintain tolerance and suppress the immune response by producing the anti-inflammatory cytokine IL-10 [66]. IL-10 functions by inhibiting the production of IL-2 by effector T cells, which in turn suppresses the development of an IFNγ-driven proinflammatory response against pathogens. A study with Salmonella-challenged broiler chickens reported a significant increase in the number of T-regs and IL-10 mRNA expression throughout the study [88]. They also found that the T-regs from infected birds suppressed T cell proliferation on days 7 and 14 post-inoculation. Moreover, Tregs from uninfected control birds did not suppress T cell proliferation [88]. In conclusion, an increase in the Treg percentage was associated with persistent S. Enteritidis infection in the cecal tonsils of broiler birds [88]. Persistent S. Enteritidis and S. Heidelberg infections were reported in chickens by the induction of CD4+CD25+ cells and by the variation in IL-10 mRNA transcription, resulting in an asymptomatic carrier state in birds 18 days after infection [89]. In vaccinated birds post-challenge with S. Enteritidis, there was a decrease in the cellular immune response, explained by the significantly higher levels of IL-10 at the same time point [90]. Overall, these findings indicate that Tregs have an impact on Salmonella infection in chickens.
5.2.3. Cytokines
The cells can be classified into Th1 and Th2 cells based on the types of cytokines they produce. Th1 cells promote the proinflammatory cell-mediated immune response against intracellular pathogens like Salmonella by producing interferon-gamma (IFNγ), IL-12, and tumor necrosis factor (TNF). Th2 cytokines, mainly IL-10, are anti-inflammatory, suppressing the Th1 response, and are associated with immune resistance to Salmonella and Salmonella carrier states in chickens [91].
In brief, exposure of ligands to PRRs on antigen-presenting effector cells like macrophages, dendritic cells, and B-cells causes the activation of APC, which in turn activates the naïve T-cells. The activated T-cells differentiate into various subsets of T-helper cells [66]. In addition, the production of IL-12 by activated macrophages, known as classical activation, induces the production of IFNγ by T cells. The production of IFNγ further induces the differentiation of T cells into Th1-type cells, marking the initiation of a cell-mediated immune response [66]. Earlier studies have shown that splenic IFNγ mRNA expression was higher in day-old chicks exposed to Salmonella [92]. The clearance of Salmonella enterica serovar Typhimurium from the spleen is associated with an increase in T-cell proliferation and IFNγ expression [93]. Another pro-inflammatory cytokine, IL-1β, was related to an acute immune response against Salmonella in young chicks [94]. A study demonstrated that IL-1β mRNA expression was increased in the ilea and cecal tonsils of S. Typhimurium-inoculated birds [92]. Furthermore, a transcriptome analysis study on the cecal mucosal immune system of layer chicks challenged with S. Typhimurium found a steady upregulation in the differentially expressed genes where IL-6 played a key role [74]. A study on the effects of killed S. Enteritidis vaccination in chickens showed increased IFNγ and IL-2 production by spleenocytes on antigen recall responses post-vaccination [95]. In birds immunized with a killed chitosan nanoparticle vaccine, the mRNA expression of TNF-α in cecal tonsils increased 0.5-fold when birds were inoculated with Salmonella. They also reported an increase in the expression of IL-17 in the cecal tonsils of immunized-challenged birds [96]. However, to date, no studies have reported the specific function of Th-17 cells in the defense against Salmonella in poultry.
Chausse et al. reported that genes linked to IFN alpha/beta were inhibited in birds resistant to Salmonella. In addition, they found that both carrier-state-resistant and susceptible birds had decreased IFNγ gene expression, suggesting suppression of the Th1 response [91]. Salmonella has developed a “unique survival strategy” for long-term colonization of the chicken intestine, resulting in persistent Salmonella infection and the development of a cecal carrier state. It was suggested that carrier status could be associated with a Th2 response, as indicated by the upregulation of cytokines like IL-4, IL-5, and IL-13 mRNA expression [91].
5.2.4. B-Cells and Immunoglobulins
B cells play a crucial role in the humoral immune response by producing antibodies. These B cells differentiate into antibody-secreting plasma cells, which produce various Ig isotypes depending on the cytokine present. For instance, IgA serves as the first line of defense against mucosal infections in MALT and is the most prevalent antibody on mucosal surfaces [66]. The exact role of antibodies in controlling Salmonella remains unclear [97]. However, recent studies have identified that humoral immunity is also important in that the enteric pathogen decreases the production of serum IgG by B cells as a mechanism to escape from the humoral immune response. Manne et al. demonstrated that Salmonella uses the protein SiiE to specifically decrease the number of IgG-producing plasma cells in bone marrow, which in turn reduces serum IgG titer [98]. In addition, they also observed that the SiiE-depleted strain of Salmonella had increased serum anti-Salmonella IgG [98]. There have been reports of a steady increase in the antigen-specific serum IgG and IgM antibodies in birds inoculated with Salmonella Typhimurium [99]. In a study on chickens looking at the impact of chemically induced B cell depletion on immunological response, it was found that the birds with B cell deficiency had higher rates of intestinal Salmonella Enteritidis shedding [100]. Other studies claim that the clearance of Salmonella enterica serovar Typhimurium infection in birds is independent of the humoral immune response. They showed that B-cell-depleted birds had the same response as control birds to clearing the Salmonella infection [101]. Withanage et al. reported that intravenous inoculation of SE in layer birds led to peak production of IgG, IgM, and IgA antibodies around 14 days post-inoculation, correlating with the decrease in Salmonella recovery rates during the same period [94]. However, further research on the role of the humoral immune response in the chicken immune system is needed to get a clear understanding of the efficiency of B-cell and Ig repertoires in controlling Salmonella infection.
6. Salmonella Control Strategies in Poultry
6.1. Biosecurity
Implementing good biosecurity measures plays a key role in combating the transmission of Salmonella and improving food safety [102]. Biosecurity controls include entry-level and site cleanliness, immunization, boot dips, and the hand hygiene of employees. In addition, better rodent and fly control, red mite control, and disinfection in between flocks are recommended to reduce the incidence of Salmonella and to halt the disease cycle in farms [103]. To reduce the prevalence of D. gallinae, a biological vector of Salmonella, appropriate biosecurity measures should be used [104].
Proper litter management has been associated with a decreased risk of Salmonella detection in poultry houses. Moreover, higher Salmonella contamination was linked to the use of fresh wood shavings than older litter [105]. Hence, proper recycling of litter using methods like composting is crucial in reducing the Salmonella count in poultry litter [106]. Additionally, the use of proper disinfectants is vital in limiting the introduction and dissemination of disease in birds [107]. Furthermore, the seroprevalence of Salmonella increased during the summer compared to the winter season [108]. Hence, strict biosecurity measures are required to combat the seasonal prevalence of Salmonella among poultry flocks.
6.2. Antibiotics
Antibiotics have been used in poultry feed since the 1940s, mainly due to their benefits on birds’ feed efficiency, enhanced growth performance, and inhibition of enteric pathogens [109]. The list of antibiotics used as feed additives to combat enteric pathogens includes small quantities of penicillin, tetracycline, and chloramphenicol [110]. However, the subtherapeutic use of antibiotics in poultry feed is being reconsidered because of the growing concern about antibiotic resistance in the human food chain [111]. Resistant Salmonella serotypes have been reported against antibiotics such as quinolones, chloramphenicol, and cephalosporins worldwide [112]. Moreover, control strategies like probiotics are given importance as a viable substitute for antibiotics in light of the European Union’s (EU) ban on their usage and the US’s restricted use of them in the production of chickens. Additionally, the use of antibiotics is associated with the destruction of beneficial gut bacteria that help fight enteric pathogens [113]. Hence, alternatives to antibiotics like probiotics, prebiotics, synbiotics, postbiotics, etc., are given more importance in the post-antibiotic era.
6.3. Prebiotics
Prebiotics and probiotics play important roles in promoting gut health and supporting the balance of beneficial intestinal flora in poultry. According to the International Scientific Association for Probiotics and Prebiotics, prebiotics can be defined as “a substrate that is selectively utilized by host microorganisms conferring a health benefit” [114]. The Food and Agricultural Organization (FAO) of the United Nations (UN) defined probiotics as a “non-viable food component that confers a health benefit on the host associated with modulation of the microbiota” [115].
The prerequisite for a potential prebiotic includes the ability to withstand hydrolysis by gastric acids and enzymes and resist absorption in the upper gastrointestinal tract [116]. An ideal prebiotic should also be metabolizable by the gut microbiota, be a selective compound that promotes the growth of beneficial intestinal flora, and have the capacity to regulate the immune response in favor of the host while suppressing pathogens, thereby improving the host’s health and performance [117].
Prebiotics like non-digestible oligosaccharides and polysaccharides have been shown to inhibit the survival and colonization of pathogens like Salmonella by producing SCFA like butyrate and acetate in the ceca, which helps lower the gut pH [118]. Yeast cell wall-derived mannan oligosaccharides (MOS) [119], fructo-oligosaccharides (FOS), inulin [120], and xylo-oligosaccharides [121] are some examples of prebiotics used in poultry production systems to control various pathogens, including Salmonella.
The composition of the yeast cell wall includes MOS (40%), β-glucan (60%), and chitin (2%) [122]. Studies have demonstrated the benefits of yeast cell product supplementation in poultry diets. Shanmugasundaram et al. reported that yeast cell wall product supplementation in layer birds reduced fecal and intestinal oocyst count, up-regulated anti-inflammatory cytokine IL-10 production, and increased proliferation of beneficial bacteria like LAB and their by-products of fermentation in the cecal tonsils during post-coccidial challenge [123]. Another study found that whole yeast cell prebiotic supplementation in the diet of broiler birds modulates the immune response by increasing the percentage of Tregs, improving IL-10 (anti-inflammatory) mRNA expression, and reducing the pro-inflammatory cytokine (IL-1) mRNA expression in the cecal tonsils of broiler birds [124]. Supplementing broilers with MOS (0.05%) and FOS (0.25%) significantly improved overall body weight gains [125]. Dietary FOS supplementation at 1% revealed a reduction in the cecal load of Salmonella Enteritidis, an upregulated ileal IgA cell titer, and increased expression of TLR-4 mRNA in layer birds challenged with SE [126]. Broiler birds supplemented with 5% trehalose and inoculated with Salmonella Typhimurium improved feed conversion ratio, favored the growth of lactobacilli in jejunum and duodenum, decreased the cecal load of ST, and decreased inflammation in GIT [127]. These results highlight the potential benefits of supplementing poultry diets with prebiotics as an alternative to antibiotic growth promoters for controlling harmful bacteria like Salmonella, ultimately improving production performance and gut health.
6.4. Probiotics
Probiotics, also known as direct-fed microbial (DFM), are defined by FAO as “live microorganisms, when administered in adequate amounts, confer a health benefit on the host” [128]. Lilly and Stillwell first used the term and defined probiotics as “growth-promoting factors produced by microorganisms” [129]. In this context, probiotics confer their beneficial effects on the host through competitive exclusion, improving barrier health and function, immunomodulation, and digestion and absorption, thereby promoting growth and performance. The qualities of potential probiotic candidates are (1) host origin; (2) non-pathogenic and beneficial to the host by adhering to the gut mucosa (biofilm formation); (3) tolerate of gastric acid and bile salts; (4) antimicrobial properties against pathogens; and (5) survive post-processing and storage stress [130,131]. Probiotic microorganisms used for poultry supplementation include spore-forming Bacillus spp. Saccharomyces yeast, Enterococcus spp. [132], Streptococcus spp. Lactobacillus spp. and Bifidobacterium spp. [133]. The available probiotics on the market include either single-species or multispecies preparations, with the latter preferred due to its ability to act on multiple sites to bring out an overall synergistic effect [134].
The administration of probiotics to layers of birds improved egg production, egg weight, and egg quality [135]. Colonization by the Bacillus subtilis CSL2 probiotic strain normalized the level of fecal microbiota and increased Lactobacillus in Salmonella-challenged Hy-line Brown laying hens [136]. Supplementation of probiotics containing Lactobacillus fermentum and Saccharomyces cerevisiae in broilers improved feed efficiency and the percentage of intestinal T-lymphocytes (CD4+ and CD8+) [137]. Continuous supplementation of Bacillus-based probiotics restored the gut microbiota, decreased Salmonella load in the internal organs, and increased the level of butyrate in free-laying birds challenged with S. Typhimurium infection [138]. Probiotic supplementation has also been associated with increased anti-Salmonella bile IgA in birds challenged with Salmonella, indicating improved humoral immunity [139]. It has been shown that feed supplementation with Lactobacillus spp. and Bifidobacterium spp. has been shown to increase the production of proinflammatory cytokines IFN-γ and TNF-α that favor the clearance of Salmonella from the gut [140]. Moreover, studies have shown that probiotic bacteria play a role in maintaining a balance between pro-inflammatory cytokines and anti-inflammatory cytokines [141]. Recent studies have reported that the combination of probiotics (Lactobacillus spp., Pediococcus spp., Saccharomyces spp., and Bacillus spp.) with the live SE vaccine enhanced the growth performance, decreased mortality rate, and reduced fecal shedding of bacteria in Salmonella-challenged broilers, thereby limiting the bacterial colonization of birds [142]. These findings suggest that probiotics could be a potential alternative to antibiotics in poultry infected with Salmonella since they positively modulate the gut microbial population and enhance the immune response against the pathogen.
However, more research needs to be performed to determine the appropriate storage and packaging conditions for probiotics and to investigate the development of antimicrobial resistance in the gut microbial community. Some studies suggest that probiotics may deteriorate when exposed to temperatures higher than room temperature [143].
6.5. Synbiotics
Synbiotics refers to the synergistic combination of prebiotics and prebiotics, a concept first used by Gibson and Roberfroid in 1995 [144]. In synbiotics, prebiotics are used to support and sustain the probiotic microorganisms by modifying the gut microflora, ultimately enhancing the ability of probiotics to survive and inhibiting colonization of the gut epithelium by pathogens. The supplementation of synbiotics has shown significant benefits to host animals compared to using prebiotics and probiotics separately [145]. The Food and Agriculture Organization (FAO) recommends using the term synbiotics only if the combined health effect is synergistic [115]. Examples of synbiotics include combinations like fructo-oligosaccharides with bifidobacteria and lactitol with lactobacilli [146].
Synbiotic supplementation has been documented to improve the production performance and alleviate the heat stress of broiler breeders. Birds fed synbiotics showed less heat stress behaviors than birds fed a regular diet [147]. Supplementation of synbiotic preparations containing Lactobacillus spp., Saccharomyces cerevisiae yeast, and inulin has shown a positive effect on the proliferation of beneficial intestinal bacteria like Bifidobacterium spp. and Lactobacillus spp. in broiler birds [148]. Shanmugasundaram et al. investigated the effect of synbiotic supplementation on the production performance, immune parameters, and cecal Salmonella load of Salmonella-challenged layer birds. They reported improved body weight gain, higher hen-day egg production both with and without a Salmonella challenge, decreased cecal Salmonella colonization, and increased bile anti-Salmonella IgA [149]. In addition, synbiotics may help to modulate lymphoid organs (bursa, spleen), increase the size of bursal follicles, and stimulate the immunoglobulins, thereby improving immunocompetence against Salmonella Typhimurium infection in broilers [150]. These findings suggest that synbiotics have promising effects and could serve as potential growth promoters in poultry production.
6.6. Postbiotics
Postbiotics are non-viable bacterial products or metabolic byproducts, either secreted by live bacteria or derived after cell lysis from probiotic microorganisms, that confer beneficial functions on the host. In general, postbiotics range from SCFA to enzymes, organic acids (propionic and 3-phenylacetic acid), peptides, plasmalogens, vitamins, teichoic acids, and muropeptides. Postbiotics mimic probiotics in their mode of action, except that they are not alive [151]. The soluble factors are obtained from probiotic microorganisms in the cell walls and cytoplasm, such as Lactobacillus spp., Bifidobacterium spp., Bacillus spp., and Saccharomyces cerevisiae. Postbiotics benefit the host by producing immunomodulatory effects, decreasing gut pH, inhibiting pathogenic bacteria in the gut (pathogen antagonism), enhancing antioxidant properties, enhancing gut health, protecting intestinal barrier integrity, and improving production performance [152]. The most common postbiotics used are metabolites and their combinations produced by strains of Lactobacillus plantarum [153].
In the context of poultry, research has shown positive effects of postbiotics on various aspects of health and performance. In broiler birds exposed to heat stress, the postbiotic supplementation obtained from L. plantarum RI11 at 0.6% (v/w) showed positive effects on genes related to gut barrier health and reduced the expression of heat shock protein 70 genes as well as the acute phase protein genes, indicating the antioxidant function of postbiotics [154]. These birds also exhibited increased growth performance, intestinal histomorphology, a higher number of cecal microflora, and a negative effect on the cecal Salmonella population following the addition of postbiotics produced by L. plantarum in broiler birds maintained under heat stress conditions [155]. Dietary inclusion of postbiotics RG14 (0.15% and 0.45%) along with prebiotics (inulin) at a concentration of 1% exhibited improved body weight gain, a higher proportion of Bifidobacterium in the cecum accompanied by decreased mRNA expression of interleukins LITAF (Lipopolysaccharide-induced tumor necrosis factor-alpha factor) and IFN and reduced pathogenic bacteria like E. coli in broilers. Nevertheless, the diet did not affect the Salmonella population [156]. Choe et al. reported that supplementing layer birds with soluble metabolite combinations of L. plantarum strains improved egg production performance, fecal population of LAB, and intestinal villi height and crypt depth [157]. Additionally, postbiotics derived from Saccharomyces cerevisiae fermentation reduced the cecal colonization of Salmonella in broilers and layer pullets, making them a potential preharvest intervention to enhance food safety and improve production performance [158,159].
As antibiotic resistance becomes a growing concern, postbiotics are a potential alternative for combating enteric pathogens like Salmonella. They can play a valuable role in preharvest intervention to enhance food safety practices and improve production performance in poultry. However, further research is needed to better understand the mechanism of action and safety of postbiotics in the poultry sector.
6.7. Phytobiotics
Phytobiotics, also known as phytogenics or phytochemicals, are biologically active compounds obtained from plants used in animal production as feed additives because they offer health benefits and promote growth in animal production, including poultry. The bioactive substances derived from plants include saponins, flavonoids, terpenoids, and alkaloids [160]. These compounds are reported to have antioxidant, antiviral, antimicrobial, anticoccidial, anti-parasitic, immunomodulatory, anti-inflammatory, and endocrine stimulatory activities [161,162]. Dietary supplementation of poultry feed with garlic powder [163], clove and cinnamon [164], peppermint powder [165], and ginger [166] has been shown to improve overall production performance, feed conversion ratio, and body weight gain. Other examples of plants used as phytobiotics include black cumin, turmeric [167], calendula, oregano, green tea, and fennel, among others [168].
Supplementing a phytobiotic named Intebio in the diet of growing birds challenged with S. Enteritidis was reported to decrease the earlier inflammatory response via the downregulation of IL6, IL8L2, CASP6, and IRF7 at day 23, thereby limiting the colonization of the pathogen [169]. Ziheng et al. found that oregano essential oil (OEO) supplementation in drinking water could inhibit and treat infection by S. Pullorum and S. Gallinarum in commercial yellow-chicken breeders. Additionally, they reported that OEO was more effective in preventing infection than the treatment [170]. A diet containing 40 mgmL−1 to 80 mgmL−1 of garlic extract revealed antimicrobial properties in broiler chicks challenged with S. Typhimurium by reducing mortality and improving body weight [171]. Phytogenic compounds like trans-cinnamaldehyde and eugenol have decreased S. Enteritidis growth and cecal colonization in challenged broiler birds after ten days of infection [172]. Moreover, natural capsaicin derived from chili pepper has been reported to control the internal organ (liver, spleen) invasion by S. Enteritidis in challenged layer birds [173]. All these studies indicate the great potential of phytobiotics as an antimicrobial substitute against Salmonella in poultry and its application in commercial farms.
6.8. Bacteriophages
Bacteriophages are viruses that infect bacteria and use the host machinery to proliferate inside the host cell. These phages penetrate their DNA into the host cell (lysogenic) and undergo multiplication, followed by the release of a large number of new bacteriophages, eventually leading to the lysis of the bacterium to release the progeny bacteriophages. In the lysogenic cycle, phage DNA integrates into the bacterial chromosome and can remain dormant for some time without causing cell lysis [174]. Bacteriophages are used as alternatives to antibiotics due to their promising target specificity, less allergic side effects, and harmlessness to the host’s normal flora [175]. The treatment of S. Enteritidis-contaminated poultry carcass with a higher number of bacteriophages was able to reduce the percentage of recoverable bacteria by 93% [176].
The application of phage cocktail (F1055S, F12013S) as an aerosol spray on fertile eggs challenged with S. Enteritidis during their transfer from incubators to hatchers reduced Salmonella’s horizontal transfer [177]. The inoculation bacteriophage cocktail made from phages isolated from chickens (UAB_Phi20, UAB_Phi87) and pigs (UAB_Phi78) decreased the Salmonella load in the cecum and mortality rate of white leghorn birds challenged with S. Typhimurium [178]. The administration of CTCBIO phage significantly reduced the S. Enteritidis load in the cloacal swab, liver, and spleen in broilers that are challenged with S. Enteritidis [179]. Broad-host-range phage (STP4-a) is beneficial over specific phages since they can inhibit multiple serovars of Salmonella with their polyvalent adsorption sites [180]. Additionally, oral inoculation of S. Enteritidis and S. Typhimurium phages reduced depression, loss of appetite, and diarrhea in a Salmonella challenge model. There is a significant decrease in the cecal colonization of Salmonella 7–15 days post-administration of phages in infected chicks [181]. In pandrug-resistant S. Typhimurium infected chicks, a phage combination (virulent and non-productive) enhanced the survival rate of chicks by 100%. It decreased the bacterial load in internal organs but did not improve body weight gain, alleviate splenomegaly, or re-establish the intestinal microbiota [182]. These data suggest that bacteriophage treatment could improve the survival rate of Salmonella-infected chickens and reduce the bacterial colonization of internal organs. The poor efficacy of phage to withstand the acidic gastric pH of the birds on oral delivery is overcome by the encapsulation technique [183]. However, the main disadvantage of phage therapy is the emergence of phage resistance [184].
6.9. Vaccination against Salmonella in Poultry
6.9.1. Live-Attenuated Vaccine
Live attenuated vaccines, as the term suggests, are vaccines with living bacterial pathogens that have been rendered inactive or avirulent using attenuation methods such as chemical and genetically engineered mutagenesis [185,186]. These vaccines mimic natural infection as they adhere to the intestinal mucosa when administered orally, eliciting potent humoral and cell-mediated immune responses [185]. In newly hatched chicks with immature immune systems, Salmonella live vaccines promote resistance to infection, inhibiting gut colonization or competitive exclusion [187].
In a study by Lin et al., a live attenuated bivalently lyophilized vaccine containing a final concentration of 6 × 108 CFU and 1 × 108 CFU ST and SE strains, respectively, was inoculated into commercial layers at day 5, week 8, and week 18 of age [188]. The birds in different treatment groups were separately challenged with SE and ST at 25 weeks of age, and the birds were humanely euthanized on day 14 post-challenge. Both vaccines successfully eliminated cloacal Salmonella shedding, as observed from cloacal swabs collected on days 7 and 14 post-challenge. Triple vaccination with the ST vaccine significantly reduced the invasion of Salmonella into the internal organs like the liver, spleen, and cecum in 90% of vaccinated birds [188].
Groves et al. showed that oral administration of a live-attenuated aroA deletion mutant S. Typhimurium vaccine (Vaxsafe ST) at a concentration of 108 CFU/250 µL decreased Salmonella load only for a temporary duration over a period of 17–25 weeks. However, the dual administration of the same vaccine through the parenteral (subcutaneous) route enhanced the cecal clearance of the bacteria [189]. In a study performed on the aroA mutant Salmonella Typhimurium live vaccine, there were no reductions in the fecal shedding of ST [190].
Many live attenuated vaccines are produced by mutating genes involved in crucial virus survival and metabolism [191]. A previous study explored the potential of an O-antigen-deficient live attenuated Salmonella Typhimurium vaccine, created by the deletion of the rfaL, lon, and cpxR genes, on the level of protection from systemic colonization and immune response. The vaccine induced significant ST-specific IgY and rapid clearance of mutant bacterial strains from the spleen and liver in about seven days [191].
Venessa et al. immunized day-old laying–type chicks with a live Salmonella Enteritidis/Typhimurium bivalent oral vaccine, followed by two booster immunizations at six weeks and 16 weeks of age. The birds immunized with the bivalent vaccine (Salmonella Duo) decreased the spleen colonization of a heterologous strain of bacteria when the birds were challenged with 4.6 × 108 CFU of Salmonella Infantis (a heterologous strain). Hence, this study has been shown to confer cross-protection against S. Infantis (serotype C strains) [192]. However, despite the ability of live-attenuated vaccines to stimulate both cell-mediated and mucosal immune responses, the biosecurity risk of virulence reversal of the live vaccine strain is the major drawback of live-attenuated vaccines [193].
6.9.2. Killed or Inactivated Vaccine
Killed bacterin vaccines made from inactivated whole-cell preparations of bacteria have been extensively used to control poultry Salmonella infection [194]. Commonly used inactivation agents for the preparation of killed vaccines include heat (60 °C for 1 h), formaldehyde, acetone, ethylene oxide, beta-propiolactone, and radiation (ultraviolet or gamma) [195]. The available Salmonella-killed vaccines are serovar-specific. Studies have shown that killed vaccines confer humoral immunity mostly and do not elicit strong cell-mediated immune responses. Therefore, booster vaccination is required for long-term protection [196,197]. Despite the absence of robust cell-mediated immunity, killed vaccines are preferred over live vaccines due to biosecurity and safety reasons [198]. Additionally, multivalent inactivated vaccines are required to contain the spread of a wide range of Salmonella serovars present in poultry [199,200].
An inactivated aluminum hydroxide-gel adjuvanted trivalent Salmonella enterica vaccine was shown to reduce the load of Salmonella Enteritidis in challenged birds by four log CFU/g, as well as demonstrate a complete reduction in bacterial dissemination to the liver when inoculated with Salmonella Infantis in vaccinated birds [194]. It has been shown that greater levels of IgA and IgY antibodies were produced in the bile and serum following the simultaneous use of live attenuated and killed Salmonella vaccines in broilers [201]. The combined use of both live and killed vaccination programs successfully reduced S. Typhimurium and S. Infantis colonization in young layers, providing broad protection [199].
6.9.3. Subunit Vaccine
Subunit vaccines, made of defined antigens, are used in poultry and are claimed to be safer than live-attenuated or inactivated vaccines [193]. Vaccines derived from the outer membrane proteins (OMPs) and flagella proteins (FliC protein) of Salmonella enterica serovar Enteritidis with adjuvants have been used to decrease bacterial shedding in poultry and to induce a significant antigen-specific immune response against Salmonella [202]. Additionally, a trivalent subunit cochleate system-based vaccine has been evaluated against three serovars of Salmonella in layers. This vaccine increased serum IgY and improved production performance [84].
Desin et al. showed the efficacy of a subunit vaccine based on the Type 3 secretion system encoded on a type 1 pathogenicity island, which showed an increased titer of IgG antibodies in birds. Bacterial colonization in the liver was reduced but not in the spleen or cecum [203]. Renu et al. experimentally designed an oral mucosal adhesive biodegradable chitosan Salmonella subunit nanoparticle (CNP) vaccine for broilers, which successfully elicited mucosal IgA production, lymphocyte recall assays, serum IgY antibody levels, and conferred cross-protection against other Salmonella enterica serovars. Further, there was a significant reduction in Salmonella Enteritidis load in the ceca and spleen of CNP-vaccinated birds at 21 days post-inoculation [204].
In an experimental vaccine challenge study performed to study the protective effect of the vaccine on Salmonella serotypes, it was shown that a direct correlation exists between the increase in serum antibodies and a decrease in bacterial loads in the intestines of vaccinated birds [84]. The vaccine in the study did not affect the production performance of the vaccinated birds. Nevertheless, the duration of the protective response and the ability of these vaccines to confer cross-protective immunity are still unclear. However, not many vaccine studies on layer performance post-vaccination are available.
6.9.4. Ghost Vaccine
In the past, Salmonella ghost vaccines have been extensively tested in rat models [205]. Bacterial ghosts are dead bacterial cell structures retaining surface antigenic LPS components without cytoplasmic contents made from Gram-negative bacteria. The lysis of Gram-negative bacteria mediated by the phage protein E produces bacterial ghosts [206]. A S. Enteritidis ghosts (SEGs) vaccine made by chemically mediated lysis was tested in rats challenged with virulent S. Enteritidis to assess its efficacy and capability to confer immune protection. They reported a significant increase in serum IgG antibody levels in SEGs-vaccinated rats [205].
Moreover, further studies have studied the safety of the ghost vaccines, as the E-gene-mediated lysis process, driven by the osmotic pressure-led bacterial cell emptying, is reportedly inefficient [207]. Chetan et al. studied the efficacy of the S. Enteritidis (SE) ghost vaccine, SE-LTB ghost, and a commercial vaccine. They found rapid clearance of the bacterial load in all groups post-SE challenge and an absence of local tissue reactions at the injection site. They also observed an increased level of IgG titer in SE-LTB-immunized birds [208].
More recently, S. Enteritidis ghost vaccines have been produced and adjuvanted with ST flagellin antigen (ST FliC). Birds immunized with the ghost vaccine demonstrated significant clearance of the SE wild-type challenge strain from the spleen and liver, increased serum IgY antibody levels, and an improved cell-mediated immune response [209]. Together, the above data indicate the paramount importance of further research into ghost vaccines as potential Salmonella vaccine candidates. However, it is important to note that since they are non-living cells, they cannot penetrate the gut epithelium and trigger a potent mucosal immune response, which could be a potential challenge when using the ghost vaccine to control Salmonella in chickens.
Salmonella, an intra-phagosomal enteropathogenic bacterium, has been used as a carrier for poultry DNA vaccines. DNA vaccines are considered safer than live vaccines because they are based on genes of antigenic proteins ligated to a plasmid, which is then transformed into attenuated S. Typhimurium and administered to birds [210]. The capacity of attenuated S. Typhimurium to elicit strong mucosal and systemic immune responses within the host is a beneficial feature for using it as a live carrier to combat a range of poultry viral infections [211,212]. Some advantages of DNA vaccines include no risk of infection and minimal interference with passive maternal antibodies [213]. DNA vaccines can be administered by intramuscular, oral, and in ovo routes in poultry [214]. Despite all its benefits, concerns about integrating DNA into the host genome and the development of anti-DNA antibodies are the central claims raised against DNA vaccines [213]. Figure 3 summarizes the control strategies against Salmonella in poultry.
Figure 3.
Salmonella control strategies in poultry. Created with Biorender.com (accessed on 26 July 2023).
7. Conclusions
With the increasing global demand for poultry meat and egg products, ensuring safe and hygienic poultry management is critical. A multi-intervention strategy that reduces the Salmonella bacterial load in birds and ultimately prevents carcass contamination in processing plants should be given more importance in the current era of multi-drug-resistant Salmonella. At present, there are a plethora of options claiming to reduce Salmonella in poultry rearing, but it is critical to exercise caution before implementing any of those approaches on a large scale. Additionally, continuous research should be performed to ensure the safety of all available novel control strategies. Further efforts should be made to study alternative vaccination strategies that can promote long-lasting immunity and understand the various virulence mechanisms of the zoonotic bacteria that can impair the effectiveness of the immune response induced in the birds.
Author Contributions
S.S.: Writing—original draft preparation; S.S.: Writing, R.S.: review, editing, and funding acquisition; R.K.S.: editing and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by USDA cooperative grant 58-6040-2-016 and hatch grant to R.K.S. and USDA ARS award number 6040-42000-046-000D to R.S.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jung B., Park S., Kim E., Yoon H., Hahn T. Salmonella Typhimurium lacking phoBR as a live vaccine candidate against poultry infection. Vet. Microbiol. 2022;266:109342. doi: 10.1016/j.vetmic.2022.109342. [DOI] [PubMed] [Google Scholar]
- 2.Majowicz S.E., Musto J., Scallan E., Angulo F.J., Kirk M., O’brien S.J., Jones T.F., Fazil A., Hoekstra R.M. International Collaboration on Enteric Disease “Burden of Illness” Studies. Glob. Burd. Nontyphoidal Salmonella Gastroenteritis Clin. Infect. Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 3.Cosby D.E., Cox N.A., Harrison M.A., Wilson J.L., Buhr R.J., Fedorka-Cray P.J. Salmonella and antimicrobial resistance in broilers: A review. J. Appl. Poult. Res. 2015;24:408–426. doi: 10.3382/japr/pfv038. [DOI] [Google Scholar]
- 4.Tajkarimi M. Salmonella spp. [(accessed on 19 June 2023)];Calif. Dep. Food Agric. 2007 :1–8. Available online: https://www.cdfa.ca.gov/ahfss/Animal_Health/PHR250/2007/25007Sal.pdf. [Google Scholar]
- 5.Howard Z.R., O’Bryan C.A., Crandall P.G., Ricke S.C. Salmonella Enteritidis in shell eggs: Current issues and prospects for control. Food Res. Int. 2012;45:755–764. doi: 10.1016/j.foodres.2011.04.030. [DOI] [Google Scholar]
- 6.Ricke S.C., Kim S.A., Shi Z., Park S.H. Molecular-based identification and detection of Salmonella in food production systems: Current perspectives. J. Appl. Microbiol. 2018;125:313–327. doi: 10.1111/jam.13888. [DOI] [PubMed] [Google Scholar]
- 7.Chousalkar K., Gast R., Martelli F., Pande V. Review of egg-related salmonellosis and reduction strategies in United States, Australia, United Kingdom and New Zealand. Crit. Rev. Microbiol. 2018;44:290–303. doi: 10.1080/1040841X.2017.1368998. [DOI] [PubMed] [Google Scholar]
- 8.Painter J.A., Hoekstra R.M., Ayers T., Tauxe R.V., Braden C.R., Angulo F.J., Griffin P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013;19:407. doi: 10.3201/eid1903.111866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gieraltowski L., Higa J., Peralta V.I., Green A., Schwensohn C., Rosen H., Libby T., Kissler B., Marsden-Haug N., Booth H. National outbreak of multidrug resistant Salmonella Heidelberg infections linked to a single poultry company. PLoS ONE. 2016;11:e0162369. doi: 10.1371/journal.pone.0162369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scharff R.L. Food Attribution and Economic Cost Estimates for Meat- and Poultry-Related Illnesses. J. Food Prot. 2020;83:959–967. doi: 10.4315/JFP-19-548. [DOI] [PubMed] [Google Scholar]
- 11.Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.E. Prebiotics can restrict Salmonella populations in poultry: A review. Anim. Biotechnol. 2022;33:1668–1677. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- 12.Bhunia A.K. Foodborne Microbial Pathogens: Mechanisms and Pathogenesis. Springer; New York, NY, USA: 2008. Salmonella enterica; pp. 271–287. [Google Scholar]
- 13.Wibisono F.M., Wibisono F.J., Effendi M.H., Plumeriastuti H., Hidayatullah A.R., Hartadi E.B., Sofiana E.D. A review of salmonellosis on poultry farms: Public health importance. Syst. Rev. Pharm. 2020;11:481–486. [Google Scholar]
- 14.Center for Disease Control and Prevention Salmonella. [(accessed on 30 June 2023)]; Available online: https://www.cdc.gov/Salmonella/index.html.
- 15.O’Bryan C.A., Ricke S.C., Marcy J.A. Public health impact of Salmonella spp. on raw poultry: Current concepts and future prospects in the United States. Food Control. 2022;132:108539. doi: 10.1016/j.foodcont.2021.108539. [DOI] [Google Scholar]
- 16.De Reu K., Grijspeerdt K., Messens W., Heyndrickx M., Uyttendaele M., Debevere J., Herman L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006;112:253–260. doi: 10.1016/j.ijfoodmicro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Padron M. Salmonella typhimurium penetration through the eggshell of hatching eggs. Avian Dis. 1990;34:463–465. [PubMed] [Google Scholar]
- 18.Kopanic R.J., Jr., Sheldon B.W., Wright C.G. Cockroaches as vectors of Salmonella: Laboratory and field trials. J. Food Prot. 1994;57:125–131. doi: 10.4315/0362-028X-57.2.125. [DOI] [PubMed] [Google Scholar]
- 19.Sparagano O. Control of poultry mites: Where do we stand? Exp. Appl. Acarol. 2009;48:1–2. doi: 10.1007/s10493-009-9259-x. [DOI] [PubMed] [Google Scholar]
- 20.Leffer A.M., Kuttel J., Martins L.M., Pedroso A.C., Astolfi-Ferreira C.S., Ferreira F., Ferreira A.J.P. Vectorial competence of larvae and adults of Alphitobius diaperinus in the transmission of Salmonella Enteritidis in poultry. Vector-Borne Zoonotic Dis. 2010;10:481–487. doi: 10.1089/vbz.2008.0089. [DOI] [PubMed] [Google Scholar]
- 21.Meerburg B.G., Kijlstra A. Role of rodents in transmission of Salmonella and Campylobacter. J. Sci. Food Agric. 2007;87:2774–2781. doi: 10.1002/jsfa.3004. [DOI] [Google Scholar]
- 22.Raufu I.A., Ahmed O.A., Aremu A., Odetokun I.A., Raji M.A. Salmonella transmission in poultry farms: The roles of rodents, lizards and formites. Savannah Vet. J. 2019;2:1–4. [Google Scholar]
- 23.Hughes L.A., Shopland S., Wigley P., Bradon H., Leatherbarrow A.H., Williams N.J., Bennett M., de Pinna E., Lawson B., Cunningham A.A. Characterisation of Salmonella enterica serotype Typhimurium isolates from wild birds in northern England from 2005–2006. BMC Vet. Res. 2008;4:4. doi: 10.1186/1746-6148-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alley M.R., Connolly J.H., Fenwick S.G., Mackereth G.F., Leyland M.J., Rogers L.E., Haycock M., Nicol C., Reed C. An epidemic of salmonellosis caused by Salmonella Typhimurium DT160 in wild birds and humans in New Zealand. N. Z. Vet. J. 2002;50:170–176. doi: 10.1080/00480169.2002.36306. [DOI] [PubMed] [Google Scholar]
- 25.Sousa E., Werther K., Berchieri Júnior A. Assessment of Newcastle and infectious bronchitis pathogens, and Salmonella spp. in wild birds captured near poultry facilities. Arq. Bras. Med. Veterinária Zootec. 2010;62:219–223. doi: 10.1590/S0102-09352010000100031. [DOI] [Google Scholar]
- 26.Fu Y., M’ikanatha N.M., Lorch J.M., Blehert D.S., Berlowski-Zier B., Whitehouse C.A., Li S., Deng X., Smith J.C., Shariat N.W. Salmonella enterica Serovar Typhimurium Isolates from Wild Birds in the United States Represent Distinct Lineages Defined by Bird Type. Appl. Environ. Microbiol. 2022;88:1979. doi: 10.1128/aem.01979-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obukhovska O. The natural reservoirs of Salmonella Enteritidis in populations of wild birds. Online J. Public Health Inform. 2013;5 doi: 10.5210/ojphi.v5i1.4569. [DOI] [Google Scholar]
- 28.Authority E.F.S. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSa J. 2018;16:e05500. doi: 10.2903/j.efsa.2018.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yavari L. Antibiotic Resistance in Salmonella enterica and the Role of Animal and Animal Food Control A literature review of Europe and USA. 2012. [(accessed on 3 July 2023)]. Available online: https://www.researchgate.net/profile/Leila-Yavari/publication/280531461_Antibiotic_Resistance_in_Salmonella_enterica_and_the_Role_of_Animal_and_Animal_Food_Control/links/55b7b5b608aed621de048679/Antibiotic-Resistance-in-Salmonella-enterica-and-the-Role-of-Animal-and-Animal-Food-Control.
- 30.Lüderitz O., Westphal O., Staub A.M., Nikaido H. Isolation and chemical and immunological characterization of bacterial lipopolysaccharides. Microb. Toxins. 2016;4:145–233. [Google Scholar]
- 31.Lüderitz O., Staub A.M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol. Rev. 1966;30:192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeves M.W., Evins G.M., Heiba A.A., Plikaytis B.D., Farmer J.J., 3rd Clonal nature of Salmonella typhi and its genetic relatedness to other salmonellae as shown by multilocus enzyme electrophoresis, and proposal of Salmonella bongori comb. nov. J. Clin. Microbiol. 1989;27:313–320. doi: 10.1128/jcm.27.2.313-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dworkin M., Falkow S., Rosenberg E., Stackebrandt E., Schleifer K. The Prokaryotes: A Handbook on the Biology of Bacteria. Volume 7 Springer; New York, NY, USA: 2006. [Google Scholar]
- 34.Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M., Roy S.L., Jones J.L., Griffin P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arya G., Holtslander R., Robertson J., Yoshida C., Harris J., Parmley J., Nichani A., Johnson R., Poppe C. Epidemiology, pathogenesis, genoserotyping, antimicrobial resistance, and prevention and control of non-typhoidal Salmonella serovars. Curr. Clin. Microbiol. Rep. 2017;4:43–53. doi: 10.1007/s40588-017-0057-7. [DOI] [Google Scholar]
- 36.Old D.C. Nomenclature of Salmonella. J. Med. Microbiol. 1992;37:361–363. doi: 10.1099/00222615-37-6-361. [DOI] [PubMed] [Google Scholar]
- 37.Chattaway M.A., Langridge G.C., Wain J. Salmonella nomenclature in the genomic era: A time for change. Sci. Rep. 2021;11:7494. doi: 10.1038/s41598-021-86243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimont P.A., Weill F. Antigenic formulae of the Salmonella serovars. WHO Collab. Cent. Ref. Res. Salmonella. 2007;9:1–166. [Google Scholar]
- 39.Carter A.J., Adams M.R., Woodward M.J., La Ragione R.M. Control strategies for Salmonella colonization of poultry: The probiotic perspective. Food Sci. Technol. 2009;5:103–115. [Google Scholar]
- 40.Hickman-Brenner F.W., Stubbs A.D., Farmer J.J., 3rd Phage typing of Salmonella enteritidis in the United States. J. Clin. Microbiol. 1991;29:2817–2823. doi: 10.1128/jcm.29.12.2817-2823.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatta D.R., Bangtrakulnonth A., Tishyadhigama P., Saroj S.D., Bandekar J.R., Hendriksen R.S., Kapadnis B.P. Serotyping, PCR, phage-typing and antibiotic sensitivity testing of Salmonella serovars isolated from urban drinking water supply systems of Nepal. Lett. Appl. Microbiol. 2007;44:588–594. doi: 10.1111/j.1472-765X.2007.02133.x. [DOI] [PubMed] [Google Scholar]
- 42.Crabb H.K., Allen J.L., Devlin J.M., Firestone S.M., Stevenson M., Wilks C.R., Gilkerson J.R. Traditional Salmonella Typhimurium typing tools (phage typing and MLVA) are sufficient to resolve well-defined outbreak events only. Food Microbiol. 2019;84:103237. doi: 10.1016/j.fm.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Ha A.J., Perez L.G.S., Kim T., Mizan M.F.R., Nahar S., Park S., Chun H., Ha S. Research Note: Identification and characterization of Salmonella spp. in mechanically deboned chickens using pulsed-field gel electrophoresis. Poult. Sci. 2021;100:100961. doi: 10.1016/j.psj.2020.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan S.A., Foley S., Stefanova R. Subtyping of Salmonella enterica Serovar Typhimurium from Clinical Samples by Multiple-Locus Variable-Number Tandem Repeat Analysis and Pulsed-Field Gel Electrophoresis. Microbiol. Infect. Dis. 2019;3:1–7. doi: 10.33425/2639-9458.1069. [DOI] [Google Scholar]
- 45.Mechesso A.F., Moon D.C., Kim S., Song H., Kang H.Y., Na S.H., Choi J., Kim H., Yoon S., Lim S. Nationwide surveillance on serotype distribution and antimicrobial resistance profiles of non-typhoidal Salmonella serovars isolated from food-producing animals in South Korea. Int. J. Food Microbiol. 2020;335:108893. doi: 10.1016/j.ijfoodmicro.2020.108893. [DOI] [PubMed] [Google Scholar]
- 46.Ferrato C., Chui L., King R., Louie M. Utilization of a molecular serotyping method for Salmonella enterica in a routine laboratory in Alberta Canada. J. Microbiol. Methods. 2017;135:14–19. doi: 10.1016/j.mimet.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 47.Mortimer C.K., Peters T.M., Gharbia S.E., Logan J.M., Arnold C. Towards the development of a DNA-sequence based approach to serotyping of Salmonella enterica. BMC Microbiol. 2004;4:31. doi: 10.1186/1471-2180-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diep B., Barretto C., Portmann A., Fournier C., Karczmarek A., Voets G., Li S., Deng X., Klijn A. Salmonella serotyping; comparison of the traditional method to a microarray-based method and an in silico platform using whole genome sequencing data. Front. Microbiol. 2019;10:2554. doi: 10.3389/fmicb.2019.02554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higginson E.E., Simon R., Tennant S.M. Animal models for salmonellosis: Applications in vaccine research. Clin. Vaccine Immunol. 2016;23:746–756. doi: 10.1128/CVI.00258-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broz P., Ohlson M.B., Monack D.M. Innate immune response to Salmonella typhimurium, a model enteric pathogen. Gut Microbes. 2012;3:62–70. doi: 10.4161/gmic.19141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobayashi N., Takahashi D., Takano S., Kimura S., Hase K. The roles of Peyer’s patches and microfold cells in the gut immune system: Relevance to autoimmune diseases. Front. Immunol. 2019;10:2345. doi: 10.3389/fimmu.2019.02345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buchmeier N.A., Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect. Immun. 1991;59:2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velge P., Cloeckaert A., Barrow P. Emergence of Salmonella epidemics: The problems related to Salmonella enterica serotyp Enteritidis and multiple antibiotic resistance in other major serotypes. Vet. Res. 2005;36:267–288. doi: 10.1051/vetres:2005005. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization . Background Document: The Diagnosis, Treatment and Prevention of Typhoid Fever. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 55.Coburn B., Grassl G.A., Finlay B.B. Salmonella, the host and disease: A brief review. Immunol. Cell Biol. 2007;85:112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 56.Bhunia A.K. Foodborne Microbial Pathogens: Mechanisms and Pathogenesis. Springer; New York, NY, USA: 2018. [Google Scholar]
- 57.Knuff K., Finlay B.B. What the SIF is happening—The role of intracellular Salmonella-induced filaments. Front. Cell. Infect. Microbiol. 2017;7:335. doi: 10.3389/fcimb.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGourty K., Thurston T.L., Matthews S.A., Pinaud L., Mota L.J., Holden D.W. Salmonella inhibits retrograde trafficking of mannose-6-phosphate receptors and lysosome function. Science. 2012;338:963–967. doi: 10.1126/science.1227037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tahoun A., Mahajan S., Paxton E., Malterer G., Donaldson D.S., Wang D., Tan A., Gillespie T.L., O’Shea M., Roe A.J. Salmonella transforms follicle-associated epithelial cells into M cells to promote intestinal invasion. Cell Host Microbe. 2012;12:645–656. doi: 10.1016/j.chom.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 60.Dieye Y., Ameiss K., Mellata M., Curtiss R. The Salmonella Pathogenicity Island (SPI) 1 contributes more than SPI2 to the colonization of the chicken by Salmonella enterica serovar Typhimurium. BMC Microbiol. 2009;9:3. doi: 10.1186/1471-2180-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jones M.A., Hulme S.D., Barrow P.A., Wigley P. The Salmonella pathogenicity island 1 and Salmonella pathogenicity island 2 type III secretion systems play a major role in pathogenesis of systemic disease and gastrointestinal tract colonization of Salmonella enterica serovar Typhimurium in the chicken. Avian Pathol. 2007;36:199–203. doi: 10.1080/03079450701264118. [DOI] [PubMed] [Google Scholar]
- 62.Pico-Rodríguez J.T., Martínez-Jarquín H., Gómez-Chávez J.D.J., Juárez-Ramírez M., Martínez-Chavarría L.C. Effect of Salmonella pathogenicity island 1 and 2 (SPI-1 and SPI-2) deletion on intestinal colonization and systemic dissemination in chickens. Vet. Res. Commun. 2023:1–12. doi: 10.1007/s11259-023-10185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merino L., Trejo F.M., De Antoni G., Golowczyc M.A. Lactobacillus strains inhibit biofilm formation of Salmonella sp. isolates from poultry. Food Res. Int. 2019;123:258–265. doi: 10.1016/j.foodres.2019.04.067. [DOI] [PubMed] [Google Scholar]
- 64.Barrow P.A. Salmonella infections: Immune and non-immune protection with vaccines. Avian Pathol. 2007;36:1–13. doi: 10.1080/03079450601113167. [DOI] [PubMed] [Google Scholar]
- 65.Mon K.K., Zhu Y., Chanthavixay G., Kern C., Zhou H. Integrative analysis of gut microbiome and metabolites revealed novel mechanisms of intestinal Salmonella carriage in chicken. Sci. Rep. 2020;10:4809. doi: 10.1038/s41598-020-60892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbas A., Lichtman A., Pillai S. Cellular and Molecular Immunology E-Book. Elsevier Health Sciences; Amsterdam, The Netherlands: 2014. [Google Scholar]
- 67.Brisbin J.T., Gong J., Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health Res. Rev. 2008;9:101–110. doi: 10.1017/S146625230800145X. [DOI] [PubMed] [Google Scholar]
- 68.Stanley D., Geier M.S., Hughes R.J., Denman S.E., Moore R.J. Highly variable microbiota development in the chicken gastrointestinal tract. PLoS ONE. 2013;8:e84290. doi: 10.1371/journal.pone.0084290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Muir W.I., Bryden W.L., Husband A.J. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 2000;24:325–342. doi: 10.1016/S0145-305X(99)00081-6. [DOI] [PubMed] [Google Scholar]
- 70.Lillehoj H.S., Trout J.M. Avian gut-associated lymphoid tissues and intestinal immune responses to Eimeria parasites. Clin. Microbiol. Rev. 1996;9:349–360. doi: 10.1128/CMR.9.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Temperley N.D., Berlin S., Paton I.R., Griffin D.K., Burt D.W. Evolution of the chicken Toll-like receptor gene family: A story of gene gain and gene loss. BMC Genom. 2008;9:62. doi: 10.1186/1471-2164-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Werling D., Hope J.C., Howard C.J., Jungi T.W. Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists. Immunology. 2004;111:41–52. doi: 10.1111/j.1365-2567.2004.01781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okamura M., Matsumoto W., Seike F., Tanaka Y., Teratani C., Tozuka M., Kashimoto T., Takehara K., Nakamura M., Yoshikawa Y. Efficacy of soluble recombinant FliC protein from Salmonella enterica serovar Enteritidis as a potential vaccine candidate against homologous challenge in chickens. Avian Dis. 2012;56:354–358. doi: 10.1637/9986-111011-Reg.1. [DOI] [PubMed] [Google Scholar]
- 74.Khan S., Chousalkar K.K. Transcriptome profiling analysis of caeca in chicks challenged with Salmonella Typhimurium reveals differential expression of genes involved in host mucosal immune response. Appl. Microbiol. Biotechnol. 2020;104:9327–9342. doi: 10.1007/s00253-020-10887-3. [DOI] [PubMed] [Google Scholar]
- 75.MacKinnon K.M., He H., Nerren J.R., Swaggerty C.L., Genovese K.J., Kogut M.H. Expression profile of toll-like receptors within the gastrointestinal tract of 2-day-old Salmonella enteriditis-infected broiler chickens. Vet. Microbiol. 2009;137:313–319. doi: 10.1016/j.vetmic.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 76.Genovese K.J., He H., Swaggerty C.L., Kogut M.H. The avian heterophil. Dev. Comp. Immunol. 2013;41:334–340. doi: 10.1016/j.dci.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 77.Ferro P.J., Swaggerty C.L., Kaiser P., Pevzner I.Y., Kogut M.H. Heterophils isolated from chickens resistant to extra-intestinal Salmonella enteritidis infection express higher levels of pro-inflammatory cytokine mRNA following infection than heterophils from susceptible chickens. Epidemiol. Infect. 2004;132:1029–1037. doi: 10.1017/S0950268804002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kogut M.H., Iqbal M., He H., Philbin V., Kaiser P., Smith A. Expression and function of Toll-like receptors in chicken heterophils. Dev. Comp. Immunol. 2005;29:791–807. doi: 10.1016/j.dci.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Kogut M.H., Chiang H., Swaggerty C.L., Pevzner I.Y., Zhou H. Gene expression analysis of Toll-like receptor pathways in heterophils from genetic chicken lines that differ in their susceptibility to Salmonella enteritidis. Front. Genet. 2012;3:121. doi: 10.3389/fgene.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jie W., Zhang Q., Sánchez A.L.B., Bo Z., Qiao W., Zheng M., Li Q., Cui H., Jie W., Zhao G. Transcriptome analysis of the spleen of heterophils to lymphocytes ratio-selected chickens revealed their mechanism of differential resistance to Salmonella. J. Integr. Agric. 2022;21:2372–2383. [Google Scholar]
- 81.Thiam M., Barreto Sánchez A.L., Zhang J., Zheng M., Wen J., Zhao G., Wang Q. Association of heterophil/lymphocyte ratio with intestinal barrier function and immune response to salmonella enteritidis infection in chicken. Animals. 2021;11:3498. doi: 10.3390/ani11123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Withanage G., Wigley P., Kaiser P., Mastroeni P., Brooks H., Powers C., Beal R., Barrow P., Maskell D., McConnell I. Cytokine and chemokine responses associated with clearance of a primary Salmonella enterica serovar Typhimurium infection in the chicken and in protective immunity to rechallenge. Infect. Immun. 2005;73:5173–5182. doi: 10.1128/IAI.73.8.5173-5182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Withanage G., Sasai K., Fukata T., Miyamoto T., Lillehoj H.S., Baba E. Increased lymphocyte subpopulations and macrophages in the ovaries and oviducts of laying hens infected with Salmonella enterica serovar Enteritidis. Avian Pathol. 2003;32:583–590. doi: 10.1080/03079450310001610631. [DOI] [PubMed] [Google Scholar]
- 84.Sáenz L., Guzmán M., Vidal S., Caruffo M., Siel D., Zayas C., Paredes R., Valenzuela C., Hidalgo H., Pérez O. Efficacy of multivalent, cochleate-based vaccine against Salmonella Infantis, S. Enteritidis and S. Typhimurium in laying hens. Vaccines. 2022;10:226. doi: 10.3390/vaccines10020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schokker D., Peters T., Hoekman A., Rebel J., Smits M.A. Differences in the early response of hatchlings of different chicken breeding lines to Salmonella enterica serovar Enteritidis infection. Poult. Sci. 2012;91:346–353. doi: 10.3382/ps.2011-01758. [DOI] [PubMed] [Google Scholar]
- 86.Meijerink N., Van den Biggelaar R.H., Van Haarlem D.A., Stegeman J.A., Rutten V.P., Jansen C.A. A detailed analysis of innate and adaptive immune responsiveness upon infection with Salmonella enterica serotype Enteritidis in young broiler chickens. Vet. Res. 2021;52:1–21. doi: 10.1186/s13567-021-00978-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sutton K.M., Morris K.M., Borowska D., Sang H., Kaiser P., Balic A., Vervelde L. Characterization of conventional dendritic cells and macrophages in the spleen using the CSF1R-reporter transgenic chickens. Front. Immunol. 2021;12:636436. doi: 10.3389/fimmu.2021.636436. [DOI] [Google Scholar]
- 88.Shanmugasundaram R., Kogut M.H., Arsenault R.J., Swaggerty C.L., Cole K., Reddish J.M., Selvaraj R.K. Effect of Salmonella infection on cecal tonsil regulatory T cell properties in chickens. Poult. Sci. 2015;94:1828–1835. doi: 10.3382/ps/pev161. [DOI] [PubMed] [Google Scholar]
- 89.Shanmugasundaram R., Acevedo K., Mortada M., Akerele G., Applegate T.J., Kogut M.H., Selvaraj R.K. Effects of Salmonella enterica ser. Enteritidis and Heidelberg on host CD4 CD25 regulatory T cell suppressive immune responses in chickens. PLoS ONE. 2021;16:e0260280. doi: 10.1371/journal.pone.0260280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Penha Filho R.A.C., Moura B.S., de Almeida A.M., Montassier H.J., Barrow P.A., Junior A.B. Humoral and cellular immune response generated by different vaccine programs before and after Salmonella Enteritidis challenge in chickens. Vaccine. 2012;30:7637–7643. doi: 10.1016/j.vaccine.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 91.Chaussé A., Grépinet O., Bottreau E., Robert V., Hennequet-Antier C., Lalmanach A., Lecardonnel J., Beaumont C., Velge P. Susceptibility to Salmonella carrier-state: A possible Th2 response in susceptible chicks. Vet. Immunol. Immunopathol. 2014;159:16–28. doi: 10.1016/j.vetimm.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Cheeseman J.H. Avian Immunology, Immunogenetics, and Host Immune Response to Salmonella Enterica Serovar Enteritidis Infection in Chickens. Iowa State University; Ames, IA, USA: 2007. [Google Scholar]
- 93.Beal R.K., Wigley P., Powers C., Hulme S.D., Barrow P.A., Smith A.L. Age at primary infection with Salmonella enterica serovar Typhimurium in the chicken influences persistence of infection and subsequent immunity to re-challenge. Vet. Immunol. Immunopathol. 2004;100:151–164. doi: 10.1016/j.vetimm.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Withanage G., Sasai K., Fukata T., Miyamoto T., Baba E., Lillehoj H.S. T lymphocytes, B lymphocytes, and macrophages in the ovaries and oviducts of laying hens experimentally infected with Salmonella enteritidis. Vet. Immunol. Immunopathol. 1998;66:173–184. doi: 10.1016/S0165-2427(98)00177-9. [DOI] [PubMed] [Google Scholar]
- 95.Okamura M., Lillehoj H.S., Raybourne R.B., Babu U.S., Heckert R.A. Cell-mediated immune responses to a killed Salmonella enteritidis vaccine: Lymphocyte proliferation, T-cell changes and interleukin-6 (IL-6), IL-1, IL-2, and IFN-γ production. Comp. Immunol. Microbiol. Infect. Dis. 2004;27:255–272. doi: 10.1016/j.cimid.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 96.Xu S., Cao X. Interleukin-17 and its expanding biological functions. Cell. Mol. Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Restif O., Goh Y.S., Palayret M., Grant A.J., McKinley T.J., Clark M.R., Mastroeni P. Quantification of the effects of antibodies on the extra-and intracellular dynamics of Salmonella enterica. J. R. Soc. Interface. 2013;10:20120866. doi: 10.1098/rsif.2012.0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Männe C., Takaya A., Yamasaki Y., Mursell M., Hojyo S., Wu T., Sarkander J., McGrath M.A., Cornelis R., Hahne S. Salmonella SiiE prevents an efficient humoral immune memory by interfering with IgG plasma cell persistence in the bone marrow. Proc. Natl. Acad. Sci. USA. 2019;116:7425–7430. doi: 10.1073/pnas.1818242116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dar M.A., Urwat U., Ahmad S.M., Ahmad R., Kashoo Z.A., Dar T.A., Bhat S.A., Mumtaz P.T., Shabir N., Shah R.A. Gene expression and antibody response in chicken against Salmonella Typhimurium challenge. Poult. Sci. 2019;98:2008–2013. doi: 10.3382/ps/pey560. [DOI] [PubMed] [Google Scholar]
- 100.Arnold J.W., Holt P.S. Response to Salmonella enteritidis infection by the immunocompromised avian host. Poult. Sci. 1995;74:656–665. doi: 10.3382/ps.0740656. [DOI] [PubMed] [Google Scholar]
- 101.Beal R.K., Powers C., Davison T.F., Barrow P.A., Smith A.L. Clearance of enteric Salmonella enterica serovar Typhimurium in chickens is independent of B-cell function. Infect. Immun. 2006;74:1442–1444. doi: 10.1128/IAI.74.2.1442-1444.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fraser R.W., Williams N.T., Powell L.F., Cook A. Reducing Campylobacter and salmonella infection: Two studies of the economic cost and attitude to adoption of on-farm biosecurity measures. Zoonoses Public Health. 2010;57:e109–e115. doi: 10.1111/j.1863-2378.2009.01295.x. [DOI] [PubMed] [Google Scholar]
- 103.Gosling R.J., Martelli F., Wintrip A., Sayers A.R., Wheeler K., Davies R.H. Assessment of producers’ response to Salmonella biosecurity issues and uptake of advice on laying hen farms in England and Wales. Br. Poult. Sci. 2014;55:559–568. doi: 10.1080/00071668.2014.949620. [DOI] [PubMed] [Google Scholar]
- 104.Sylejmani D., Musliu A., Ramadani N., Sparagano O., Hamidi A. Associations between the level of biosecurity and occurrence of Dermanyssus gallinae and Salmonella spp. in layer farms. Avian Dis. 2016;60:454–459. doi: 10.1637/11327-111415-Reg. [DOI] [PubMed] [Google Scholar]
- 105.Volkova V.V., Wills R.W., Hubbard S.A., Magee D.L., Byrd J.A., Bailey R.H. Risk factors associated with detection of Salmonella in broiler litter at the time of new flock placement. Zoonoses Public Health. 2011;58:158–168. doi: 10.1111/j.1863-2378.2009.01323.x. [DOI] [PubMed] [Google Scholar]
- 106.Eid S., Hassan H.M., Al-Atfeehy N.M., Selim K.M., El Oksh A.S. Composting: A biosecurity measure to maximize the benefit of broilers’ litter. Anim. Res. 2023;10:458–468. doi: 10.5455/javar.2023.j699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdulghaffar T.A., El Bahgy H.E. Effect of some disinfectants on some pathogenic microorganisms isolated from poultry farm. Benha Vet. Med. J. 2016;31:154–158. [Google Scholar]
- 108.Meher M.M., Sharif M.A., Al Bayazid A. Seroprevalence of Salmonella spp. infection in different types of poultry and biosecurity measures associated with Salmonellosis. Int. J. Agric. Environ. Food Sci. 2022;6:557–567. doi: 10.31015/jaefs.2022.4.8. [DOI] [Google Scholar]
- 109.Alagawany M., Abd El-Hack M.E., Farag M.R., Sachan S., Karthik K., Dhama K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018;25:10611–10618. doi: 10.1007/s11356-018-1687-x. [DOI] [PubMed] [Google Scholar]
- 110.Marshall B.M., Levy S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eckert N.H., Lee J.T., Hyatt D., Stevens S.M., Anderson S., Anderson P.N., Beltran R., Schatzmayr G., Mohnl M., Caldwell D.J. Influence of probiotic administration by feed or water on growth parameters of broilers reared on medicated and nonmedicated diets. J. Appl. Poult. Res. 2010;19:59–67. doi: 10.3382/japr.2009-00084. [DOI] [Google Scholar]
- 112.Karon A.E., Archer J.R., Sotir M.J., Monson T.A., Kazmierczak J.J. Human multidrug-resistant Salmonella newport infections, Wisconsin, 2003–2005. Emerg. Infect. Dis. 2007;13:1777. doi: 10.3201/eid1311.061138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Palamidi I., Fegeros K., Mohnl M., Abdelrahman W., Schatzmayr G., Theodoropoulos G., Mountzouris K.C. Probiotic form effects on growth performance, digestive function, and immune related biomarkers in broilers. Poult. Sci. 2016;95:1598–1608. doi: 10.3382/ps/pew052. [DOI] [PubMed] [Google Scholar]
- 114.Gibson G.R., Hutkins R., Sanders M.E., Prescott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 115.Pineiro M., Asp N., Reid G., Macfarlane S., Morelli L., Brunser O., Tuohy K. FAO Technical meeting on prebiotics. J. Clin. Gastroenterol. 2008;42:S156–S159. doi: 10.1097/MCG.0b013e31817f184e. [DOI] [PubMed] [Google Scholar]
- 116.Ricke S.C., Lee S.I., Kim S.A., Park S.H., Shi Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020;99:670–677. doi: 10.1016/j.psj.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hajati H., Rezaei M. The application of prebiotics in poultry production. Int. J. Poult. Sci. 2010;9:298–304. doi: 10.3923/ijps.2010.298.304. [DOI] [Google Scholar]
- 118.Bogusławska-Tryk M., Piotrowska A., Burlikowska K. Dietary fructans and their potential beneficial influence on health and performance parametrs in broiler chickens. [(accessed on 10 November 2023)];J. Cent. Eur. Agric. 2012 13 doi: 10.5513/JCEA01/13.2.1045. Available online: https://hrcak.srce.hr/83284. [DOI] [Google Scholar]
- 119.Fomentini M., Haese D., Kill J.L., Sobreiro R.P., Puppo D.D., Haddade I.R., Lima A.L., Saraiva A. Prebiotic and antimicrobials on performance, carcass characteristics, and antibody production in broilers. Ciência Rural. 2016;46:1070–1075. doi: 10.1590/0103-8478cr20150133. [DOI] [Google Scholar]
- 120.Adhikari P.A., Kim W.K. Overview of prebiotics and probiotics: Focus on performance, gut health and immunity—A review. Ann. Anim. Sci. 2017;17:949–966. doi: 10.1515/aoas-2016-0092. [DOI] [Google Scholar]
- 121.Pourabedin M., Guan L., Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3:15. doi: 10.1186/s40168-015-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fathima S., Shanmugasundaram R., Sifri M., Selvaraj R. Yeasts and Yeast-based Products in Poultry Nutrition. J. Appl. Poult. Res. 2023;32:100345. doi: 10.1016/j.japr.2023.100345. [DOI] [Google Scholar]
- 123.Markazi A.D., Perez V., Sifri M., Shanmugasundaram R., Selvaraj R.K. Effect of whole yeast cell product supplementation (CitriStim®) on immune responses and cecal microflora species in pullet and layer chickens during an experimental coccidial challenge. Poult. Sci. 2017;96:2049–2056. doi: 10.3382/ps/pew482. [DOI] [PubMed] [Google Scholar]
- 124.Shanmugasundaram R., Selvaraj R.K. Effect of killed whole yeast cell prebiotic supplementation on broiler performance and intestinal immune cell parameters. Poult. Sci. 2012;91:107–111. doi: 10.3382/ps.2011-01732. [DOI] [PubMed] [Google Scholar]
- 125.Kim G., Seo Y.M., Kim C.H., Paik I.K. Effect of dietary prebiotic supplementation on the performance, intestinal microflora, and immune response of broilers. Poult. Sci. 2011;90:75–82. doi: 10.3382/ps.2010-00732. [DOI] [PubMed] [Google Scholar]
- 126.Adhikari P., Cosby D.E., Cox N.A., Franca M.S., Williams S.M., Gogal R.M., Jr., Ritz C.W., Kim W.K. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis. Poult. Sci. 2018;97:2525–2533. doi: 10.3382/ps/pey101. [DOI] [PubMed] [Google Scholar]
- 127.Wu Y., Yang W., Wu Y.S., Chen J., Chen Y. Modulations of growth performance, gut microbiota, and inflammatory cytokines by trehalose on Salmonella Typhimurium-challenged broilers. Poult. Sci. 2020;99:4034–4043. doi: 10.1016/j.psj.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morelli L., Capurso L. FAO/WHO guidelines on probiotics: 10 years later. J. Clin. Gastroenterol. 2012;46:S1–S2. doi: 10.1097/MCG.0b013e318269fdd5. [DOI] [PubMed] [Google Scholar]
- 129.Lilly D.M., Stillwell R.H. Probiotics: Growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 130.Sánchez B., Delgado S., Blanco-Míguez A., Lourenço A., Gueimonde M., Margolles A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017;61:1600240. doi: 10.1002/mnfr.201600240. [DOI] [PubMed] [Google Scholar]
- 131.Fathima S., Shanmugasundaram R., Adams D., Selvaraj R.K. Gastrointestinal microbiota and their manipulation for improved growth and performance in chickens. Foods. 2022;11:1401. doi: 10.3390/foods11101401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Simon O., Jadamus A., Vahjen W. Probiotic feed additives-effectiveness and expected modes of action. J. Anim. Feed. Sci. 2001;10:51–68. doi: 10.22358/jafs/70012/2001. [DOI] [Google Scholar]
- 133.Kabir S.L., Rahman M.M., Rahman M.B., Rahman M.M., Ahmed S.U. The dynamics of probiotics on growth performance and immune response in broilers. Int. J. Poult. Sci. 2004;3:361–364. [Google Scholar]
- 134.Krysiak K., Konkol D., Korczyński M. Overview of the use of probiotics in poultry production. Animals. 2021;11:1620. doi: 10.3390/ani11061620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Menconi A., Kallapura G., Latorre J.D., Morgan M.J., Pumford N.R., Hargis B.M., Tellez G. Identification and characterization of lactic acid bacteria in a commercial probiotic culture. Biosci. Microbiota Food Health. 2014;33:25–30. doi: 10.12938/bmfh.33.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oh J.K., Pajarillo E.A.B., Chae J.P., Kim I.H., Kang D. Protective effects of Bacillus subtilis against Salmonella infection in the microbiome of Hy-Line Brown layers. Asian-Australas. J. Anim. Sci. 2017;30:1332. doi: 10.5713/ajas.17.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bai S.P., Wu A.M., Ding X.M., Lei Y., Bai J., Zhang K.Y., Chio J.S. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 2013;92:663–670. doi: 10.3382/ps.2012-02813. [DOI] [PubMed] [Google Scholar]
- 138.Khan S., Chousalkar K.K. Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J. Anim. Sci. Biotechnol. 2020;11:29. doi: 10.1186/s40104-020-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shanmugasundaram R., Applegate T.J., Selvaraj R.K. Effect of Bacillus subtilis and Bacillus licheniformis probiotic supplementation on cecal Salmonella load in broilers challenged with salmonella. J. Appl. Poult. Res. 2020;29:808–816. doi: 10.1016/j.japr.2020.07.003. [DOI] [Google Scholar]
- 140.El-Sharkawy H., Tahoun A., Rizk A.M., Suzuki T., Elmonir W., Nassef E., Shukry M., Germoush M.O., Farrag F., Bin-Jumah M. Evaluation of Bifidobacteria and Lactobacillus probiotics as alternative therapy for Salmonella typhimurium infection in broiler chickens. Animals. 2020;10:1023. doi: 10.3390/ani10061023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Menconi A., Bielke L.R., Hargis B.M., Tellez G. Immuno-modulation and anti-inflammatory effects of antibiotic growth promoters versus probiotics in the intestinal tract. J. Microbiol. Res. Rev. 2014;2:62–67. [Google Scholar]
- 142.El-Shall N.A., Awad A.M., El-Hack M.E.A., Naiel M.A., Othman S.I., Allam A.A., Sedeik M.E. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals. 2019;10:70. doi: 10.3390/ani10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dianawati D., Mishra V., Shah N.P. Effect of drying methods of microencapsulated Lactobacillus acidophilus and Lactococcus lactis ssp. cremoris on secondary protein structure and glass transition temperature as studied by Fourier transform infrared and differential scanning calorimetry. J. Dairy Sci. 2013;96:1419–1430. doi: 10.3168/jds.2012-6058. [DOI] [PubMed] [Google Scholar]
- 144.Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 145.Khomayezi R., Adewole D. Probiotics, prebiotics, and synbiotics: An overview of their delivery routes and effects on growth and health of broiler chickens. Worlds Poult. Sci. J. 2022;78:57–81. doi: 10.1080/00439339.2022.1988804. [DOI] [Google Scholar]
- 146.Yadav A.S., Kolluri G., Gopi M., Karthik K., Singh Y. Exploring alternatives to antibiotics as health promoting agents in poultry—A review. J. Exp. Biol. 2016;4:368–383. [Google Scholar]
- 147.Mohammed A.A., Jacobs J.A., Murugesan G.R., Cheng H.W. Effect of dietary synbiotic supplement on behavioral patterns and growth performance of broiler chickens reared under heat stress. Poult. Sci. 2018;97:1101–1108. doi: 10.3382/ps/pex421. [DOI] [PubMed] [Google Scholar]
- 148.Śliżewska K., Markowiak-Kopeć P., Żbikowski A., Szeleszczuk P. The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Sci. Rep. 2020;10:4281. doi: 10.1038/s41598-020-61256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Luoma A., Markazi A., Shanmugasundaram R., Murugesan G.R., Mohnl M., Selvaraj R. Effect of synbiotic supplementation on layer production and cecal Salmonella load during a Salmonella challenge. Poult. Sci. 2017;96:4208–4216. doi: 10.3382/ps/pex251. [DOI] [PubMed] [Google Scholar]
- 150.Villagrán-de la Mora Z., Vázquez-Paulino O., Avalos H., Ascencio F., Nuño K., Villarruel-López A. Effect of a synbiotic mix on lymphoid organs of broilers infected with salmonella typhimurium and clostridium perfringens. Animals. 2020;10:886. doi: 10.3390/ani10050886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aguilar-Toalá J.E., Garcia-Varela R., Garcia H.S., Mata-Haro V., González-Córdova A.F., Vallejo-Cordoba B., Hernández-Mendoza A. Postbiotics: An evolving term within the functional foods field. Trends Food Sci. Technol. 2018;75:105–114. doi: 10.1016/j.tifs.2018.03.009. [DOI] [Google Scholar]
- 152.Abd El-Ghany W.A. Paraprobiotics and postbiotics: Contemporary and promising natural antibiotics alternatives and their applications in the poultry field. Open Vet. J. 2020;10:323–330. doi: 10.4314/ovj.v10i3.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Van Thu T., Foo H.L., Loh T.C., Bejo M.H. Inhibitory activity and organic acid concentrations of metabolite combinations produced by various strains of Lactobacillus plantarum. Afr. J. Biotechnol. 2011;10:1359–1363. [Google Scholar]
- 154.Humam A.M., Loh T.C., Foo H.L., Izuddin W.I., Zulkifli I., Samsudin A.A., Mustapha N.M. Supplementation of postbiotic RI11 improves antioxidant enzyme activity, upregulated gut barrier genes, and reduced cytokine, acute phase protein, and heat shock protein 70 gene expression levels in heat-stressed broilers. Poult. Sci. 2021;100:100908. doi: 10.1016/j.psj.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Humam A.M., Loh T.C., Foo H.L., Samsudin A.A., Mustapha N.M., Zulkifli I., Izuddin W.I. Effects of feeding different postbiotics produced by Lactobacillus plantarum on growth performance, carcass yield, intestinal morphology, gut microbiota composition, immune status, and growth gene expression in broilers under heat stress. Animals. 2019;9:644. doi: 10.3390/ani9090644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kareem K.Y., Loh T.C., Foo H.L., Asmara S.A., Akit H. Influence of postbiotic RG14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 2017;96:966–975. doi: 10.3382/ps/pew362. [DOI] [PubMed] [Google Scholar]
- 157.Choe D.W., Loh T.C., Foo H.L., Hair-Bejo M., Awis Q.S. Egg production, faecal pH and microbial population, small intestine morphology, and plasma and yolk cholesterol in laying hens given liquid metabolites produced by Lactobacillus plantarum strains. Br. Poult. Sci. 2012;53:106–115. doi: 10.1080/00071668.2012.659653. [DOI] [PubMed] [Google Scholar]
- 158.Chaney W.E., Naqvi S.A., Gutierrez M., Gernat A., Johnson T.J., Petry D. Dietary Inclusion of a Saccharomyces cerevisiae-Derived Postbiotic Is Associated with Lower Salmonella enterica Burden in Broiler Chickens on a Commercial Farm in Honduras. Microorganisms. 2022;10:544. doi: 10.3390/microorganisms10030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chaney W.E., McBride H., Girgis G. Effect of a Saccharomyces cerevisiae Postbiotic Feed Additive on Salmonella Enteritidis Colonization of Cecal and Ovarian Tissues in Directly Challenged and Horizontally Exposed Layer Pullets. Animals. 2023;13:1186. doi: 10.3390/ani13071186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang C., Chowdhury M.K., Hou Y., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arain M.A., Nabi F., Shah Q.A., Alagawany M., Fazlani S.A., Khalid M., Soomro F., Khand F.M., Farag M.R. The role of early feeding in improving performance and health of poultry: Herbs and their derivatives. Worlds Poult. Sci. J. 2022;78:499–513. doi: 10.1080/00439339.2022.2043133. [DOI] [Google Scholar]
- 162.Mohammadi Gheisar M., Kim I.H. Phytobiotics in poultry and swine nutrition—A review. Ital. J. Anim. Sci. 2018;17:92–99. doi: 10.1080/1828051X.2017.1350120. [DOI] [Google Scholar]
- 163.Pourali M., Mirghelenj S.A., Kermanshahi H. Effects of garlic powder on productive performance and immune response of broiler chickens challenged with Newcastle Disease Virus. Glob. Vet. 2010;4:616–621. [Google Scholar]
- 164.Chalghoumi R., Belgacem A., Trabelsi I., Bouatour Y., Bergaoui R. Effect of dietary supplementation with probiotic or essential oils on growth performance of broiler chickens. Int. J. Poult. Sci. 2013;12:538–544. doi: 10.3923/ijps.2013.538.544. [DOI] [Google Scholar]
- 165.Asadi N., Husseini S.D., Tohidian M., Abdali N., Mimandipoure A., Rafieian-Kopaei M., Bahmani M. Performance of broilers supplemented with peppermint (Mentha piperita L.) powder. J. Evid. Based Complement. Altern. Med. 2017;22:703–706. doi: 10.1177/2156587217700771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mohamed A.B., Al-Rubaee M.A., Jalil A.G. Effect of Ginger (Zingiber officinale) on Performance and. Int. J. Poult. Sci. 2012;11:143–146. doi: 10.3923/ijps.2012.143.146. [DOI] [Google Scholar]
- 167.Ghosh T., Kumar A., Sati A., Mondal B.C., Singh S.K., Kumar R. Effect of dietary supplementation of herbal feed additives (black cumin, garlic and turmeric) in combination with linseed oil on production performance of white leghorn laying chickens. J. Entomol. Zool. Stud. 2020;8:478–482. [Google Scholar]
- 168.El-Ghany A. Phytobiotics in poultry industry as growth promoters, antimicrobials and immunomodulators—A review. J. World’s Poult. Res. 2020;10:571–579. doi: 10.36380/jwpr.2020.65. [DOI] [Google Scholar]
- 169.Laptev G.Y., Filippova V.A., Kochish I.I., Yildirim E.A., Ilina L.A., Dubrovin A.V., Brazhnik E.A., Novikova N.I., Novikova O.B., Dmitrieva M.E. Examination of the expression of immunity genes and bacterial profiles in the caecum of growing chickens infected with Salmonella Enteritidis and fed a phytobiotic. Animals. 2019;9:615. doi: 10.3390/ani9090615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu Z., Wang C., Li C., Wang M., Chen W., Zhou C., Wei P. The effect of oregano essential oil on the prevention and treatment of Salmonella pullorum and Salmonella gallinarum infections in commercial Yellow-chicken breeders. Front. Vet. Sci. 2022;9:1058844. doi: 10.3389/fvets.2022.1058844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Salem W.M., El-Hamed D.M.S., Sayed W.F., Elamary R.B. Alterations in virulence and antibiotic resistant genes of multidrug-resistant Salmonella serovars isolated from poultry: The bactericidal efficacy of Allium sativum. Microb. Pathog. 2017;108:91–100. doi: 10.1016/j.micpath.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 172.Kollanoor-Johny A., Mattson T., Baskaran S.A., Amalaradjou M.A., Babapoor S., March B., Valipe S., Darre M., Hoagland T., Schreiber D. Reduction of Salmonella enterica serovar Enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012;78:2981–2987. doi: 10.1128/AEM.07643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vicente J.L., Lopez C., Avila E., Morales E., Hargis B.M., Tellez G. Effect of dietary natural capsaicin on experimental Salmonella enteritidis infection and yolk pigmentation in laying hens. Int. J. Poult. Sci. 2007;6:393–396. doi: 10.3923/ijps.2007.393.396. [DOI] [Google Scholar]
- 174.Voyles B.A. The Biology of Viruses. Mosby-Year Book. Inc.; St. Louis, MO, USA: 1993. [Google Scholar]
- 175.Iqbal A., Hasni S., Sajjad-ur-Rahman, Aslam R., Khan K. Preparation and evaluation of bacteriophage lysate specific for Salmonella typhimurium. Int. J. Curr. Microbiol. Appl. Sci. 2016;5:828–835. doi: 10.20546/ijcmas.2016.511.094. [DOI] [Google Scholar]
- 176.Higgins J.P., Higgins S.E., Guenther K.L., Huff W., Donoghue A.M., Donoghue D.J., Hargis B.M. Use of a specific bacteriophage treatment to reduce Salmonella in poultry products. Poult. Sci. 2005;84:1141–1145. doi: 10.1093/ps/84.7.1141. [DOI] [PubMed] [Google Scholar]
- 177.Henriques A., Sereno R., Almeida A. Reducing Salmonella horizontal transmission during egg incubation by phage therapy. Foodborne Pathog. Dis. 2013;10:718–722. doi: 10.1089/fpd.2012.1363. [DOI] [PubMed] [Google Scholar]
- 178.Bardina C., Spricigo D.A., Cortés P., Llagostera M. Significance of the bacteriophage treatment schedule in reducing Salmonella colonization of poultry. Appl. Environ. Microbiol. 2012;78:6600–6607. doi: 10.1128/AEM.01257-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kimminau E.A., Russo K.N., Karnezos T.P., Oh H.G., Lee J.J., Tate C.C., Baxter J.A., Berghaus R.D., Hofacre C.L. Bacteriophage in-feed application: A novel approach to preventing Salmonella Enteritidis colonization in chicks fed experimentally contaminated feed. J. Appl. Poult. Res. 2020;29:930–936. doi: 10.1016/j.japr.2020.09.003. [DOI] [Google Scholar]
- 180.Li M., Lin H., Jing Y., Wang J. Broad-host-range Salmonella bacteriophage STP4-a and its potential application evaluation in poultry industry. Poult. Sci. 2020;99:3643–3654. doi: 10.1016/j.psj.2020.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nabil N.M., Tawakol M.M., Hassan H.M. Assessing the impact of bacteriophages in the treatment of Salmonella in broiler chickens. Infect. Ecol. Epidemiol. 2018;8:1539056. doi: 10.1080/20008686.2018.1539056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hao G., Li P., Huang J., Cui K., Liang L., Lin F., Lu Z., Sun S. Research Note: Therapeutic effect of a Salmonella phage combination on chicks infected with Salmonella Typhimurium. Poult. Sci. 2023;102:102715. doi: 10.1016/j.psj.2023.102715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Malik D.J. Bacteriophage encapsulation using spray drying for phage therapy. Curr. Issues Mol. Biol. 2021;40:303–316. doi: 10.21775/cimb.040.303. [DOI] [PubMed] [Google Scholar]
- 184.Luong T., Salabarria A., Roach D.R. Phage therapy in the resistance era: Where do we stand and where are we going? Clin. Ther. 2020;42:1659–1680. doi: 10.1016/j.clinthera.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 185.Jia S., McWhorter A.R., Andrews D.M., Underwood G.J., Chousalkar K.K. Challenges in vaccinating layer hens against Salmonella typhimurium. Vaccines. 2020;8:696. doi: 10.3390/vaccines8040696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tennant S.M., Levine M.M. Live attenuated vaccines for invasive Salmonella infections. Vaccine. 2015;33:C36–C41. doi: 10.1016/j.vaccine.2015.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Van Immerseel F., Methner U., Rychlik I., Nagy B., Velge P., Martin G., Foster N., Ducatelle R., Barrow P.A. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: Exploitation of innate immunity and microbial activity. Epidemiol. Infect. 2005;133:959–978. doi: 10.1017/S0950268805004711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin C., Lu T., Chen Y., Yu H., Wu C., Yang W. Safety of bivalent live attenuated Salmonella vaccine and its protection against bacterial shedding and tissue invasion in layers challenged with Salmonella. Poult. Sci. 2022;101:101943. doi: 10.1016/j.psj.2022.101943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Groves P.J., Sharpe S.M., Muir W.I., Pavic A., Cox J.M. Live and inactivated vaccine regimens against caecal Salmonella Typhimurium colonisation in laying hens. Aust. Vet. J. 2016;94:387–393. doi: 10.1111/avj.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tan S., Gyles C.L., Wilkie B.N. Evaluation of an aroA mutant Salmonella typhimurium vaccine in chickens using modified semisolid Rappaport Vassiliadis medium to monitor faecal shedding. Vet. Microbiol. 1997;54:247–254. doi: 10.1016/S0378-1135(96)01279-5. [DOI] [PubMed] [Google Scholar]
- 191.Senevirathne A., Hewawaduge C., Park S., Park J., Kirthika P., Lee J.H. O-antigen-deficient, live, attenuated Salmonella typhimurium confers efficient uptake, reduced cytotoxicity, and rapid clearance in chicken macrophages and lymphoid organs and induces significantly high protective immune responses that protect chickens against Salmonella infection. Dev. Comp. Immunol. 2020;111:103745. doi: 10.1016/j.dci.2020.103745. [DOI] [PubMed] [Google Scholar]
- 192.Eeckhaut V., Haesebrouck F., Ducatelle R., Van Immerseel F. Oral vaccination with a live Salmonella Enteritidis/Typhimurium bivalent vaccine in layers induces cross-protection against caecal and internal organ colonization by a Salmonella Infantis strain. Vet. Microbiol. 2018;218:7–12. doi: 10.1016/j.vetmic.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 193.Kang X., Huang T., Shen H., Meng C., Jiao X., Pan Z. Salmonella Enteritidis Subunit Vaccine Candidate Based on SseB Protein Co-Delivered with Simvastatin as Adjuvant. Pathogens. 2022;11:443. doi: 10.3390/pathogens11040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Crouch C.F., Nell T., Reijnders M., Donkers T., Pugh C., Patel A., Davis P., van Hulten M.C., de Vries S.P. Safety and efficacy of a novel inactivated trivalent Salmonella enterica vaccine in chickens. Vaccine. 2020;38:6741–6750. doi: 10.1016/j.vaccine.2020.08.033. [DOI] [PubMed] [Google Scholar]
- 195.Rabie N.S., Amin Girh Z. Bacterial vaccines in poultry. Bull. Natl. Res. Cent. 2020;44:15. doi: 10.1186/s42269-019-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Singh B.R. Salmonella vaccines for animals and birds and their future perspective. Open Vaccine J. 2009;2:100–112. doi: 10.2174/1875035400902010100. [DOI] [Google Scholar]
- 197.Deguchi K., Yokoyama E., Honda T., Mizuno K. Efficacy of a novel trivalent inactivated vaccine against the shedding of Salmonella in a chicken challenge model. Avian Dis. 2009;53:281–286. doi: 10.1637/8516-110908-Reg.1. [DOI] [PubMed] [Google Scholar]
- 198.Acevedo-Villanueva K.Y., Akerele G.O., Al Hakeem W.G., Renu S., Shanmugasundaram R., Selvaraj R.K. A Novel approach against Salmonella: A review of polymeric nanoparticle vaccines for broilers and layers. Vaccines. 2021;9:1041. doi: 10.3390/vaccines9091041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huberman Y.D., Caballero-García M., Rojas R., Ascanio S., Olmos L.H., Malena R., Lomónaco J., Nievas P., Chero P., Lévano-Gracía J. The efficacy of a trivalent inactivated salmonella vaccine combined with the live s. gallinarum 9R vaccine in young layers after experimental infections with s. enteritidis, s. typhimurium, and s. infantis. Vaccines. 2022;10:1113. doi: 10.3390/vaccines10071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Marouf S., Ibrahim H.M., El-Naggar M.S., Swelum A.A., Alqhtani A.H., El-Saadony M.T., El-Tarabily K.A., Salem H.M. Inactivated pentavalent vaccine against mycoplasmosis and salmonellosis for chickens. Poult. Sci. 2022;101:102139. doi: 10.1016/j.psj.2022.102139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Acevedo-Villanueva K.Y., Renu S., Shanmugasundaram R., Akerele G.O., Gourapura R.J., Selvaraj R.K. Salmonella chitosan nanoparticle vaccine administration is protective against Salmonella Enteritidis in broiler birds. PLoS ONE. 2021;16:e0259334. doi: 10.1371/journal.pone.0259334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Acevedo-Villanueva K., Akerele G., Al-Hakeem W., Adams D., Gourapura R., Selvaraj R. Immunization of broiler chickens with a killed chitosan nanoparticle Salmonella vaccine decreases Salmonella enterica serovar enteritidis load. Front. Physiol. 2022;13:920777. doi: 10.3389/fphys.2022.920777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Desin T.S., Wisner A.L.S., Lam P.S., Berberov E., Mickael C.S., Potter A.A., Köster W. Evaluation of Salmonella enterica serovar Enteritidis pathogenicity island-1 proteins as vaccine candidates against S. Enteritidis challenge in chickens. Vet. Microbiol. 2011;148:298–307. doi: 10.1016/j.vetmic.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 204.Renu S., Markazi A.D., Dhakal S., Lakshmanappa Y.S., Shanmugasundaram R., Selvaraj R.K., Renukaradhya G.J. Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens. Int. J. Nanomed. 2020;15:761–777. doi: 10.2147/IJN.S238445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vinod N., Oh S., Kim S., Choi C.W., Kim S.C., Jung C. Chemically induced Salmonella enteritidis ghosts as a novel vaccine candidate against virulent challenge in a rat model. Vaccine. 2014;32:3249–3255. doi: 10.1016/j.vaccine.2014.03.090. [DOI] [PubMed] [Google Scholar]
- 206.Jalava K., Hensel A., Szostak M., Resch S., Lubitz W. Bacterial ghosts as vaccine candidates for veterinary applications. J. Control. Release. 2002;85:17–25. doi: 10.1016/S0168-3659(02)00267-5. [DOI] [PubMed] [Google Scholar]
- 207.Haidinger W., Szostak M.P., Jechlinger W., Lubitz W. Online monitoring of Escherichia coli ghost production. Appl. Environ. Microbiol. 2003;69:468–474. doi: 10.1128/AEM.69.1.468-474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jawale C.V., Lee J.H. Comparative evaluation of Salmonella Enteritidis ghost vaccines with a commercial vaccine for protection against internal egg contamination with Salmonella. Vaccine. 2014;32:5925–5930. doi: 10.1016/j.vaccine.2014.08.072. [DOI] [PubMed] [Google Scholar]
- 209.Senevirathne A., Hewawaduge C., Lee J.H. Immunization of chicken with flagellin adjuvanted Salmonella enteritidis bacterial ghosts confers complete protection against chicken salmonellosis. Poult. Sci. 2021;100:101205. doi: 10.1016/j.psj.2021.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jazayeri S.D., Poh C.L. Recent advances in delivery of veterinary DNA vaccines against avian pathogens. Vet. Res. 2019;50:78. doi: 10.1186/s13567-019-0698-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gao X., Xu K., Yang G., Shi C., Huang H., Wang J., Yang W., Liu J., Liu Q., Kang Y. Construction of a novel DNA vaccine candidate targeting F gene of genotype VII Newcastle disease virus and chicken IL-18 delivered by Salmonella. J. Appl. Microbiol. 2019;126:1362–1372. doi: 10.1111/jam.14228. [DOI] [PubMed] [Google Scholar]
- 212.Yu X., Jia R., Huang J., Shu B., Zhu D., Liu Q., Gao X., Lin M., Yin Z., Wang M. Attenuated Salmonella typhimurium delivering DNA vaccine encoding duck enteritis virus UL24 induced systemic and mucosal immune responses and conferred good protection against challenge. Vet. Res. 2012;43:56. doi: 10.1186/1297-9716-43-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Oshop G.L., Elankumaran S., Heckert R.A. DNA vaccination in the avian. Vet. Immunol. Immunopathol. 2002;89:1–12. doi: 10.1016/S0165-2427(02)00189-7. [DOI] [PubMed] [Google Scholar]
- 214.Li J., Jiang Y., Zhao S., Chang X., Liu J., Zeng X., Li Y., Chen H. Protective efficacy of an H5N1 DNA vaccine against challenge with a lethal H5N1 virus in quail. Avian Dis. 2012;56:937–939. doi: 10.1637/10150-040812-ResNote.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.