Abstract
The relationship between growth rate and rRNA content in a marine Synechococcus strain was examined. A combination of flow cytometry and whole-cell hybridization with fluorescently labeled 16S rRNA-targeted oligonucleotide probes was used to measure the rRNA content of Synechococcus strain WH8101 cells grown at a range of light-limited growth rates. The sensitivity of this approach was sufficient for the analysis of rRNA even in very slowly growing Synechococcus cells (μ = 0.15 day−1). The relationship between growth rate and cellular rRNA content comprised three phases: (i) at low growth rates (<∼0.7 day−1), rRNA cell−1 remained approximately constant; (ii) at intermediate rates (∼0.7 − 1.6 day−1), rRNA cell−1 increased proportionally with growth rate; and (iii) at the highest, light-saturated rates (>∼1.6 day−1), rRNA cell−1 dropped abruptly. Total cellular RNA (as measured with the nucleic acid stain SYBR Green II) was well correlated with the probe-based measure of rRNA and varied in a similar manner with growth rate. Mean cell volume and rRNA concentration (amount of rRNA per cubic micrometer) were related to growth rate in a manner similar to rRNA cell−1, although the overall magnitude of change in both cases was reduced. These patterns are hypothesized to reflect an approximately linear increase in ribosome efficiency with increasing growth rate, which is consistent with the prevailing prokaryotic model at low growth rates. Taken together, these results support the notion that measurements of cellular rRNA content might be useful for estimating in situ growth rates in natural Synechococcus populations.
Synechococcus spp. are ubiquitous and abundant components of the photosynthetic picoplankton in a wide range of marine environments (32). These unicellular cyanobacteria and their close relatives, Prochlorococcus spp., can account for a large share of total community photosynthetic biomass and primary production, particularly in open-ocean settings (29, 33). Measurement of the in situ growth rates of these organisms, and of natural microbial populations generally, remains a major challenge for microbial ecologists. Traditional methods involving incubations are problematic, owing to the inevitable disruption of natural conditions that such incubations entail (13). This problem is particularly well documented in marine environments, many of which are characterized by tightly coupled microbial food webs and by extraordinarily low ambient concentrations of nutrients and trace metals (e.g., reference 12). One alternative, nonincubation approach for measuring natural microbial growth rates involves the use of biochemical “indexes” that are correlated with growth rate and can be measured in the population of interest (13). The utility of this sort of biochemical approach will clearly depend on the robustness of the correlation with growth rate and on the ease with which the index can be measured in the population of interest. In the present study, we examine the relationship between rRNA content and growth rate in the marine Synechococcus strain WH8101. Our goal is to establish the general nature of this relationship in cyanobacteria compared with that in better-studied heterotrophic bacteria and to evaluate the possibility of using cellular rRNA content as an index for growth rate in this important group of photosynthetic picoplankton.
The relationships between gross macromolecular composition (e.g., RNA, DNA, and protein content) and growth rate in Escherichia coli and other enteric bacteria are well established (9, 14). The fact that these relationships can be described in simple mathematical terms, and appear to be independent of the specific environmental factor that determines the growth rate, supports the idea of using gross biochemical composition to estimate the in situ growth rate of natural microbial populations (11, 16). In particular, ribosome (or rRNA) content is expected a priori to be particularly tightly coupled to growth rate: ribosomes act as catalysts for protein synthesis, and at steady state (and assuming that protein turnover is insignificant), specific protein synthesis rate equals specific growth rate (9). Therefore, cellular rRNA content is a good candidate as a biochemical index for growth rate in natural microbial populations (3, 16, 19).
The relationship between cellular rRNA content and growth rate in cyanobacteria has not been determined. Of the few studies that have examined growth rate-related changes in total cellular RNA, most have involved the freshwater Synechococcus strain PCC6301 (formerly Anacystis nidulans) (22, 25, 30). Although RNA cell−1 is generally observed to increase with growth rate, a consistent picture of the quantitative relationship between these two parameters has not yet emerged. Thus, the increase in cellular RNA has been reported to be exponential, sigmoidal, or linear (see Discussion).
In this study, we used fluorescently labeled 16S rRNA-targeted oligonucleotide probes to specifically analyze the cellular rRNA content of a coastal Synechococcus isolate growing at a range of light-limited growth rates. Additionally, total RNA was measured with the nucleic acid stain SYBR Green II. Flow cytometry provided rapid, sensitive, and high-resolution analysis of hybridized and stained cells in these laboratory studies and in the future should facilitate the extension of this analysis to natural Synechococcus populations in mixed microbial communities.
MATERIALS AND METHODS
Culturing and sampling.
Synechococcus strain WH8101 was obtained from J. Waterbury (Woods Hole Oceanographic Institution) and grown on SN medium (32). Cultures of 25 ml were maintained at 25°C under constant light from Cool-White fluorescent lamps. Light intensity was measured with a scalar PAR meter (Biospherical Instruments, Inc.). Photon flux densities ranging between 4 and 240 μmol m−2 s−1 were achieved by careful placement of culture tubes in different locations within the incubator and by shading with black nylon window screening. Culture growth was monitored by in vivo fluorescence (8), using a Turner Designs model 10 fluorometer equipped with a chlorophyll analysis accessory kit. Specific growth rates (day−1) were calculated from the slope of the regression of natural log (in vivo fluorescence) versus time. In order to maintain semicontinuous growth conditions, cells were diluted into fresh medium prior to the onset of stationary phase, resulting in constant exponential growth rates. Cultures were maintained at a given light level and growth rate for the equivalent of at least 10 generations prior to sampling. Cells were sampled, preserved in methanol, and stored at −20°C as described previously (6). In preliminary hybridization studies with WH8101, this simple methanol fixation appeared to be as good or better than the combined paraformaldehyde and methanol fixation used in previous studies with heterotrophic bacteria (2, 31). Data in this study derive from three independent growth experiments that were performed over the course of 1.5 years (indicated in figures where appropriate).
Whole-cell hybridizations.
Methanol-preserved cells were centrifuged (16,000 × g, 10 min, 4°C) and resuspended in phosphate-buffered saline (PBS; pH 7.5). Standard hybridization conditions were as follows. Five-microliter aliquots of these cell suspensions were combined with 50 μl of hybridization buffer (900 mM NaCl, 20 mM Tris [pH 7.2]) and 1.6 μl of probe solution (50 ng of probe μl−1; final concentration of 1.4 μg ml−1) and incubated at 45°C overnight. Hybridization suspensions were then diluted with 500 μl of hybridization buffer, incubated at 48°C for 20 min, centrifuged, resuspended in 500 μl of PBS, and held on ice prior to analysis.
Two 16S rRNA-targeted oligonucleotide probes, EUB338 and NON338, were used. The former (Oligonucleotide Probe Database designation S-D-Bact-0338-a-A-18 [1]) is specific for the domain Bacteria (2); the latter is the complement of EUB338 and is used as a negative control (31). These oligonucleotides were synthesized with 5′-end amino groups and labeled with BODIPY FL-X (Molecular Probes, Inc.), using the manufacturer’s ONLY oligonucleotide labeling kit.
Cell staining.
Aliquots of methanol-fixed cells were resuspended in PBS (pH 7.9), and incubated for 30 min at 37°C with RNase I (100 U ml−1 [final concentration]; Boehringer Mannheim Corp.). Duplicate cell suspensions were incubated without RNase under the same conditions. Our staining protocol, modified from that of Marie et al. (23), was as follows. Samples were resuspended in PBS plus potassium citrate (30 mM [final concentration]), stained with SYBR Green II (Molecular Probes) at a final concentration of 0.01% of the stock solution, and analyzed flow cytometrically. SYBR Green fluorescence of RNase-treated cells was taken to reflect DNA content and compared to Hoechst fluorescence (see below). The difference between this DNA-derived fluorescence and the fluorescence of cells not treated with RNase was taken to reflect RNA content and was compared with probe-conferred fluorescence. Digestion of cellular DNA would in theory yield a direct measure of RNA content, but we found that DNase did not fully degrade cellular DNA in our samples (data not shown). For comparative purposes, cells in some samples were stained with Hoechst 33342 (0.5 μg ml−1 [final concentration]) as described previously (5).
Flow cytometric analysis.
Probed or stained samples were analyzed on an EPICS 753 flow cytometer (Coulter Corp.) equipped with a 5-W argon ion laser and modified for high sensitivity as described previously (7). Blue excitation (488 nm, 750 mW) was used for probed and SYBR Green-stained samples. Green fluorescence from BODIPY or SYBR Green was collected through a 525-nm band-pass filter. Red fluorescence from phycocyanin was collected through a 680-nm band-pass filter and, together with forward angle light scatter was used to unambiguously identify Synechococcus cells. For Hoechst-stained samples, 125 mW of UV excitation was used, blue fluorescence from the stain was collected through a 408-nm long-pass and a 470-nm short-pass filter, and red fluorescence was collected as described above. BODIPY and SYBR Green fluorescence was normalized to that of standard fluorescent latex beads (0.474-μm diameter; Polysciences, Inc.) which were added to each sample. Hoechst fluorescence was normalized to genome equivalents (6). All data were collected as list modes and analyzed with CYCLOPS (Cytomation, Inc.), WIN-MDI (Joseph Trotter, The Scripps Research Institute), or WinList (Verity Software House, Inc.) software.
Cell volume determinations.
Methanol-fixed cells were washed in filtered (0.2-μm-pore-size filter) artificial seawater, diluted in the same seawater as necessary, and analyzed with a Multisizer II Coulter Counter (Coulter Corp.) equipped with a 15-μm aperture. Methanol fixation does not significantly affect cell volume in Synechococcus strain WH8101 (data not shown). Reported mean cell volumes are derived from a Gaussian curve fit to raw cell size distribution, using ModFit software (Verity Software House), in order to reduce the effects of noise in the lower channels of the instrument.
RESULTS
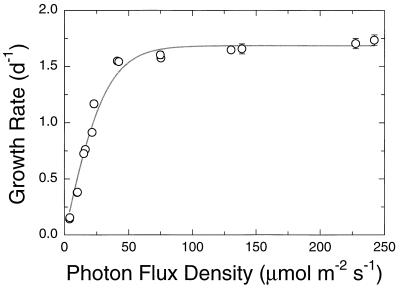
WH8101 grew at specific growth rates between 0.15 and 1.83 day−1 under the range of light intensities used in this study. The relationship between growth rate and light intensity was well described by a hyperbolic tangent function (15), with no evidence of photoinhibition (Fig. 1).
FIG. 1.
Relationship between specific growth rate and light intensity for Synechococcus strain WH8101. Shown are the means and standard errors for at least four consecutive transfers of a given culture (corresponding to ∼16 generations); line is the best-fit hyperbolic tangent function (α = 2.97, μmax = 1.69; r2 = 0.98) (15). Error bars not shown are contained within the symbols. d, day.
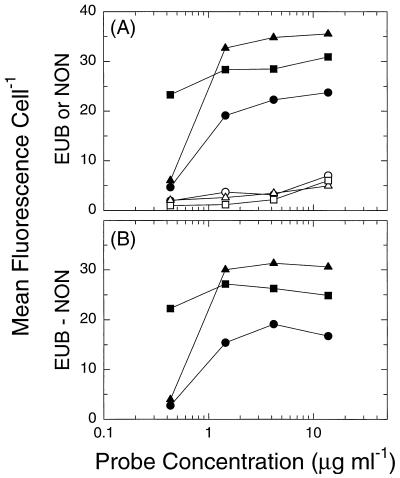
Preliminary hybridization experiments established that a single 5′-end BODIPY-labeled probe resulted in a detectable fluorescence signal, well in excess of the negative control (Fig. 2). For these methanol-fixed cells, inclusion of 0.1% sodium dodecyl sulfate in the hybridization buffer (31) resulted in increased nonspecific binding as well as increased concentrations of small particulates which interfered with our flow cytometric analysis (not shown). Sodium dodecyl sulfate was therefore excluded from the hybridization mix in subsequent experiments. Optimum probe concentration was determined for cells growing at three different growth rates (Fig. 3). Whereas fluorescence was relatively insensitive to probe concentration in rapidly growing cells, it dropped off sharply below 1.4 μg of probe ml−1 in cells growing at moderate and low rates. Above this probe concentration, mean fluorescence increased slowly in all cells. Owing to a parallel increase in nonspecific binding (as reflected by NON338 fluorescence), however, the difference between specific and nonspecific fluorescence remained approximately constant at these higher probe concentrations (Fig. 3B). We therefore routinely used a final probe concentration of 1.4 μg ml−1. Under these conditions, specific fluorescence and nonspecific fluorescence were insensitive to cell density in the hybridization mix over a factor of at least 10 (not shown). EUB338-conferred fluorescence was insensitive to DNase treatment but was reduced to NON338 levels by RNase treatment, confirming that EUB338 fluorescence was in fact associated with cellular RNA (data not shown). The standard deviation of separate analyses performed on different days with aliquots of the same sample corresponded to approximately 7% of the mean.
FIG. 2.
Flow cytometric signature of hybridized Synechococcus cells. (A) Two-parameter histogram of red fluorescence (derived from phycobiliprotein autofluorescence) and forward angle light scatter (related to cell size) showing cells and latex bead standards. Contour lines indicate the relative number of cells having the specified combination of red fluorescence and light scatter and correspond to 0.01%, 0.02%, 0.04%, 0.08%, etc., of the total population within the corresponding histogram bin. (B) Frequency distribution of BODIPY fluorescence for the cells identified in panel A and hybridized with EUB338 (dark line) or NON338 (light line).
FIG. 3.
Effect of probe concentration on mean cellular fluorescence in cells growing at 0.40 (○, •), 1.17 (▵, ▴), and 1.75 (□, ■) day−1. (A) Cells hybridized with EUB338 (closed symbols) or NON338 (open symbols). (B) The difference between EUB338- and NON338-conferred fluorescence for each cell type. Data shown represent individual determinations.
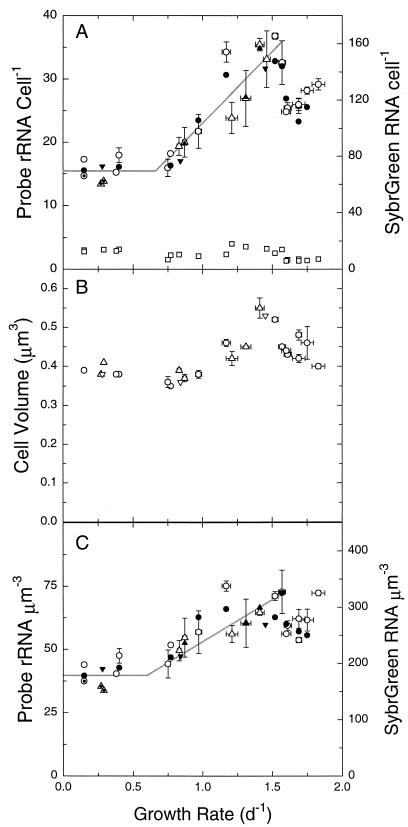
The relationship between growth rate and rRNA content in WH8101 appeared to comprise three phases (Fig. 4A). At the lowest rates (<∼0.7 day−1), rRNA cell−1 remained approximately constant; at intermediate growth rates (∼0.7 to 1.6 day−1), it increased linearly and proportionally to growth rate; at the highest growth rates (>∼1.6 day−1), it appeared to undergo a downshift. Nonspecific fluorescence decreased slightly as growth rate increased and was always insignificant (10% on average) compared to EUB338-conferred fluorescence.
FIG. 4.
Variation of cellular rRNA, total RNA, and volume with growth rate in Synechococcus strain WH8101. (A) Cellular rRNA content, as reflected by EUB338-conferred fluorescence, in cells from two different experiments (○, ▵); NON338-conferred fluorescence (□); and total cellular RNA as measured with SYBR Green II in cells from three different experiments (•, ▴, ▾). EUB338-conferred fluorescence represents the mean and standard error of replicate cultures at a given light intensity (except for dotted symbols, which represent data from single cultures). Data for NON338-conferred fluorescence and SYBR Green RNA represent individual determinations. Growth rates and associated error bars as in Fig. 1. Both y axes are relative scales; the relationship between the two scales is arbitrary. (B) Mean cell volume of cells from three different experiments (○, ▵, ▿). Error bars are as in panel A. (C) rRNA and RNA concentrations, as calculated from the data in panels A and B. Symbols, error bars, and axis scaling are as in panel A. Lines in panels A and C were drawn by eye. d, day.
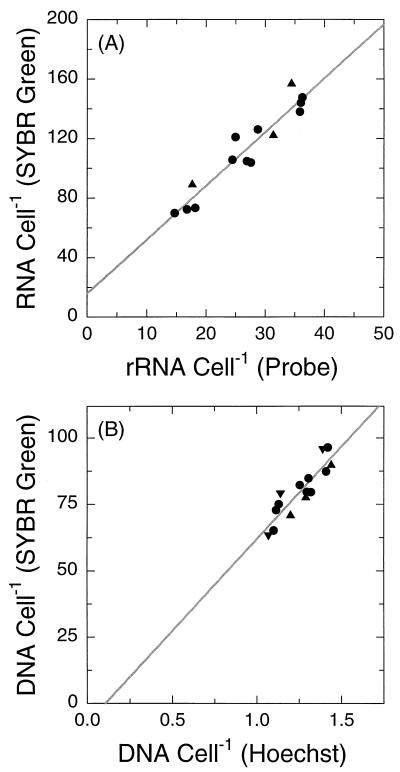
Measurements of total cellular RNA and DNA based on SYBR Green II were very well correlated with probe-based rRNA measurements and Hoechst-based DNA measurements, respectively (r = 0.95 and 0.90) (Fig. 5). The relationship between growth rate and RNA cell−1 as measured with SYBR Green II was very similar to the relationship described above for rRNA cell−1 (Fig. 4A).
FIG. 5.
Comparison of SYBR Green II-based measurements of cellular RNA (A) and DNA (B) with probe-based measurements of rRNA and Hoechst-based measurements of DNA, respectively. Points represent individual determinations on the same sample; lines are least-square regressions, with SYBR Green determinations taken as the dependent variable in both cases. Symbols are as in Fig. 4B. The Hoechst-based DNA measurement is expressed in genome equivalents; all other axis scales represent relative fluorescence.
Mean cell volume varied with growth rate in a manner that was qualitatively similar to the pattern for rRNA cell−1, remaining approximately constant (or perhaps decreasing slightly) at low growth rates, increasing linearly at intermediate rates, and dropping at rates greater than ∼1.6 day−1 (Fig. 4B). The overall magnitude of these changes, however, was somewhat less than for rRNA cell−1. These two data sets were combined to calculate cellular rRNA concentration (rRNA per cubic micrometer), which is in many ways the relevant physiological parameter (see Discussion). Again, the relationship between rRNA concentration and growth rate was qualitatively similar to but of smaller magnitude than that between rRNA cell−1 and growth rate (Fig. 4C). In particular, the downshift in cellular rRNA at the highest growth rates was significantly reduced when the data were expressed on a per-cell-volume basis.
DISCUSSION
The relationship that we report here between rRNA content and growth rate in Synechococcus strain WH8101 is similar in many respects to that observed in E. coli. This likely reflects fundamental similarities in the regulation of ribosome synthesis and activity with respect to growth rate in these two organisms. The concordance between measurements obtained by using fluorescently labeled 16S rRNA-targeted oligonucleotide probes and the nucleic acid stain SYBR Green II indicates that both of these methods can be used to estimate RNA content in Synechococcus cells. Note that a strong correlation between rRNA and total RNA is to be expected, given that rRNA generally represents a large proportion of total cellular RNA in prokaryotic cells (9).
In E. coli and related heterotrophic bacteria growing at moderate to high growth rates, total cellular RNA and rRNA increase approximately exponentially with growth rate (9). When normalized to cell mass or protein, the increase in rRNA concentration with growth rate is linear (9, 14). At lower growth rates, RNA concentration has been observed variously to continue to decrease linearly (but with a positive, non-zero intercept) or to level off at a constant, low value (14, 17).
Our observations of relatively constant rRNA cell−1 at the lowest growth rates and a linear increase at intermediate growth rates in Synechococcus strain WH8101 are reminiscent of these patterns in E. coli. However, decreases in rRNA cell−1 at high growth rates as observed here in Synechococcus have not been reported in E. coli. As this decrease occurs in those cells incubated under the highest light intensities (Fig. 1), it may reflect a shift in cellular regulation that occurs as growth shifts from a light-limited to a light-saturated condition. A corresponding downshift in cell volume in the same range of growth rates supports this notion. Furthermore, this downshift in volume can explain at least in part the downshift in rRNA cell−1 (compare Fig. 4A and C) (see below). The overall pattern observed here in cell volume is strikingly similar to the pattern reported for cellular protein content in light-limited cultures of Synechococcus strain PCC6301 (25; but see also reference 22).
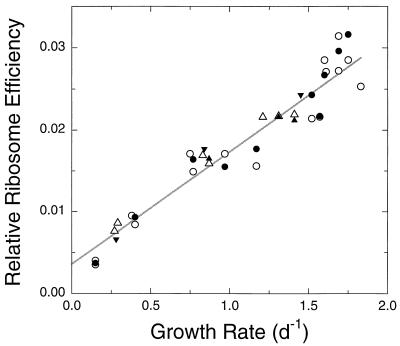
Variations in cellular rRNA content can be interpreted in the context of ribosome efficiency, or average protein synthesis rate per ribosome. Under conditions of balanced growth, and assuming negligible or constant protein turnover, ribosome efficiency can be shown to equal μ · P/R, where μ is specific growth rate and P and R are cellular protein and ribosome content, respectively (9, 28). If we assume that protein content is proportional to cell volume, a relative ribosome efficiency can be calculated as μ · V/R, where V is cell volume. In Synechococcus strain WH8101, relative ribosome efficiency so calculated increases linearly with growth rate over the entire range of growth rates examined in this study (Fig. 6). This linear relationship may in part be a reflection of the fact that growth rate appears in the numerator of the ordinate. Nevertheless, the calculation clearly indicates that the observed changes in ribosome concentration are insufficient to support the corresponding changes in growth rate without a concomitant increase in the average per-ribosome protein synthesis rate. In contrast, in E. coli growing at moderate to high growth rates, ribosome efficiency has been found to be constant or nearly so (9, 14). At growth rates below ∼0.5 doubling h−1, however, ribosome efficiency in E. coli decreases linearly with decreasing growth rate, as observed here in Synechococcus, apparently reflecting a decrease in the proportion of ribosomes that are actively involved in protein synthesis in slowly growing cells (14, 17). We hypothesize that a similar phenomenon underlies the decreasing relative ribosome efficiency observed here in Synechococcus strain WH8101.
FIG. 6.
Relative ribosome efficiency versus growth rate. Ribosome efficiency was calculated from the probe-based measurements of rRNA (open symbols) and SYBR Green-based measurements of total RNA (closed symbols) shown in Fig. 4C (see text). Relative scaling of these two calculated efficiencies is arbitrary but consistent with scaling in Fig. 4C. Line shows linear regression of probe-based efficiencies versus growth rate. Symbols are as in Fig. 4. d, day.
In comparisons of growth rate-related behavior in Synechococcus with that in E. coli, it is not immediately obvious whether relative growth rates (normalized to μmax for each organism) or absolute growth rates represent the more appropriate basis for comparison. Should we expect the behavior of Synechococcus strain WH8101 growing near its maximum growth rate (∼1.8 day−1) to be analogous to E. coli growing at near its maximum rate (∼40 day−1), or to E. coli growing at the same absolute rate (1.8 day−1)? In this study, the growth rate-related behavior of ribosome efficiency in Synechococcus is dramatically different from that in E. coli when the comparison is based on relative growth rates but is quite consistent when the comparison is based on absolute growth rates. Thus, it appears that our understanding of growth rate regulation of rRNA in Synechococcus (and other ecologically relevant, relatively slowly growing prokaryotes) may be best served by reference to the slow-growth behavior of E. coli and other well-studied prokaryotic models. Similar conclusions have been drawn with respect to growth rate regulation of DNA replication in Synechococcus strain WH8101 (4, 5).
Previous studies examining the relationship between macromolecular composition and growth rate in cyanobacteria have largely involved the freshwater Synechococcus strain PCC6301. In these studies, total RNA cell−1 has generally been shown to increase with growth rate, although the quantitative relationship between RNA content and growth rate has not been well established. Thus, while Mann and Carr (22) observed an exponential increase in RNA cell−1 with increasing growth rate under light limitation, Parrott and Slater (25) observed a sigmoidal relationship reminiscent of the pattern observed in the present study. Under sulfate limitation, RNA cell−1 appears to increase sigmoidally with growth rate as well (calculated from Table 1 in reference 30), while the relationship is linear under CO2 limitation (25). Magnesium limitation represents the single exception to the general observation that RNA cell−1 increases with growth rate, likely reflecting a disruption of protein synthesis (i.e., a dramatic decrease in ribosome efficiency) at severely limiting Mg concentrations (30).
In the one relevant study of a marine Synechococcus strain (WH7803) of which we are aware, RNA cell−1 appears to remain approximately constant at low growth rates, to increase dramatically as growth rate reaches ∼0.7 day−1, and then to decrease again at higher growth rates (18). Although this pattern is not identical to that observed here in Synechococcus strain WH8101, the similarities are intriguing and suggest the presence of a common regulatory pattern in marine Synechococcus strains.
Whole-cell hybridization with 16S rRNA-targeted probes has been used successfully to quantify rRNA content in other bacteria (10, 20, 21, 26, 27, 31). That the patterns in rRNA cell−1 observed in this study are not influenced by growth rate-related changes in probe binding behavior (e.g., changes in cell membrane permeability, rRNA target accessibility, or background binding) is evidenced by (i) the lack of significant signal from the NON338 probe (Fig. 4), (ii) the sensitivity of the signal to RNase but not to DNase, and (iii) the excellent correspondence between the probe-based measurement of rRNA and the SYBR Green-based measurement of total RNA (Fig. 4 and 5), the latter of which does not require access to one specific target on the rRNA molecule and is unlikely to behave the same as oligonucleotide probes with respect to cell permeability. Interference by the photosynthetic pigments in Synechococcus is also unlikely to have influenced our results: pigment-derived fluorescence spillover into the signal channel would be present in both NON338- and EUB338-hybridized cells. Again, the former signal was on the order of 10% of the latter at all growth rates (Fig. 4). Note that such spillover could represent a more significant problem in Synechococcus strains containing phycoerythrin, which fluoresces at lower wavelengths than do the phycobiliproteins in WH8101. The possibility of fluorescence quenching by photosynthetic pigments cannot be entirely eliminated, but in no sample was there any indication of an inverse relationship between phycobiliprotein content (as reflected by cellular red fluorescence) and probe-conferred fluorescence. Furthermore, it is unlikely that any fluorescence quenching would affect SYBR Green fluorescence in quantitatively the same way as it affected probe-conferred fluorescence. Finally, the pattern observed here is in many ways consistent with previous observations, based on more traditional biochemical analyses, of growth rate-regulated changes in cellular RNA in Synechococcus spp. (see above).
The data presented here support the notion that measurements of cellular rRNA may prove useful for estimating growth rates in natural Synechococcus populations. Mean cellular rRNA content and rRNA concentration both varied coherently and systematically with light-limited growth rate in Synechococcus strain WH8101. Even at growth rates as low as 0.15 day−1 (corresponding to doubling times of 4.6 days), cellular rRNA was clearly measurable by the analytical approach that we used (Fig. 4). It is important to note, however, that at extreme growth rates, both low and high, the relationship between cellular rRNA and growth rate departs from linearity. rRNA-based estimates of growth rate would therefore be more ambiguous under these conditions than at intermediate growth rates. Furthermore, the magnitude of change in rRNA with respect to changes in growth rate was somewhat lower than might have been hoped for. Over a 12-fold range of growth rates, rRNA cell−1 varied by a factor of ∼2.3.
The successful application of fluorescently labeled probes and flow cytometry to the measurement of rRNA in Synechococcus cells greatly improves the potential for using rRNA-based analyses to study natural microbial populations. Flow cytometric analysis can be used to discriminate between groups within a mixed assemblage based on cellular light scattering and fluorescence properties (24), allowing the analysis of the specific populations of interest rather than the microbial community as a whole. Taxonomic specificity could in theory be sharpened further by the use of group- or species-specific rRNA-targeted probes, rather than the general probes used in the present study (2).
Although the data presented here are encouraging, the usefulness of an rRNA-based approach for estimating in situ microbial growth rates will ultimately depend on the generality of the relationship between rRNA content and growth rate with respect to both taxonomy and environmental conditions. Both of these issues are under investigation.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Department of Energy (DE-FG02-93-ER61694.A000) and the National Science Foundation (OCE-9711306).
We gratefully acknowledge the Office of the Vice President for Research, University of Georgia, for providing time on the flow cytometer.
REFERENCES
- 1.Alm E W, Oerther D B, Larsen N, Stahl D A, Raskin L. The Oligonucleotide Probe Database. Appl Environ Microbiol. 1996;62:3557–3559. doi: 10.1128/aem.62.10.3557-3559.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann R I, Binder B J, Olson R J, Chisholm S W, Devereux R, Stahl D A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amann R I, Ludwig W, Schleifer K-H. Phylogenetic identification and in situ detection of individual cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armbrust E V, Bowen J D, Olson R J, Chisholm S W. The effects of light on the cell cycle of a marine Synechococcus strain. Appl Environ Microbiol. 1989;55:425–432. doi: 10.1128/aem.55.2.425-432.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binder B J, Chisholm S W. Cell cycle regulation in marine Synechococcus sp. strains. Appl Environ Microbiol. 1995;61:708–717. doi: 10.1128/aem.61.2.708-717.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder B J, Chisholm S W. Relationship between DNA cycle and growth rate in Synechococcus sp. strain PCC 6301. J Bacteriol. 1990;172:2313–2319. doi: 10.1128/jb.172.5.2313-2319.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder B J, Chisholm S W, Olson R J, Frankel S L, Worden A Z. Dynamics of pico-phytoplankton, ultra-phytoplankton, and bacteria in the central equatorial Pacific. Deep-Sea Res Part II Top Stud Oceanogr. 1996;43:907–931. [Google Scholar]
- 8.Brand L E, Guillard R R L, Murphy L S. A method for rapid and precise determination of acclimated phytoplankton reproduction rates. J Plankton Res. 1981;3:193–201. [Google Scholar]
- 9.Bremer H, Dennis P P. Modulation of chemical composition and other parameters of the cell by growth rate. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C: ASM Press; 1996. pp. 1553–1569. [Google Scholar]
- 10.DeLong E F, Wickham G S, Pace N R. Phylogenetic stains: ribosomal RNA-based probes for the identification of single cells. Science. 1989;243:1360–1363. doi: 10.1126/science.2466341. [DOI] [PubMed] [Google Scholar]
- 11.Dortch Q, Roberts T L, Clayton J R, Ahmed S I. RNA/DNA ratios and DNA concentrations as indicators of growth rate and biomass in planktonic marine organisms. Mar Ecol Prog Ser. 1983;13:61–71. [Google Scholar]
- 12.Fitzwater S E, Knauer G A, Martin J H. Metal contamination and its effect on primary production measurement. Limnol Oceanogr. 1982;27:544–551. [Google Scholar]
- 13.Furnas M J. In situ growth rates of marine phytoplankton: approaches to measurement, community and species growth rates. J Plankton Res. 1990;12:1117–1151. [Google Scholar]
- 14.Ingraham J L, Maaløe O, Neidhardt F C. Growth of the bacterial cell. Sunderland, Mass: Sinauer Associates, Inc.; 1983. [Google Scholar]
- 15.Jassby A T, Platt T. Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr. 1976;21:540–547. [Google Scholar]
- 16.Kemp P F, Lee S, LaRoche J. Estimating the growth rate of slowly growing marine bacteria from RNA content. Appl Environ Microbiol. 1993;59:2594–2601. doi: 10.1128/aem.59.8.2594-2601.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch A L. Overall controls on the biosynthesis of ribosomes in growing bacteria. J Theor Biol. 1970;28:203–231. doi: 10.1016/0022-5193(70)90053-6. [DOI] [PubMed] [Google Scholar]
- 18.Kramer J G, Morris I. Growth regulation in irradiance limited marine Synechococcus sp. WH 7803. Arch Microbiol. 1990;154:286–293. [Google Scholar]
- 19.Kramer J G, Singleton F L. Measurement of rRNA variations in natural communities of microorganisms on the southeastern U.S. continental shelf. Appl Environ Microbiol. 1993;59:2430–2436. doi: 10.1128/aem.59.8.2430-2436.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S H, Kemp P F. Single-cell RNA content of natural marine planktonic bacteria measured by hybridization with multiple 16S rRNA-targeted fluorescent probes. Limnol Oceanogr. 1994;39:869–879. [Google Scholar]
- 21.Lee S H, Malone C, Kemp P F. Use of multiple 16S rRNA-targeted fluorescent probes to increase signal strength and measure cellular RNA from natural planktonic bacteria. Mar Ecol Prog Ser. 1993;101:193–201. [Google Scholar]
- 22.Mann N, Carr N G. Control of macromolecular composition and cell division in the blue-green alga Anacystis nidulans. J Gen Microbiol. 1974;83:399–405. doi: 10.1099/00221287-83-2-399. [DOI] [PubMed] [Google Scholar]
- 23.Marie D, Patensky F, Jacquet S, Vaulot D. Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Appl Environ Microbiol. 1997;63:186–193. doi: 10.1128/aem.63.1.186-193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olson R J, Zettler E R, Chisholm S W, Dusenberry J A. Advances in oceanography through flow cytometry. In: Demers S, editor. Particle analysis in oceanography. Berlin, Germany: Springer-Verlag; 1991. pp. 351–399. [Google Scholar]
- 25.Parrott L M, Slater J H. The DNA, RNA and protein composition of the cyanobacterium Anacystis nidulans grown in light- and carbon dioxide-limited chemostats. Arch Microbiol. 1980;127:53–58. doi: 10.1007/BF00414355. [DOI] [PubMed] [Google Scholar]
- 26.Poulsen L K, Ballard G, Stahl D A. Use of rRNA fluorescence in situ hybridization for measuring the activity of single cells in young and established biofilms. Appl Environ Microbiol. 1993;59:1354–1360. doi: 10.1128/aem.59.5.1354-1360.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruimy R, Breittmayer V, Boivin V, Christen R. Assessment of the state of activity of individual bacterial cells by hybridization with a ribosomal RNA targeted fluorescently labelled oligonucleotide probe. FEMS Microbiol Ecol. 1994;15:207–214. [Google Scholar]
- 28.Schleif R. Control of production of ribosomal protein. J Mol Biol. 1967;27:41–55. doi: 10.1016/0022-2836(67)90350-6. [DOI] [PubMed] [Google Scholar]
- 29.Stockner J G, Antia N J. Algal picoplankton from marine and freshwater ecosystems: a multidisciplinary perspective. Can J Fish Aquat Sci. 1986;43:2472–2503. [Google Scholar]
- 30.Utkilen H C. Magnesium-limited growth of the cyanobacterium Anacystis nidulans. J Gen Microbiol. 1982;128:1849–1862. [Google Scholar]
- 31.Wallner G, Amann R, Beisker W. Optimizing fluorescent in situ hybridization with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry. 1993;14:136–143. doi: 10.1002/cyto.990140205. [DOI] [PubMed] [Google Scholar]
- 32.Waterbury J B, Watson S W, Valois F W, Franks D G. Biological and ecological characterization of the marine unicellular cyanobacteria Synechococcus. Can Bull Fish Aquat Sci. 1986;214:71–120. [Google Scholar]
- 33.Weisse T. Dynamics of autotrophic picoplankton in marine and freshwater ecosystems. Adv Microb Ecol. 1993;13:327–370. [Google Scholar]