Abstract
To evaluate the potential of a bacterial strain as a fungal disease control agent and plant growth promoter, its inhibitory effects on phytopathogens such as Bipolaris sorokiniana, Botrytis cinerea, Colletotrichum capsici, Fusarium graminearum, F. oxysporum, Neocosmospora rubicola, Rhizoctonia solani, and Verticillium dahliae were investigated. The results showed that the inhibitory rates in dual-culture and sterile filtrate assays against these eight phytopathogens ranged from 57% to 83% and from 36% to 92%. The strain was identified as Bacillus velezensis based on morphological and physiological characterization as well as phylogenetic analyses of 16S rRNA and the gyrase subunit A protein (gyrA) regions. The results demonstrated that B. velezensis was able to produce fungal cell-wall-degrading enzymes, namely, protease, cellulase, and β-1,3-glucanase, and the growth-promotion substances indole-3-acetic acid (IAA) and siderophore. Furthermore, B. velezensis BV01 had significant control effects on wheat root rot and pepper Fusarium wilt in a greenhouse. Potted growth-promotion experiments displayed that BV01 significantly increased the height, stem diameter, and aboveground fresh and dry weights of wheat and pepper. The results imply that B. velezensis BV01, a broad-spectrum biocontrol bacterium, is worth further investigation regarding its practical applications in agriculture.
Keywords: Bacillus, antifungal activity, fungal phytopathogens, wheat root rot, Fusarium wilt, greenhouse pot experiment
1. Introduction
Crop diseases caused by phytopathogens have resulted in a decrease in agricultural yields and quality, leading to significant economic losses [1]. In particular, soil-borne fungal infections of important crops such as wheat, corn, rice, and pepper cause large economic losses [2]. The United Nations 2030 Sustainable Development Goals suggested that the world should ensure sustainable consumption and production patterns, promote sustainable agriculture, and reduce environmental pollution [3]. For a long time, synthetic chemical pesticides were commonly used in traditional agriculture to combat plant diseases, but they often caused environmental pollution and residual toxic effects in animals and humans [4]. Thus, discovery of eco-friendly, long-lasting, and effective methods are required for disease prevention and management in agriculture. The use of microbial and biochemical agents has been explored as a practical alternative approach [5].
The plant-growth-promoting rhizobacteria (PGPRs) are often used for the production of bioactive substances that can protect plants by suppressing pathogens, inducing systemic resistance, or improving resistance to environmental stresses, by facilitating nutrient acquisition and modulating phytohormone levels in plants [6,7]. In recent years, Bacillus subtilis and its closest relatives B. amyloliquefaciens, B. velezensis, B. cereus, and B. licheniformis have been widely used as biofertilizers and biofungicides [8,9]. Bacillus velezensis FZB42, the classical PGPR strain, was successfully used as a biocontrol agent in potato, strawberry, wheat, and cabbage [10,11,12,13,14]. The most prevalent plant fungal diseases, such as grey mold, Fusarium head blight, anthracnose, and root rot, etc., are mainly caused by species of Botrytis, Fusarium, Colletotrichum, and Rhizoctonia [15]. This can be attributed to their broad host range, genetic diversity, rapid adaptation to plant disease resistance, and production of toxins [16]. Previous studies have shown that B. velezensis is a promising agent for control of Rhizoctonia solani [17], Gaeumannomyces graminis var. tritici [18], Fusarium oxysporum f. sp. niveum [19], Botrytis cinerea, Colletotrichum gloeosporioides, and Phytophthora infestans [20], and it has attracted widespread attention in agricultural disease research. Nevertheless, studies on its biocontrol mechanism, screening of excellent strains, analyses of transcriptomics, proteomics, metabolomics, and research on industrial and commercial applications of B. velezensis are needed [21].
In this study, we aimed to assess the potential of a newly isolated bacterial strain, B. velezensis BV01, as a broad-spectrum biocontrol agent and investigate its capacity to control plant diseases and promote wheat and pepper development. The findings are of great significance for reducing the use of chemical fungicides to control soil-borne fungal diseases, thereby improving the ecological environment, and for providing technical support for food safety and sustainable development.
2. Materials and Methods
2.1. Tested Strains
Information on the 12 bacterial and fungal strains used is listed in Table 1. Bacillus velezensis BV01 was isolated from a contaminated potato dextrose agar (PDA) plate in the laboratory. Bacillus velezensis JDF and B. subtilis L01 and BS208 were isolated from three commercially available bacterial agents NongBaoShengWu®, LvLong®, and GuanLan®, respectively, and the eight fungal plant pathogen strains were provided by colleagues from Beijing Academy Agriculture and Forestry Sciences, China Academy Agricultural Sciences, Guangxi Academy Agricultural Sciences, Nanjing Agricultural University, and our institute (Table 1). All strains were deposited in the China General Microbiological Culture Collection Center (CGMCC) and the State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences.
Table 1.
The bacterial and fungal strains tested in this study.
| Strain | Characteristics Relevant to This Work | Source |
|---|---|---|
| Bacillus velezensis CGMCC 1.60184 | Isolated from the State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences (116.38982° E, 40.01076° N) on 6 November 2019 | This work |
| B. velezensis JDF | Registration of broad-spectrum antagonism, stress resistance and growth-promoting ability, used as a positive control | Isolated from NongBaoShengWu® bacterial agent |
| B. subtilis L01 | Registration of antimicrobial activity and strong stress resistance, used as a positive control | Isolated from LvLong® bacterial agent |
| B. subtilis BS208 | Registration of prevention and control of gray mold and powdery mildew, used as a positive control | Isolated from GuanLan® bacterial agent |
| Bipolaris sorokiniana PP12 | Causes wheat root rot | Provided by Prof. Niu Yongchun of Chinese Academy of Agricultural Sciences |
| Botrytis cinerea PP1 | Causes tomato gray mold | Provided by Prof. Qiu Jiyan of Beijing Academy of Agricultural Sciences |
| Colletotrichum capsici PP6 | Causes ring rot disease | |
| Fusarium graminearum PP15 | Causes Fusarium head blight | Provided by Prof. Ma Zhengqiang of Nanjing Agricultural University |
| F. oxysporum F6 | Causes blight disease | Provided by Prof. Li Qili of Guangxi Academy of Agricultural Sciences |
| Neocosmospora rubicola PP23 | Causes root and stem rot | Provided by Dr. Fu Shenzhan of Institute of Microbiology, Chinese Academy of Sciences |
| Rhizoctonia solani PP11 | Causes wilt disease | Provided by Prof. Niu Yongchun of Chinese Academy of Agricultural Sciences |
| Verticillium dahliae PP8 | Causes greensickness |
2.2. Evaluation of Antagonistic Abilities In Vitro
All bacterial strains were tested for their antagonistic abilities against eight phytopathogens in dual-culture assays [18]. The bacterial strains were grown in nutrient broth (NB: peptone 10.0 g, beef paste 3.0 g, NaCl 5.0 g, dH2O 1000 mL) at 28 °C for 2 d, and the cell suspension was adjusted to 5 × 108 CFU/mL. The fungal strains were grown on PDA at 25 °C for 5 d. Mycelial plugs (5 mm in diam.) were placed in the center of each new PDA plate (90 mm in diam.); then, four sterile filter papers (5 mm in diam.), which contained 6 μL of a bacterial suspension, were placed evenly around individual mycelial plugs. Sterile water was used as the control. All plates were incubated at 25 °C for 5 d. Each treatment had three replicates. Measurements were calculated with the following formula: y = (A − B)/A × 100% (y: percentage of inhibition, A: colony diameter of pathogen on control plate, and B: colony diameter of pathogen in experimental group) [22].
2.3. Assessment of Antifungal Activity of Bacterial Sterile Filtrate
The antifungal activity of bacterial sterile filtrate was evaluated by measuring the diameter of the fungal colony [23]. Four bacterial strains were incubated in NB with shaking at 180 rpm at 28 °C for 2 d, and then the cultures were centrifuged at 8000 rpm at 4 °C for 20 min to collect the supernatant. The supernatant was filtered through a 0.22 μm filter and then mixed into molten PDA at 45–50 °C with a concentration of 20% (v/v). The mycelium plug (5 mm in diam.) of each pathogen was placed at the center of each PDA plate with bacterial filtrate and incubated for 5 d at 25 °C. The PDA plate without bacterial filtrate was used as the control. Three replicates were set up for each treatment. The inhibition percentage (y%) was calculated with the following formula: y = (A − C)/A × 100% (A: growth radius of pathogen in control and C: growth radius of pathogen in different treatments).
2.4. Morphological Observation and Molecular Identification of Strain BV01
The morphological characteristics of strain BV01 were recorded after incubation on a nutrient agar (NA: peptone 10.0 g, beef paste 3.0 g, NaCl 5.0 g, agar 18.0 g, dH2O 1000 mL) plate at 28 °C for 2 d. A single colony was then taken and evenly spread onto a glass slide, dry fixed, and Gram stained for 1 min [24]. The microscopic photographs were taken with a Zeiss AxioCam MRc 5 digital camera (Carl Zeiss, Jena, Germany) attached to Zeiss Axioskop 2 plus microscope (Carl Zeiss, Göttingen, Germany).
Genomic DNA was extracted from fresh cultures using a bacterial genomic DNA kit (ZOMAN, Beijing, China) according to the manufacturer’s instructions. The 16S rRNA and the gyrase subunit A protein (gyrA) regions were amplified using the primer pairs of 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) and gyrA-F (5′-CAGTCAGGAAATGCGTACGTCTT-3′) and gyrA-R (5′-CAAGGTAATGCTCCAGGCATTGCT-3′), respectively. PCR reactions were conducted using an ABI 2720 Thermal Cycler (Applied Biosciences, Foster City, CA, USA), and the products were then sequenced using an ABI3730XL DNA Sequencer (Applied Biosciences, Foster City, CA, USA). The newly obtained sequences and those of the ex-type strain as well as the related ones retrieved from GenBank were aligned using BioEdit 7.2 [25] and analyzed with the neighbor-joining (NJ) method [26] via MEGA X [27]. The topological confidence of the resulting tree and the statistical supports of the branches were tested using the neighbor-joining bootstrap proportion (NJBP) with 1000 replications, each with 10 replicates of random addition of taxa [28].
2.5. Determination of Enzyme Activity and Secondary Metabolites
Protease, cellulase, and β-1,3-glucanase were detected on skim milk, carboxymethyl cellulose (CMC), and glucose agar, respectively [29]. Siderophore and indole-3-acetic acid (IAA) were tested using modified chrome azurol S and Salkowski reagent agar, respectively [30]. Enzyme activity and production of metabolites were observed based on the presence of clear zones around bacterial colonies after incubation at 28 °C for 3 d. All treatments were repeated three times.
2.6. Biocontrol Activity of Strain BV01 In Vivo
After evaluation of the antagonistic abilities of the four bacterial strains, two strains, namely, JDF and BV01, were further tested for their biocontrol and plant-growth-promotion abilities in a pot experiment. Four germinated wheat seeds were sowed per pot containing a mixture of podsolic soil and vermiculite (v:v = 1:1). On the 14th day, the roots of the plants were punctured and inoculated nearby the wounds with pathogen mycelium blocks (5 mm in diam.). After 24 h, the roots were irrigated with 30 mL (2.5 × 108 CFU/mL) of bacterial suspensions per wheat plant, and the same amount of NB was used as the control. The plants were kept in a greenhouse with a 14 h/10 h photoperiod/dark period at 26 ± 1 °C. The incidence of disease of wheat treated with BV01, JDF, and the non-treated control was recorded and calculated at 20 d after B. sorokiniana inoculation. Infection types (ITs) of B. sorokiniana on wheat were evaluated based on the area of a brown or black lesion at the stem base, with scores varying from 0 to 4 (IT0: no lesion, IT1: coverage of necrotic lesion less than 1/4, IT2: lesion coverage between 1/4 and 1/2, IT3: coverage between 1/2 and 2/3, and IT4: coverage between 2/3 and total). The disease index = ∑ (di × li) / (L × dimax) × 100% (di: infection type, li: number of plants with each infection type, and L: number of wheat plants investigated) [31].
Three true leaves at the age of 40 d were detached from pepper seedlings and punctured in two places with a sterile needle on both sides of the leaf. Mycelium blocks (5 mm in diam.) of F. graminearum were placed on the two wounds and then sprayed with 2 mL of either BV01 or JDF fermentation broth at a concentration of 2.5 × 108 CFU/mL, and the same volume of NB was used as the non-inoculated control at 24 h after F. graminearum inoculation. The treatments were kept in a greenhouse at 26 ± 1 °C with a 14 h/10 h photoperiod/dark period for 6 d; sterile water was added once to keep the treatment moist. There were 3 leaves in each pot and 3 replicates in each treatment. The diameters of diseased spots were recorded and calculated at the 6th day after F. graminearum inoculation. The inhibition rate was calculated as follows: (DCK − Di)/DCK × 100% (DCK: the control group’s average colony diameter and Di: the treatment group’s average colony diameter).
2.7. Plant-Growth-Promoting Assays in a Greenhouse
Sterilized wheat and pepper seeds were soaked in suspensions of BV01 or JDF at a concentration of 2.5 × 108 CFU/mL and then in sterile water for 10 min. NB was used as the control. The plants were kept in a greenhouse with a 14 h/10 h photoperiod/dark period at 26 ± 1 °C. The wheat and peppers were harvested at the 21st and 35th days, respectively. Plant height, fresh weight, dry weight, and leaf width were recorded, and the strong seedling index (SSI) was calculated [32].
2.8. Statistical Analysis
Statistical analysis was performed using SPSS 21 (Armonk, NY, USA). ANOVA was performed, and mean values were compared using Duncan’s multiple range test with p < 0.05 as the level of significance. All analyses were conducted using GraphPad Prism 8 (San Diego, CA, USA).
3. Results
3.1. Inhibitory Effects of Four Tested Bacterial Strains against Eight Fungal Phytopathogens
Strain BV01 exhibited varying degrees of antagonism against different phytopathogens, and the inhibition rates ranged from 57% to 83% (Table 2) with the highest potential inhibitory effects against B. cinerea PP1 (Figure 1, Treatment 1). The inhibition rates showed that BV01 had significantly higher inhibitory effects than JDF, L01, and BS208 on six of the eight tested fungal phytopathogens.
Table 2.
Antifungal activities of four bacterial strains.
| Strains | Dual-Culture Inhibition Rate (%) | Fermentation Broth Inhibition Rate (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| CGMCC 1.60184 | JDF | L01 | BS208 | CGMCC 1.60184 | JDF | L01 | BS208 | |
| B. sorokiniana PP12 | 80.68 ± 1.23 a | 79.55 ± 0.93 a | 77.27 ± 1.05 b | 27.27 ± 2.47 c | 92.26 ± 0.68 a | 76.77 ± 0.23 b | 70.32 ± 1.03 c | 8.39 ± 1.89 d |
| B. cinerea PP1 | 82.95 ± 1.23 b | 85.23 ± 2.23 a | 85.23 ± 2.14 a | 0.00 ± 0.83 c | 81.82 ± 0.82 a | 64.77 ± 1.00 c | 71.59 ± 0.58 b | 2.27 ± 1.44 d |
| C. capsici PP6 | 79.55 ± 0.64 a | 75.00 ± 1.25 b | 77.27 ± 1.07 b | 26.14 ± 1.68 c | 72.73 ± 0.75 a | 71.59 ± 0.86 a | 72.73 ± 0.42 a | 4.55 ± 1.45 b |
| F. graminearum PP15 | 61.36 ± 1.09 b | 65.91 ± 0.49 a | 62.50 ± 0.62 b | 0.00 ± 0.62 c | 59.09 ± 1.66 a | 43.18 ± 0.52 c | 47.53 ± 0.66 b | 13.64 ± 0.97 d |
| F. oxysporum F6 | 65.91 ± 0.66 a | 62.50 ± 1.03 b | 52.27 ± 0.71 c | 32.95 ± 1.27 d | 56.79 ± 1.23 a | 13.58 ± 1.06 b | 12.04 ± 0.69 b | 8.95 ± 0.46 c |
| N. rubicola PP23 | 56.52 ± 1.02 a | 50.72 ± 0.93 b | 50.72 ± 1.23 b | 42.03 ± 1.24 c | 36.13 ± 1.19 a | 12.18 ± 0.99 b | 12.18 ± 0.52 b | 4.19 ± 0.74 c |
| R. solani PP11 | 69.32 ± 0.71 a | 54.55 ± 0.86 b | 53.41 ± 1.23 b | 0.00 ± 1.7 c | 46.59 ± 1.23 a | 0.00 ± 1.08 c | 0.00 ± 1.21 c | 5.68 ± 0.56 b |
| V. dahliae PP8 | 70.15 ± 1.18 a | 58.21 ± 0.73 b | 55.22 ± 1.35 c | 32.84 ± 0.88 d | 71.72 ± 1.18 a | 59.07 ± 0.73 b | 61.03 ± 1.35 b | 33.95 ± 0.88 c |
The inhibition rates (%) (n = 3, mean ± SE). Different letters indicate significantly different groups (p < 0.05, ANOVA, Tukey HSD).
Figure 1.
Inhibitory effects of BV01 against fungal phytopathogens. CK: only pathogen on PDA at 25 °C for 5 d; Treatment 1: dual culture of BV01 against pathogen on PDA at 25 °C for 5 d; Treatment 2: pathogen on PDA amended with fermentation broth of BV01 at 25 °C for 5 d.
Antifungal assay by fermentation broth test showed that BV01 had relatively high inhibitory effects against different pathogens (Table 2), and the highest inhibitory rate reached 92% (against B. sorokiniana PP12) (Figure 1, Treatment 2). Overall, the effects of BV01 were better than those of JDF and L01 and were significantly superior to those of BS208.
3.2. Identification of Strain BV01
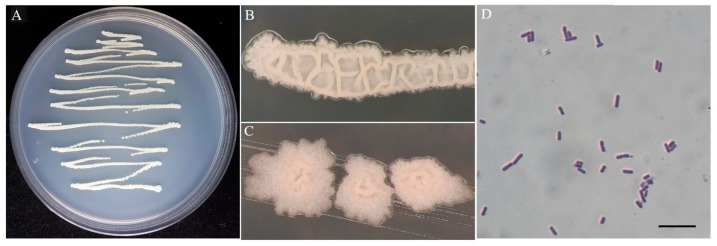
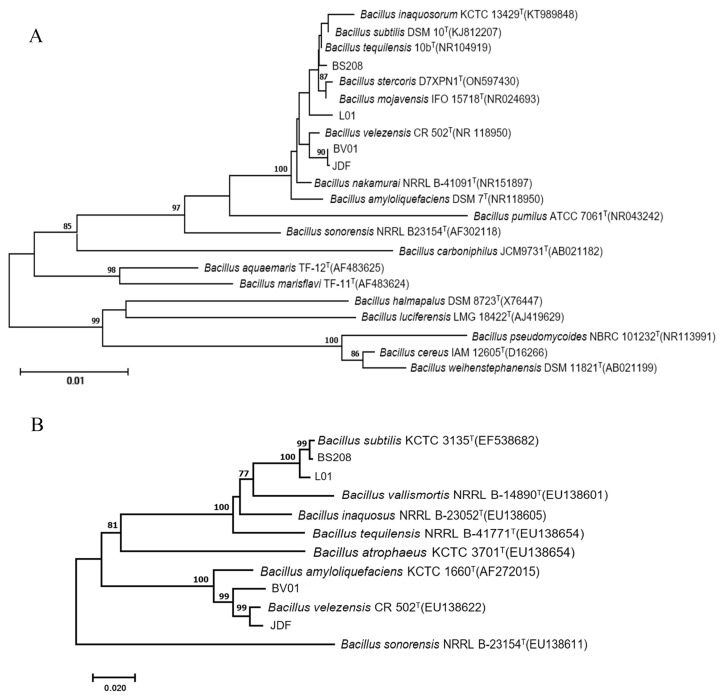
The colony of BV01 was ivory white and non-transparent with a rough surface on NA medium (Figure 2A–C). The cells were Gram-positive (Figure 2D), rod-shaped, 1.43–2.53 µm long, 0.66–0.88 µm wide, and occurred singly, in pairs, or occasionally in short chains. The analysis of 16S rRNA sequences showed that strain BV01 shared 99% identity with the type strain of B. velezensis (CR502) according to a BLAST search. The resulting NJ trees based on sequences of 16S rRNA and gyrA (Figure 3) showed that BV01 clustered with Bacillus species and grouped with the type strain of B. velezensis, which confirmed its taxonomic position.
Figure 2.
Colony and microscopic characteristics of BV01. (A–C) General colonies on nutrient agar; (D) Gram-stained cells. Bar: D = 10 µm.
Figure 3.
Phylogenetic trees generated based on sequences of 16S rRNA (A) and gyrA (B) regions of Bacillus species. NJBP values greater than 75% are shown at the nodes.
3.3. Detection of Antagonism-Related Lytic Enzymes
Clear zones detected around the colony of BV01 indicated that the strain produced protease, cellulase, and β-1,3-glucanase (Figure 4A–C) as well as siderophore (Figure 4D) and IAA (Figure 4E), which suggested its high potential in biological control. The production of IAA reached 12.17 mg/mL after incubation for 6 d.
Figure 4.
Detection of extracellular enzyme production and growth-promotion traits of BV01. (A) protease; (B) cellulase; (C) β-1,3-glucanase; (D) siderophore; (E) indole-3-acetic acid (IAA); Gp1: BV01 suspension, CK1: 10 mg/mL IAA, CK2: sterilize distilled water, CK3: NB.
3.4. Biocontrol Effects of Bacterial Strains BV01 and JDF on Wheat Root Rot
Lesions at the stem bases of wheat were obviously brown in the non-treated control, while those treated with BV01 and JDF were very slightly infected (Figure 5A). The disease indices of CK, BV01, and JDF were 76.4, 40.8, and 53.6, respectively (Figure 5B). In the BV01 treatment, infection with wheat root rot was significantly (p < 0.05) reduced, the relative control efficacy was 47% (Figure 5C), and the fresh and dry weights (Figure 5D) and plant height (Figure 5E) were increased by 91%, 34%, and 24%, respectively.
Figure 5.
Inhibitory effect of Bacillus strains on wheat root rot disease caused by B. sorokiniana PP12. (A) Symptoms of B. sorokiniana on wheat roots with different treatments; (B) disease index of B. sorokiniana in the treatments with BV01 and JDF; (C) inhibition rates of BV01 and JDF; (D) fresh and dry weights of wheat; (E) shoot biomass (cm) measured by plant height of wheat. The results were observed after 40 d of incubation. Values are the means ± SEs, n = 27 plants, ** p < 0.001, and * p < 0.05.
3.5. Biocontrol Effect of Strain BV01 on Fusarium Wilt
The symptoms on pepper leaves of the control were severe, on those treated with JDF were moderate, and on those treated with BV01 were weak (Figure 6A). The average diameter of a spot was 2.31, 0.99, and 1.76 cm in the CK, BV01, and JDF treatments (Figure 6B). The control effect reached 57% and 24% for the treatments with BV01 and JDF, respectively (Figure 6C).
Figure 6.
Effect of Bacillus strains on disease symptoms caused by F. graminearum PP15 on leaves. (A) Symptoms of F. graminearum on leaves with different treatments; (B) spot diameter after treatment with BV01 or JDF; (C) inhibition rates of BV01 and JDF. Values are the means ± SEs, n = 9 leaves, ** p < 0.001, and * p < 0.05.
3.6. Growth-Promotion Effects of Strain BV01 on Wheat and Pepper
Wheat treated with BV01 exhibited an increase in height of 13% (Figure 7A), while the fresh weight and dry weight were improved by 10% and 5% (Figure 7B). Pepper treated with BV01 exhibited increases in the fresh weight, leaf width, and stem thickness of 20%, 9%, and 9%, respectively. The dry weight and plant height were improved by 12% and 2% (Figure 7C,D). The SSI for treatment with BV01 and JDF increased by 19% and 10%. These findings suggest that strain BV01 was more effective in promoting plant growth than JDF.
Figure 7.
Growth-promotion effects of BV01 and JDF on wheat and pepper. Wheat growth (A) and experimental conditions (B) in CK, BV01, and JDF pot experiments, n = 12 plants. Pepper growth (C) and experimental conditions (D) in CK, BV01, and JDF pot experiments, n = 9 plants. Values are the means ± SEs, different letters show significant differences (Fisher’s LSD, p < 0.05).
4. Discussion
For a long time, Bacillus amyloliquefaciens and B. subtilis were known to have biocontrol functions against various plant pathogens [33]. Recently, B. velezensis was reported as a biocontrol agent against many phytopathogens. For example, B. velezensis strain F21 can control Fusarium wilt on watermelon [19], and strain BR-01 has strong antagonistic effects on rice pathogens [34], while strain CE100 increases fruit yield of strawberries by controlling fungal diseases [35]. The star strain FZB42 was initially established in 1998, and successive studies on its antimicrobial substances, interactions between plants and bacteria, regulatory small RNAs, and biocontrol enzymes have been carried out [33]. In previous studies, antagonistic strains of B. velezensis were often isolated from water, soil, air, plant roots, plant surfaces, and animal intestines [7]. In the present study, strain BV01 was derived from a PDA plate in the laboratory and speculated to be an air source strain. Based on morphological characteristics and phylogenetic evidence, strain BV01 was identified as B. velezensis; further exploration of its biological control potential was then performed. Its dual-culture inhibition rates against different pathogens were greater than 56%, and the fermentation broth inhibition rates were reduced by more than 36% when compared to the control. The results indicate that BV01 produces a special antibacterial substance. Some lipopeptide extract components of B. amyloliquefaciens have been demonstrated as key substances in controlling the growth of Xanthomonas citri subsp. citri [36]. Zhou et al. [34] proved that the relative inhibition rate of B. velezensis BR-01 against F. fujikuroi was 57%, while the strain showed no antagonistic ability against R. solani. The results of the current study revealed that strain BV01 possessed very strong antagonistic activity and broad-spectrum biological ability against B. cinerea, F. oxysporum, C. capsici, V. dahliae, R. solani, B. sorokiniana, F. graminearum, and N. rubicola.
Many Bacillus species produce a variety of hydrolytic enzymes, such as cellulase, β-1,3-glucanase, and protease, which are responsible for the degradation of diverse components of fungal pathogens [35,37]. The detection of cellulase, protease, and β-1,3-glucanase in BV01 supports its association with the growth suppression of several fungal phytopathogens. Our results also revealed that strain BV01 effective in vitro against fungal pathogens was also able to produce siderophores, which are related to indirect antagonistic processes such as plant defenses and growth promotion [30]. Moreover, some members of Bacillus invade the rhizosphere of plants and promote plant growth by producing plant hormones, such as IAA, cytokinins, and gibberellins, and chelating minerals and siderophores. Many plant-growth-promoting bacteria produce IAA, which promotes the development of plant roots, and are usually utilized as bioinoculants [38,39,40,41,42,43,44,45]. In a previous study, B. velezensis BY6 was reported to significantly increase the dry and fresh mass and plant height of Pdpap poplar seedlings [46]. In the present study, B. velezensis BV01 produced IAA during its growth. Moreover, our pot experiment results revealed that pepper and wheat treated with strain BV01 possessed higher fresh weight, dry weight, plant height, leaf width, stem thickness, and SSI than controls. Both the antifungal activity assay and greenhouse pot experiment indicated that the strain BV01 has biocontrol and plant-growth-promotion potential.
Wheat and pepper are two of the most commonly grown crops and vegetables in the world. Several pathogens cause severe diseases of them and thus reduce significantly their yields. For example, wheat root rot caused by B. sorokiniana, Fusarium spp., and other pathogens alone or in combination generally can lead to wheat yield reductions of 20%–30%, with severe cases of more than 50% [47,48]. Previous studies revealed that B. subtilis and B. amyloliquefaciens can prevent and control wheat root rot [47]. However, there are few studies on the effects of B. velezensis on wheat root rot caused by B. sorokiniana. Bacillus velezensis strains CC09 and NEAU-242-2 could be used as potential biocontrol agents to control wheat disease [49,50]. In this study, B. velezensis strain BV01 was able to effectively control wheat root rot caused by B. sorokiniana in a greenhouse, with a control rate of 47%. The occurrence of pepper wilt is increasing currently and seriously affects the quality of pepper. For example, the incidence of pepper wilt disease in China is generally 15%–30%, with severe cases decreasing quality by 70%–80% [51]. The main pathogen, Fusarium graminearum, is a highly destructive phytopathogen, not only lowering crop yields but also producing mycotoxins and affecting crop quality. Previous studies have confirmed that B. velezensis could control pepper root rot [52], wheat spikes [53], corn stalk rot [54], and corn head blight [55]. To our knowledge, the present study is the first report that B. velezensis can serve as a potential biocontrol agent for controlling pepper wilt induced by F. graminearum. Bacillus velezensis BV01 not only promotes the growth of wheat and pepper seedlings but also significantly controls wheat root rot and pepper wilt. In summary, Bacillus velezensis BV01 has good control effects in both dual-culture and fermentation broth tests against B. sorokiniana and F. graminearum, and it obviously reduced the disease symptoms and promoted the growth of wheat and pepper.
5. Conclusions
Bacillus velezensis BV01 showed protease, cellulase, and β-1,3-glucanase activities, which are related to phytopathogen cell wall degradation, and produced growth-promotion substances such as IAA and siderophore. This strain also suppressed the growth of eight phytopathogens both in dual-culture and sterile filtrate assays and significantly reduced the disease incidence of wheat root rot and Fusarium wilt in greenhouse settings. Moreover, it significantly promoted wheat and pepper growth. In conclusion, BV01 exhibits broad and effective antagonistic activity against several phytopathogens, promotes plant growth, and is worthy of further exploration of its biocontrol applications in eco-friendly agriculture practices.
Acknowledgments
The authors would like to thank Yongchun Niu, Jiyan Qiu, Zhengqiang Ma, Qili Li, and Shenzhan Fu for providing the phytopathogens used in this study and Hongjun Chen for corrections to the language.
Author Contributions
Conceptualization, Z.Z. and W.Z.; resources, W.Z. and Z.Z.; data curation, T.H.; writing—original draft preparation, T.H.; writing—review and editing, Y.Z., W.Z., Z.Y. and Z.Z.; visualization, T.H. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All the data relevant to this manuscript are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was supported by the National Natural Science Foundation of China (32270009, 31870012, 31750001), the Biological Resources Programme, Chinese Academy of Sciences (KFJ-BRP-017-082), and the Frontier Key Program of the Chinese Academy of Sciences (QYZDY-SSW-SMC029).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Savary S., Willocquet L., Pethybridge S.J., Esker P., McRoberts N., Nelson A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019;3:430–439. doi: 10.1038/s41559-018-0793-y. [DOI] [PubMed] [Google Scholar]
- 2.Xu S., Liu Y.X., Cernava T., Wang H., Zhou Y., Xia T., Cao S., Berg G., Shen X.X., Wen Z., et al. Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts. Nat. Microbiol. 2022;7:831–843. doi: 10.1038/s41564-022-01131-x. [DOI] [PubMed] [Google Scholar]
- 3.Wennersten R., Qie S. United nations sustainable development goals for 2030 and resource use. In: Filho W.L., editor. Handbook of Sustainability Science and Research. Springer International Publishing; Cham, Switzerland: 2018. pp. 317–339. [Google Scholar]
- 4.Mesnage R., Defarge N., Spiroux de Vendômois J., Séralini G. Major pesticides are more toxic to human cells than their declared active principles. BioMed Res. Int. 2014;2014:179691. doi: 10.1155/2014/179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borriss R. Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents in agriculture. In: Maheshwari D., editor. Bacteria in Agrobiology: Plant Growth Responses. Springer; Berlin/Heidelberg, Germany: 2011. pp. 41–76. [Google Scholar]
- 6.Jing J.Y., Cong W.F., Bezemer T.M. Legacies at work: Plant-soil-microbiome interactions underpinning agricultural sustainability. Trends Plant Sci. 2022;27:781–792. doi: 10.1016/j.tplants.2022.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Chowdhury S.P., Hartmann A., Gao X.W., Borriss R. Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42—A review. Front. Microbiol. 2015;6:780. doi: 10.3389/fmicb.2015.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan B., Blom J., Klenk H.P., Borriss R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form an “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017;8:22. doi: 10.3389/fmicb.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solano-Alvarez N., Valencia-Hernández J.A., Rico-García E., Torres-Pacheco I., Ocampo-Velázquez R.V., Escamilla-Silva E.M., Romero-García A.L., Alpuche-Solís Á.G., Guevara-González R.G. A novel isolate of Bacillus cereus promotes growth in tomato and inhibits Clavibacter michiganensis infection under greenhouse conditions. Plants. 2021;10:506. doi: 10.3390/plants10030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmiedeknecht G., Bochow H., Junge H. Use of Bacillus subtilis as biocontrol agent. II. Biological control of potato diseases. J. Plant Dis. Protect. 1998;105:376–386. [Google Scholar]
- 11.Sylla J., Alsanius B.W., Krüger E., Reineke A., Strohmeier S., Wohanka W. Leaf microbiota of strawberries as affected by biological control agents. Phytopathology. 2013;103:1001–1011. doi: 10.1094/PHYTO-01-13-0014-R. [DOI] [PubMed] [Google Scholar]
- 12.Talboys P.J., Owen D.W., Healey J.R., Withers P.J.A., Jones D.L. Auxin secretion by Bacillus amyloliquefaciens FZB42 both stimulates root exudation and limits phosphorus uptake in Triticum aestivum. BMC Plant Biol. 2014;14:51. doi: 10.1186/1471-2229-14-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhury S.P., Dietel K., Rändler M., Schmid M., Junge H., Borriss R., Hartmann A., Grosch R. Effects of Bacillus amyloliquefaciens FZB42 on lettuce growth and health under pathogen pressure and its impact on the rhizosphere bacterial community. PLoS ONE. 2013;8:68818. doi: 10.1371/journal.pone.0068818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mácha H., Marešová H., Juříková T., Švecová M., Benada O., Škríba A., Baránek M., Novotný Č., Palyzová A. Killing effect of Bacillus velezensis FZB42 on a Xanthomonas campestris pv. campestris (Xcc) strain newly isolated from cabbage Brassica oleracea Convar. capitata (L.): A metabolomic study. Microorganisms. 2021;9:1410. doi: 10.3390/microorganisms9071410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Guo Y.J., Luo Z., Gao L.W., Li R., Zhang Y.X., Kalaji H.M., Qiang S., Chen S.G. Recent advances in alternaria phytotoxins: A review of their occurrence, structure, bioactivity, and biosynthesis. J. Fungi. 2022;8:168. doi: 10.3390/jof8020168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean R., Van Kan J.A., Pretorius Z.A., Hammond-Kosack K.E., Di Pietro A., Spanu P.D., Rudd J.J., Dickman M., Kahmann R., Ellis J., et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali S.A.M., Sayyed R.Z., Mir M.I., Khan M.Y., Hameeda B., Alkhanani M.F., Haque S., Mohammad Al Tawaha A.R., Poczai P. Induction of systemic resistance in maize and antibiofilm activity of surfactin from Bacillus velezensis MS20. Front. Microbiol. 2022;13:879739. doi: 10.3389/fmicb.2022.879739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang X.X., Guo Y., Leng S., Xiao L., Wang L.H., Xue Y.R., Liu C.H. Comparative transcriptome profiling of Gaeumannomyces graminis var. tritici in wheat roots in the absence and presence of biocontrol Bacillus velezensis CC09. Front. Microbiol. 2019;10:1474. doi: 10.3389/fmicb.2019.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang C.H., Yao X.F., Mi D.D., Li Z.J., Yang B.Y., Zheng Y., Qi Y.J., Guo J.H. Comparative transcriptome analysis reveals the biocontrol mechanism of Bacillus velezensis F21 against Fusarium wilt on watermelon. Front. Microbiol. 2019;10:652. doi: 10.3389/fmicb.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan H.H., Qiu Y., Yang S., Wang Y.Q., Wang K.Y., Jiang L.L., Wang H.Y. Antagonistic activity of Bacillus velezensis SDTB038 against Phytophthora infestans in potato. Plant Dis. 2021;105:1738–1747. doi: 10.1094/PDIS-08-20-1666-RE. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D.F., Gao Y.X., Wang Y.J., Liu C., Shi C.B. Advances in taxonomy, antagonistic function and application of Bacillus velezensis. Microbiol. China. 2020;47:3634–3649. [Google Scholar]
- 22.Xu W., Zhang L.Y., Goodwin P.H., Xia M.C., Zhang J., Wang Q., Liang J., Sun R.H., Wu C., Yang L.R. Isolation, identification, and complete genome assembly of an endophytic Bacillus velezensis YB-130, potential biocontrol agent against Fusarium graminearum. Front. Microbiol. 2020;11:598285. doi: 10.3389/fmicb.2020.598285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z., Guo B., Wan K., Cong M., Huang H., Ge Y. Effects of bacteria-free filtrate from Bacillus megaterium strain L2 on the mycelium growth and spore germination of Alternaria alternata. Biotechnol. Biotechnol. Equip. 2015;29:1062–1068. doi: 10.1080/13102818.2015.1068135. [DOI] [Google Scholar]
- 24.Tripathi N., Sapra A. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Gram staining. [PubMed] [Google Scholar]
- 25.Tippmann H.F. Analysis for free: Comparing programs for sequence analysis. Brief. Bioinform. 2004;5:82–87. doi: 10.1093/bib/5.1.82. [DOI] [PubMed] [Google Scholar]
- 26.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 29.Syed-Ab-Rahman S.F., Carvalhais L.C., Chua E., Xiao Y.W., Wass T.J., Schenk P.M. Identification of soil bacterial isolates suppressing different Phytophthora spp. and promoting plant growth. Front. Plant Sci. 2018;9:1502. doi: 10.3389/fpls.2018.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demange P., Bateman A., Mertz C., Dell A., Piémont Y., Abdallah M.A. Bacterial siderophores: Structures of pyoverdins Pt, siderophores of Pseudomonas tolaasii NCPPB 2192, and pyoverdins Pf, siderophores of Pseudomonas fluorescens CCM 2798. Identification of an unusual natural amino acid. Biochemistry. 1990;29:11041–11051. doi: 10.1021/bi00502a005. [DOI] [PubMed] [Google Scholar]
- 31.Dong N., Liu X., Lu Y., Du L., Xu H., Liu H., Xin Z., Zhang Z. Overexpression of TaPIEP1, a pathogen-induced ERF gene of wheat, confers host-enhanced resistance to fungal pathogen Bipolaris sorokiniana. Funct. Integr. Genom. 2010;10:215–226. doi: 10.1007/s10142-009-0157-4. [DOI] [PubMed] [Google Scholar]
- 32.Anwar A., Yan Y., Liu Y.M., Li Y.S., Yu X.C. 5-aminolevulinic acid improves nutrient uptake and endogenous hormone accumulation, enhancing low-temperature stress tolerance in cucumbers. Int. J. Mol. Sci. 2018;19:3379. doi: 10.3390/ijms19113379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan B., Wang C., Song X.F., Ding X.L., Wu L.M., Wu H.J., Gao X.W., Borriss R. Bacillus velezensis FZB42 in 2018: The gram-positive model strain for plant growth promotion and biocontrol. Front. Microbiol. 2018;9:2491. doi: 10.3389/fmicb.2018.02491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J.P., Xie Y.Q., Liao Y.H., Li X.Y., Li Y.M., Li S.P., Ma X.G., Lei S.M., Lin F., Jiang W., et al. Characterization of a Bacillus velezensis strain isolated from Bolbostemmatis rhizoma displaying strong antagonistic activities against a variety of rice pathogens. Front. Microbiol. 2022;13:983781. doi: 10.3389/fmicb.2022.983781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong S., Kim T.Y., Won S.J., Moon J.H., Ajuna H.B., Kim K.Y., Ahn Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms. 2022;10:365. doi: 10.3390/microorganisms10020365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Liang L., Shao H., Ye X., Yang X., Chen X., Shi Y., Zhang L., Xu L., Wang J. Isolation of the novel strain Bacillus amyloliquefaciens F9 and identification of lipopeptide extract components responsible for activity against Xanthomonas citri subsp. citri. Plants. 2022;11:457. doi: 10.3390/plants11030457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jing R.X., Li N., Wang W.P., Liu Y. An endophytic strain JK of genus Bacillus isolated from the seeds of super hybrid rice (Oryza sativa L., Shenliangyou 5814) has antagonistic activity against rice blast pathogen. Microb. Pathog. 2020;147:104422. doi: 10.1016/j.micpath.2020.104422. [DOI] [PubMed] [Google Scholar]
- 38.Idris E.E., Iglesias D.J., Talon M., Borriss R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant Microbe Interact. 2007;20:619–626. doi: 10.1094/MPMI-20-6-0619. [DOI] [PubMed] [Google Scholar]
- 39.Kloepper J.W., Ryu C.M., Zhang S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- 40.Yao A.V., Bochow H., Karimov S.A., Boturov U., Sanginboy S., Sharipov A. Effect of FZB24® Bacillus subtilis as a biofertilizer on cotton yields in field tests. Arch. Phytopathol. Plant Prot. 2006;39:323–328. doi: 10.1080/03235400600655347. [DOI] [Google Scholar]
- 41.Bloemberg G.V., Lugtenberg B.J.J. Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr. Opin. Plant Biol. 2001;4:343–350. doi: 10.1016/S1369-5266(00)00183-7. [DOI] [PubMed] [Google Scholar]
- 42.Compant S., Duffy B., Nowak J., Clement C., Barka E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microb. 2005;71:4951–4959. doi: 10.1128/AEM.71.9.4951-4959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi Y.J., Luan P.Y., Wang K., Li G.L., Yin Y.A., Yang Y.H., Zhang Q.Y., Liu Y. Antifungal activity and plant growth-promoting properties of Bacillus mojovensis B1302 against Rhizoctonia cerealis. Microorganisms. 2022;10:1682. doi: 10.3390/microorganisms10081682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Balderas-Ruíz K.A., Bustos P., Santamaria R.I., González V., Cristiano-Fajardo S.A., Barrera-Ortíz S., Mezo-Villalobos M., Aranda-Ocampo S., Guevara-García Á.A., Galindo E., et al. Bacillus velezensis 83 a bacterial strain from mango phyllosphere, useful for biological control and plant growth promotion. AMB Express. 2020;10:163. doi: 10.1186/s13568-020-01101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hernandez J., Tamez-Guerra P., Gomez-Flores R., Delgado C., Robles-Hernandez L., Gonzalez-Franco A.C., Infante-Ramirez R. Pepper growth promotion and biocontrol against Xanthomonas euvesicatoria by Bacillus cereus and Bacillus thuringiensis formulations. Peer J. 2023;11:e14633. doi: 10.7717/peerj.14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P., Xie G., Wang L., Xing Y. Bacillus velezensis BY6 promotes growth of poplar and improves resistance contributing to the biocontrol of Armillaria solidipes. Microorganisms. 2022;10:2472. doi: 10.3390/microorganisms10122472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Sadi A.M. Bipolaris sorokiniana-induced black point, common root rot, and spot blotch diseases of wheat: A review. Front. Cell. Infect. Microbiol. 2021;11:584899. doi: 10.3389/fcimb.2021.584899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H., Ren Z.H., Zu X., Yu X.Y., Zhu H.J., Li X.J., Zhong J., Liu E.M. Efficacy of plant growth-promoting bacteria Bacillus cereus YN917 for biocontrol of rice blast. Front. Microbiol. 2021;12:684888. doi: 10.3389/fmicb.2021.684888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang X., Zhang W., Cai X., Zhu T., Xue Y., Liu C. Bacillus velezensis CC09: A potential ‘Vaccine’ for controlling wheat diseases. Mol. Plant Microbe Interact. 2018;31:623–632. doi: 10.1094/MPMI-09-17-0227-R. [DOI] [PubMed] [Google Scholar]
- 50.Zhao T., Zhang L., Qi C., Bing H., Ling L., Cai Y., Guo L., Wang X., Zhao J., Xiang W. A seed-endophytic bacterium NEAU-242-2: Isolation, identification, and potential as a biocontrol agent against Bipolaris sorokiniana. Biol. Control. 2023;185:105312. doi: 10.1016/j.biocontrol.2023.105312. [DOI] [Google Scholar]
- 51.Zhang J.Q., Zheng A.K., Gao L.H., Sun Y.M., Jiang Z.Y. Isolation and identification of the main pathogens causing Capsicum wilt and screening of agricultural antagonisms. J. Anhui Agric. Sci. 2021;49:134–137. [Google Scholar]
- 52.Pei D., Zhang Q., Zhu X., Yao X., Zhang L. The complete genome sequence resource of rhizospheric soil-derived Bacillus velezensis Yao, with biocontrol potential against Fusarium solani-induced pepper root rot. Phytopathology. 2023;113:580–583. doi: 10.1094/PHYTO-03-22-0101-A. [DOI] [PubMed] [Google Scholar]
- 53.Cantoro R., Palazzini J.M., Yerkovich N., Miralles D.J., Chulze S.N. Bacillus velezensis RC 218 as a biocontrol agent against Fusarium graminearum: Effect on penetration, growth and TRI5 expression in wheat spikes. BioControl. 2020;66:259–270. doi: 10.1007/s10526-020-10062-7. [DOI] [Google Scholar]
- 54.Wang S., Sun L., Zhang W., Chi F., Hao X., Bian J.Y., Li Y. Bacillus velezensis BM21, a potential and efficient biocontrol agent in control of corn stalk rot caused by Fusarium graminearum. Egypt. J. Biol. Pest Control. 2020;30:9. doi: 10.1186/s41938-020-0209-6. [DOI] [Google Scholar]
- 55.Kim J.A., Song J.S., Kim P.I., Kim D.H., Kim Y. Bacillus velezensis TSA32-1 as a promising agent for biocontrol of plant pathogenic fungi. J. Fungi. 2022;8:1053. doi: 10.3390/jof8101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data relevant to this manuscript are available on request from the corresponding author.